Abstract
Background/Aims
The insulin-like growth factor (IGF) system has been implicated in tumor growth, invasion, and metastasis. However, reports on the IGF-1 receptor (IGF-1R) based on radioimmunoassays are conflicting, and its prognostic implications in non-small-cell lung cancer (NSCLC) are still controversial.
Methods
Seventy-one paraffin-embedded tissue sections from stage I NSCLC patients were stained using a mouse monoclonal antibody against human IGF-1R.
Results
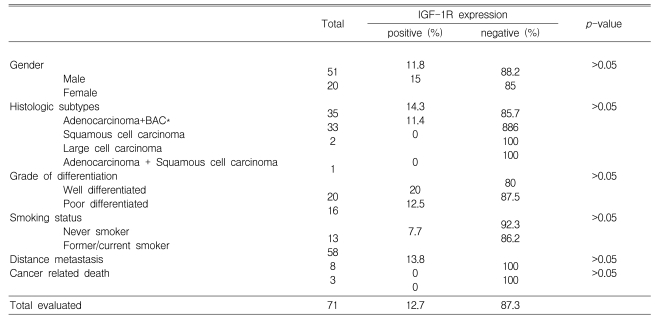
The intensity and frequency of IGF-1R expression on the membrane and cytoplasm of cancer cells was evaluated and scored using a semiquantitative system. IGF-1R expression was detected in nine of 71 (12.7%) cases. No significant relationship was found between clinical/histopathological parameters and IGF-1R expression. None of the patients whose tumor expressed IGF-1R had experienced distant metastasis or cancer-related death, although the difference did not reach statistical significance.
Conclusions
We conclude that IGF-1R expression may not be a major prognostic factor for stage I NSCLC.
Keywords: IGF, IGF-1R, Immunohistochemistry, NSCLC
INTRODUCTION
Non-small-cell lung cancer (NSCLC) accounts for approximately 75~80% of all lung cancers and is the leading cause of cancer-related deaths worldwide1). However, 20% of stage I and 30% of stage II NSCLC patients who are treated with curative intent experience recurrence of their cancer, which is often incurable at the time of discovery. This statistic indicates the urgent need to elucidate the carcinogenesis of lung cancer and to devise new approaches for its prevention and treatment, such as chemoprevention.
The insulin-like growth factor (IGF) system plays a critical role in the growth and development of many tissues and regulates overall growth, particularly prenatal growth. It has also been implicated in various pathophysiological conditions and is thought to play a prominent role in tumorigenesis. The insulin-like growth factor-1 receptor (IGF-1R) is a glycosylated heterotetramer composed of two extracellular α- and β-subunits that have intrinsic tyrosine kinase activity, and it shares 70% homology with the insulin receptor. It is encoded by the IGF-1R gene located on chromosome 15q262, 3). IGF-1R mainly mediates the effect of IGFs, which are potent mitogens that regulate cell proliferation, differentiation, and protection from apoptosis4). Clinical and epidemiological data suggest that the serum levels of IGF-1 and IGF-binding proteins (IGFBPs) are related to the risk of solid tumors, such as breast, prostate, endometrial, ovarian, and colon cancer5). In breast cancer, IGF-1R expression and activation have been linked to disease progression, increased resistance to radiotherapy, and a poor prognosis6, 7). The expression of a dominant-negative truncated IGF-1R in colon cancer cells reduced the expression of vascular endothelial growth factor, impaired tumor progression in nude mice, and increased tumor cell apoptosis8). More focused studies of IGF-1R expression in the breast and prostate that used immunohistochemistry or matched cell lines corresponding to normal and tumor tissue revealed that normal epithelium and early-stage tumors both express abundant IGF-1R and that its expression is reduced significantly in advanced metastatic cancer9-13).
Although several commercial anti-IGF-1R antibodies are available, no scoring system for IGF-1R expression in formalin-fixed, paraffin-embedded tissue has been established. Here, we report the prognostic implications of IGF-1R expression as determined immunohistochemically using archived materials of stage I NSCLC. We evaluated its expression in cancer cell membranes and the cytoplasm and correlated its expression with various clinicopathological parameters.
MATERIALS AND METHODS
Study population and samples
We examined 71 tissue specimens from stage I NSCLC patients who had undergone curative surgical removal of primary lesions between 1995 and 1998 at Yonsei Medical Center. The pathological evaluation established the histological classification and stage in all patients. None of the patients had either radiotherapy or chemotherapy before or after surgery until the disease recurred. All clinical and pathological information and follow-up data were obtained from reports from our registry service. The study was reviewed and approved by the institution's Surveillance Committee to allow us to obtain pertinent information from the patients' files.
