Abstract
Background/Aims
The aim of this study was to examine the recent clinical trends and antibiotic susceptibilities of the causative microorganisms in renal and perirenal abscesses, and to elucidate the factors associated with treatment strategies.
Methods
We retrospectively analyzed 56 patients who were diagnosed with renal and perirenal abscesses at our hospital from January 2000 to September 2007.
Results
The mean age of the patients was 53.5 years, and a female predominance of patients (75%) was observed. Diabetes mellitus (44.6%) was the most common predisposing condition. The mean duration of symptoms before diagnosis was 11.6 days, and fever (75%) was the most common symptom. Escherichia coli (44%) and Klebsiella pneumoniae (28%) were common pathogens, and the rates of susceptibility of E. coli isolates to ampicillin, cephalothin, cefotaxime, trimethoprim-sulfamethoxazole, ciprofloxacin, gentamicin, and imipenem were 18.2%, 27.3%, 72.7%, 72.7%, 63.6%, 63.6%, and 100%, respectively. Abscesses were classified according to the location as follows: renal abscess (n=31, 55.4%) and perirenal abscess±renal abscess (n=25, 44.6%). In the renal abscess group, the infection rate of gram-negative organisms was higher than in the perirenal abscess group. Patients were also divided according to the treatment modality: antibiotics only (n=20, 35.7%) and percutaneous intervention or surgery (n=36, 64.3%). Patients who had a perirenal abscess or a large renal abscess required more invasive treatment.
Conclusion
This study revealed somewhat different results from those of previous studies. Clinical and microbial differences were observed between the renal and perirenal abscess groups. Abscess location and the size of the renal abscess were the factors associated with treatment strategies.
Keywords: Kidney, Abscess
INTRODUCTION
Renal and perirenal abscesses are rare disease entities resulting from infections in or surrounding the kidneys. In the past, they were associated with significant morbidity and mortality, which was in part due to their obscure symptoms and lack of detection using low-quality imaging systems1-5). Recently, computed tomography (CT) and magnetic resonance imaging (MRI) have become more available, and the quality of renal ultrasound examinations has increased. These advances in imaging techniques have led to earlier diagnoses of renal and perirenal abscesses. Furthermore, novel antibiotics and current percutaneous drainage techniques have reduced surgery-related morbidity and mortality6-10).
In the pre-antibiotics era, hematogenous spread of Staphylococcus aureus was the most common cause of renal and perirenal abscesses. Since the 1960s, however, infection by aerobic gram-negative bacilli has become the most common cause of infection. Escherichia coli and Klebsiella pneumoniae are presently the most frequently isolated organisms11, 12).
Since 1980, several Korean studies have examined the clinical features of renal and perirenal abscesses9, 12-14). The latest report was an analysis of 34 patients with renal and perirenal abscesses by Choi et al. in 200215).
The purpose of our study was to examine the recent clinical trends of renal and perirenal abscesses that were diagnosed after the year 2000 in Korea and to compare our results with those of recent foreign studies. Furthermore, we examined the causative organisms along with their antimicrobial susceptibilities, and compared these data with those of several recent studies that analyzed community-acquired acute pyelonephritis (CA-APN). This study was also planned to explore the factors that affect treatment modalities for renal and perirenal abscesses.
MATERIALS AND METHODS
Subjects
This study was performed retrospectively and was based on the medical records of patients who admitted to our hospital from January 2000 to December 2007. Fifty-nine patients were diagnosed with renal and perirenal abscesses during this period. Fifty-six patients were enrolled for the final analysis after excluding three who had tuberculous abscesses.
Collections of records and analyses
Age, gender, the underlying conditions, the duration of symptoms prior to diagnosis, the symptoms and physical findings at admission, and the laboratory results were obtained from the records. Furthermore, the radiological features of the abscesses, the causative organisms and their sensitivities to antibiotics, the diagnostic and treatment modalities, and the clinical outcomes were also collected.
On the basis of the radiologic findings, a renal abscess was defined as "an abscess confined only to the renal parenchyma," and a perirenal abscess was defined as "an abscess between the renal capsule and Gerota's fascia." A renal abscess together with a perirenal abscess was termed a mixed abscess.
The abscess size was determined radiologically by measuring the longest diameter of the largest abscess. The abscesses of three patients were not measurable due to their extensive involvement or lack of a radiologic image. Renal abscesses were classified into three groups according to the size of abscess as follows: <3 cm, 3~5 cm, and >5 cm.
The patients were divided according to the location of abscess into the renal abscess group and the perirenal abscess group, and we compared the clinical characteristics between the two groups. The renal abscess group consisted of patients with renal abscess alone, and the perirenal abscess group included patients with perirenal abscess alone and mixed abscess.