Immunohistochemical staining for IGF-1R
Paraffin-embedded, 4-µm-thick tissue sections were stained with mouse monoclonal antibody against human IGF-1R (BioSource International, Camarillo, CA, USA). The sections were deparaffinized in a xylene bath, rehydrated using a graded alcohol series, and retrieved in 10 mM sodium citrate buffer via microwave heating for 15 min at 95℃. The sections were then treated with 0.3% hydrogen peroxidase for 10 min to block endogenous peroxidase activity. Subsequently, the sections were incubated with the primary anti-IGF-1R antibody (1:50) for 30 min at room temperature and processed using standard avidin-biotin immunohistochemical techniques according to the manufacturer's recommendations (Vector Laboratories, Burlingame, CA, USA). Diaminobenzidine (DAB) was used as a chromogen, and commercial hematoxylin was used for counterstaining. Each time, placenta and breast cancer tissues were stained to serve as positive controls. Adjacent normal-appearing bronchial epithelium within each tissue section served as an internal reference. The intensity of IGF-1R immunostaining of the membrane and cytoplasm of the invasive cancer components was evaluated. Cell membrane and cytoplasm staining were scored on a scale of 0, 1+, 2+, and 3+, where scores of 1+ and above were classified as positive. All slides were evaluated and scored independently by two pathologists who were blind to the subjects' clinical information.
Statistical analysis
In the univariate analysis, independent sample t-tests and Fisher's exact tests were used for continuous and categorical variables, respectively. The Kaplan-Meier estimator was used to compute survival probability as a function of time. The log-rank test was used to compare survival time between groups. All statistical tests were two-sided. p<0.05 was considered significant.
RESULTS
Patient demographic characteristics
The NSCLC cases included 51 (71.8%) males and 20 (28.2%) females with a median age of 61.8 (31-83) years, which is similar to the distribution in the large database of patients with stage I NSCLC from our institution (data not shown). Thirty-five (49.3%) patients were diagnosed with adenocarcinoma, 33 (46.5%) with squamous cell carcinoma, and three (4.2%) with other histological subtypes. Fifty-eight (81.7%) patients had a history of smoking. The general clinical characteristics of the patients are shown in Table 1.
Table 1.
Analysis of the patients with stage I NSCLC according to the expression status of IGF-1R
*Bronchioloalveolar carcinoma
IGF-1R immunohistochemistry
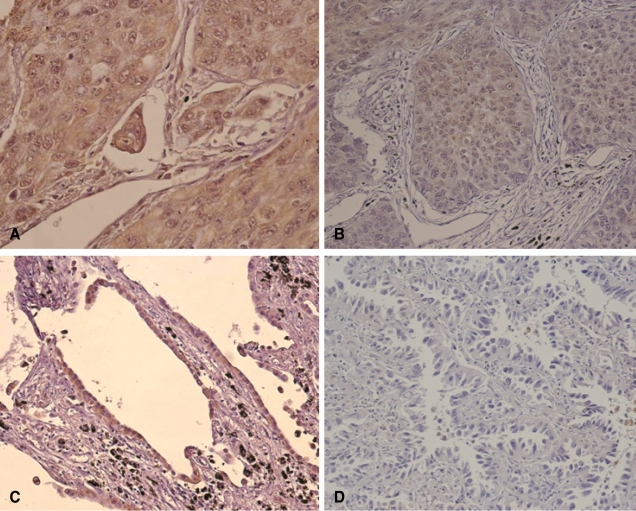
IGF-1R expression was granular, heterogeneous, and discrete, i.e., mainly localized to the cytoplasm of NSCLC cells (Figure 1A and B). Some bronchioloalveolar carcinoma tissue showed distinct cytoplasmic IGF-1R expression (Fig. 1C). However, the number of NSCLC tissues expressing IGF-1R was small. There were 62 (87.3%), seven (9.9%), two (2.8%), and zero (0.0%) NSCLC cases with IGF-1R staining scores of 0, 1+, 2+, and 3+, respectively. Cases with scores more than 1+ were defined as positive and were used for the subsequent statistical analysis. In the normal-appearing adjacent lung tissues, the cytoplasm of bronchial/bronchiolar epithelium showed uniform, constant IGF-1R expression. The immunohistochemical staining of IGF-1R in breast cancer and placenta tissues was also stable and reproducible (data not shown).
Figure 1.
Immunohistochemical analysis of IGF-1R expression in stage I NSCLC tissues. A. Poorly differentiated adenocarcinoma with 2+ IGF-1R expression (×400). B. Squamous cell carcinoma with 1+ IGF-1R expression (×200). C. Bronchioloalveolar carcinoma with 2+ IGF-1R expression (×200). D. Another bronchioloalveolar carcinoma that did not express IGF-1R (×200).