Additionally, we classified the patients into two groups to explore the factors that were involved in deciding treatment modalities: the antibiotics-only group and the intervention group (i.e., percutaneous drainage, aspiration, or surgical treatment).
Statistics
All continuous variables were expressed as means±SD, and the proportions were expressed as numbers (%). Comparisons between two groups were performed using the Mann-Whitney U-test and Fisher's exact test, as appropriate. Comparisons among three groups were performed using the Kruskal-Wallis test and the chi-square test. The computer software used for statistical analysis was SPSS® (version 11.5; SPSS Inc., Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics (Table 1)
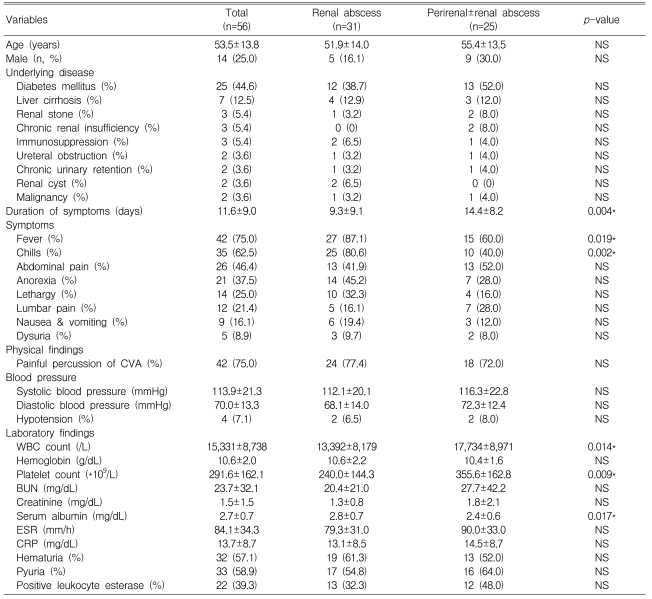
Table 1.
Patients' characteristics
CVA, costovertebral angle; WBC, white blood cell; BUN, blood urea nitrogen; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; NS, not significant
*Indicates statistically significant differences between the renal and perirenal abscess groups.
The average age of the patients was 53.5±13.8 years. There were 14 males (25%) and 42 females (75%) with a male-to-female ratio of 1:3.
Twenty-five patients (44.6%) had diabetes mellitus, which was the most common predisposing condition. The other predisposing conditions were as follows: liver cirrhosis (n=7, 12.5%), renal stones (n=3, 5.4%), chronic renal insufficiency (n=3, 5.4%), an immunosuppressed state (n=3, 5.4%), ureteral obstruction (n=2, 3.6%), chronic urinary retention (n=2, 3.6%), renal cysts (n=2, 3.6%), and malignancy (n=2, 3.6%).
The average duration of symptoms prior to admission was 11.6±9.0 days. The most common presenting symptoms were fever (n=42, 75%) and chills (n=35, 62.5%). Other presenting symptoms were as follows: abdominal pain (n=26, 46.4%), anorexia (n=21, 37.5%), and dysuria (n=5, 8.9%). Upon physical examination, a knocking tenderness of the costovertebral angle (CVA) was the most common physical finding (n=42, 75%). The mean systolic and diastolic blood pressures at admission were 113.9±21.3 mmHg and 70.0±13.3 mmHg, respectively, and four patients (7.1%) were in a hypotensive state with their systolic blood pressures less than 90 mmHg.
The mean blood leukocyte count was 15,331±8,738/L, the mean hemoglobin level was 10.6±2.0 g/dL, and the platelet count was 291.6±162.1×109/L. The mean serum chemistry values were as follows: blood urea nitrogen, 23.7±32.1 mg/dL; creatinine, 1.5±1.5 mg/dL; and albumin, 2.7±0.7 mg/dL. The mean erythrocyte sedimentation rate was 84.1±34.3 mm/h, and the C-reactive protein level was 13.7±8.7 mg/dL. Urine analysis of 56 patients showed that 33 (58.9%) had pyuria, 32 (57.1%) had hematuria, and 22 patients (39.3%) were positive for leukocyte esterase.
Radiologic findings (Tables 2, 3)
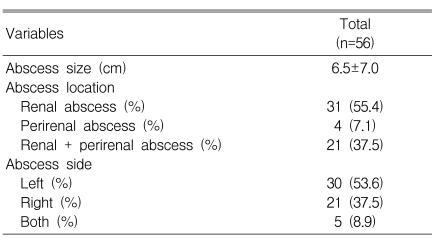
Table 2.
Radiographic findings of renal and perirenal abscesses
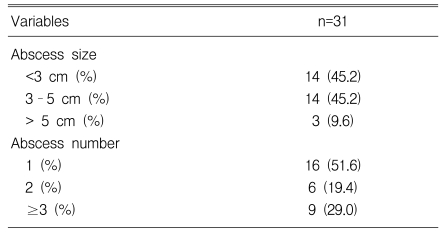
Table 3.