Correlation of IGF-1R expression with the clinicopathological parameters
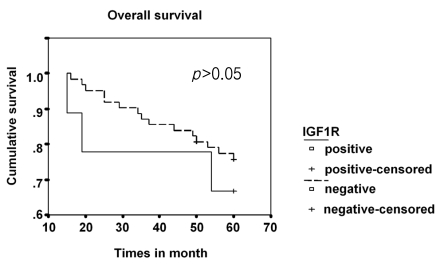
IGF-1R expression was positive in nine (12.7%) cases and negative in 62 (87.3%). IGF-1R expression was not associated with the clinical or pathological characteristics tested in this study (Table 1). The overall survival curves did not differ significantly between the group of patients with tumors expressing IGF-1R and those with negative IGF-1R expression (Figure 2). None of the patients with positive IGF-1R expression experienced distant metastasis or cancer-related death, although the difference did not reach significance (Table 1).
Figure 2.
Survival curve for patients with stage I NSCLC stratified by IGF-1R expression. There was no significant difference between the curves for patients with and without IGF-1R expression.
DISCUSSION
IGFs are potent mitogens in several types of cancer, including NSCLC and small-cell lung cancer15, 16). Furthermore, IGF-1R can upregulate several aspects of the malignant phenotype and metastasis in vitro17). In breast cancer, IGF-1R expression and activation have been linked to disease progression, increased resistance to radiotherapy, and a poor prognosis18, 19). For lung cancer, no data are available on IGF-1R expression, so we focused on the expression of IGF-1R in patients with stage I NSCLC and its significance to the clinical outcome. We tested the prognostic significance of IGF-1R expression in formalin-fixed, paraffin-embedded tissues.
We found that IGF-1R expression was downregulated in a significant fraction of patients with stage I NSCLC. Overall, nine of 71 tumors expressed IGF-1R, and its expression was localized to the membrane and cytoplasm, rather than the nucleus of NSCLC cells. Our data indicate that IGF-1R expression is not a prognostic factor in patients with stage I NSCLC. None of the patients expressing IGF-1R experienced distant metastasis or cancer-related death, although the difference was not significant. Our study did not show a better survival with IGF-1R expression. Other studies have shown that IGF-1R expression was significantly associated with a better survival in breast cancer and highly malignant soft tissue sarcoma20, 21), although other studies found the opposite22, 23). Early studies showed that functionality of the IGF-1R was a prerequisite for neoplastic transformation and for survival of the transformed cell in vitro and in vivo24). More recent evidence has pointed to putative roles for IGF-1R in cell adhesion and cancer cell dedifferentiation25). The significant association with survival observed in this study is of particular relevance, and should be confirmed in additional cohorts of patients.
In summary, in a homologous population of 71 patients with stage I NSCLC, we showed that IGF-1R expression is neither frequent nor a prognostic factor in stage I NSCLC. More comprehensive studies of the mechanism of IGF-1R expression in NSCLC are necessary to define the role of IGF-1R in lung carcinogenesis.
Footnotes
This study was supported in part by a Korea Research Foundation Grant funded by the government of the Republic of Korea (MOEHRD, KRF-2007-331-E00079) and an Institutional Grant from Yonsei University College of Medicine (6-2007) through the Human Barrier Research Institute.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Ward CW, Garrett TP, McKern NM, Lou M, Cosgrove LJ, Sparrow LG, Frenkel MJ, Hoyne PA, Elleman TC, Adams TE, Lovrecz GO, Lawrence LJ, Tulloch PA. The three dimentional structure of the type I insulin-like growth factor receptor. Mol Pathol. 2001;54:125–132. doi: 10.1136/mp.54.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakesley VA, Stannard BS, Kalebic T, Helman LJ, LeRoith D. Role of the IGF-I receptor in mutagenesis and tumor promotion. J Endocrinol. 1997;152:339–344. doi: 10.1677/joe.0.1520339. [DOI] [PubMed] [Google Scholar]
- 4.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 6.Rocha RL, Hilsenbeck SG, Jackson JG, VanDenBerg CL, Weng C, Lee AV, Yee D. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin Cancer Res. 1997;3:103–109. [PubMed] [Google Scholar]
- 7.Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, Gumbs AA, Kaplan L, Burgaud JL, Carter D, Baserga R, Glazer PM. Insulin-like growth factor-1 receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57:3079–3083. [PubMed] [Google Scholar]
- 8.Reinmuth N, Liu W, Fan F, Jung YD, Ahmad SA, Stoeltzing O, Bucana CD, Radinsky R, Ellis LM. Blockade of insulin-like growth factor I receptor function inhibits growth and angiogenesis of colon cancer. Clin Cancer Res. 2002;8:3259–3269. [PubMed] [Google Scholar]
- 9.Tennant MK, Trasher JB, Twomey PA, Drivdahl RH, Birnbaum RS, Plymate SR. Protein and messenger ribonucleic acid (mRNA) for the type I insulin-like growth factor (IGF) receptor is decreased and IGF-II mRNA is increased in human prostate carcinoma compared to benign prostate epithelium. J Clin Endocrinol Metab. 1996;81:3774–3782. doi: 10.1210/jcem.81.10.8855837. [DOI] [PubMed] [Google Scholar]
- 10.Happerfield LC, Miles DW, Barnes DM, Thomsen LL, Smith P, Hanby A. The localization of the insulin-like growth factor receptor 1 (IGFR-1) in benign and malignant breast tissue. J Pathol. 1997;183:412–417. doi: 10.1002/(SICI)1096-9896(199712)183:4<412::AID-PATH944>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Chott A, Sun Z, Morganstern D, Pan J, Li T, Susani M, Mosberger I, Upton MP, Bubley GJ, Balk SP. Tyrosin kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. 1999;155:1271–1279. doi: 10.1016/S0002-9440(10)65229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnarr B, Strunz K, Oham J, Benner A, Wacker J, Mayer D. Down-regulation of insulin-like growth factor-I receptor and insulin receptor substrate-1 expression in advanced human breast cancer. Int J Cancer. 2000;89:506–513. doi: 10.1002/1097-0215(20001120)89:6<506::aid-ijc7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 13.Damon SE, Plymate SR, Carroll JM, Sprenger CC, Dechsukhum C, Ware JL, Roberts CT., Jr Transcriptional regulation of insulin-like growth factor-I receptor gene expression in prostate cancer cells. Endocrinology. 2001;142:21–27. doi: 10.1210/endo.142.1.7890. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Specificity of Hercep Test in determing HER-2/neu status of breast cancers sing the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999;17:1983–1987. doi: 10.1200/JCO.1999.17.7.1983. [DOI] [PubMed] [Google Scholar]
- 15.Quinn KA, Treston AM, Unsworth EJ, Miller MJ, Vos M, Grimley C, Battery J, Mulshine JL, Cuttitta F. Insulin-like growth factor expression in human cancer cell lines. J Biol Chem. 1996;271:11477–11483. doi: 10.1074/jbc.271.19.11477. [DOI] [PubMed] [Google Scholar]
- 16.Furstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 17.Mauro L, Salerno M, Morelli C, Boterberg T, Bracke ME, Surmacz E. Role of the IGF-I receptor in the regulation of cell-cell adhesion: implications in cancer development and progression. J Cell Physiol. 2003;194:108–116. doi: 10.1002/jcp.10207. [DOI] [PubMed] [Google Scholar]
- 18.Rocha RL, Hilsenbeck SG, Jackson JG, VanDenBerg CL, Weng C, Lee AV, Yee D. Insulin-like growth factor binding protein-3 and insulin receptor substrate-1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin Cancer Res. 1997;3:103–109. [PubMed] [Google Scholar]
- 19.Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, Gumbs AA, Kaplan L, Burgaud JL, Carter D, Baserga R, Glazer PM. Insulin-like growth factor-1 receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57:3079–3083. [PubMed] [Google Scholar]
- 20.Resnik JL, Reichart DB, Huey K, Webster NJ, Seely BL. Elevated insulin-like growth factor I receptor autophosphorylation and kinase activity in human breast cancer. Cancer Res. 1998;58:1159–1164. [PubMed] [Google Scholar]
- 21.Ahlén J, Wejde J, Brosjö O, von Rosen A, Weng WH, Girnita L, Larsson O, Larsson C. Insulin-like growth factor type 1 receptor expression correlates to good prognosis in highly malignant soft tissue sarcoma. Clin Cancer Res. 2005;11:206–216. [PubMed] [Google Scholar]
- 22.All-Ericsson C, Girnita L, Seregard S, Bartolazzi A, Jager MJ, Larsson O. Insulin-like growth factor-1 receptor in uveal melanoma: a predictor for metastatic disease and a potential therapeutic target. Invest Ophthalmol Vis Sci. 2002;43:1–8. [PubMed] [Google Scholar]
- 23.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, Karl RC, Coppola D. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–1133. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 24.Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995;55:249–252. [PubMed] [Google Scholar]
- 25.Playford MP, Bicknell D, Bodmer WF, Macaulay VM. Insulin-like growth factor 1 regulates the location, stability and transcriptional activity of β-catenin. Proc Natl Acad Sci U S A. 2000;97:12103–12108. doi: 10.1073/pnas.210394297. [DOI] [PMC free article] [PubMed] [Google Scholar]