Characteristics of renal abscesses
Nine patients (16.1%) were diagnosed using ultrasonography (US) alone, and 33 (58%) were diagnosed by CT alone. 11 patients (19.6%) needed both US and CT for making the diagnosis, and MRI was useful in three patients (5.4%) to differentiate abscesses from other renal diseases. An intravenous pyelogram (IVP) was acquired in only one patient.
For three patients, it was impossible to measure the size of abscess because one patient had a ruptured renal abscess with extensive peritonitis, and two patients had only radiologic reports without images. Excluding these three patients, the mean size of the abscesses was 6.5±7.0 cm (range: 0.5-32 cm).
Of the 56 patients, renal abscesses were found in 31 patients (55.4%), perirenal abscesses were found in four patients (7.1%), and 21 patients (37.5%) had mixed abscesses. Thirty patients (53.6%) had left-sided abscesses, 21 patients (37.5%) had right-sided abscesses, and both kidneys were involved in five patients (8.9%). For the 31 cases of renal abscesses, 14 patients (45.2%) had abscesses smaller than 3 cm, 14 (45.2%) had lesions ranging from 3 to 5 cm, and three patients (9.6%) had lesions greater than 5 cm. Sixteen patients (51.6%) had a single lesion, six patients (19.4%) had two lesions, and multiple abscesses (three or more) were found in nine patients (29.0%).
Results of culture and antimicrobial susceptibility(Table 4, 5)
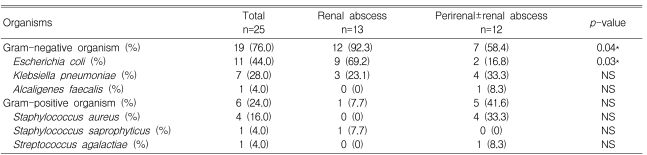
Table 4.
Results of culture in renal and perirenal abscesses
*Indicates statistically significant differences between the renal and perirenal abscess groups.
Table 5.
Antimicrobial susceptibility of the cultured organisms
AMP, ampicillin; CPT, cephalothin; CTX, cefotaxime; BAC, trimethoprim-sulfamethoxazole; CFN, ciprofloxacin; GM, gentamicin; IMP, imipenem; S, sensitive; R, resistant
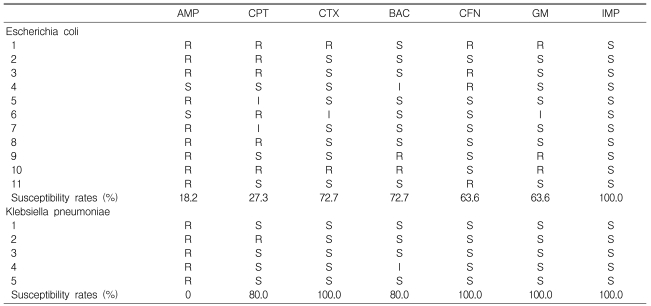
The microbial culture results from the urine, blood, and abscess were available for 52, 48, and 30 patients, respectively. Urine cultures were positive in 14 patients (26.9%), blood cultures were positive in eight patients (16.7%), and 15 patients (50.0%) had positive culture reports from the abscesses. The most frequently isolated pathogen was E. coli (11 cases, 44%). Others were K. pneumoniae (n=7, 28%) and S. aureus (n=4, 16%). Three cases were caused by Alcaligenes faecalis, Staphylococcus saprophyticus, or Streptococcus agalactiae, respectively.
The rates of antimicrobial susceptibility 11 E. coli isolates to ampicillin, cephalothin, cefotaxime, trimethoprim-sulfamethoxazole, ciprofloxacin, gentamicin, and imipenem were 18.2%, 27.3%, 72.7%, 72.7%, 63.6%, 63.6%, and 100%, respectively. In five K. pneumoniae isolates, the rates were 0%, 80.0%, 100.0%, 80.0%, 100.0%, 100.0%, and 100.0%, respectively. All four S. aureus were resistant to penicillin, but were susceptible to methicillin.
Comparison of antimicrobial susceptibility between the abscess and CA-APN in E. coli (Table 6)
Table 6.
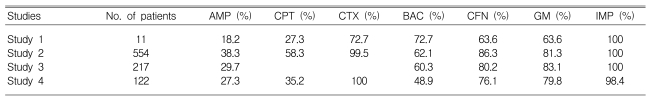
Comparison of the antimicrobial susceptibility rates (%) of Escherichia coli among our study and previous studies investigating community-acquired acute pyelonephritis
We compared our antimicrobial susceptibility results from the E. coli isolates with those of recent Korean studies that investigated CA-APN26-28). The E. coli isolates from our study were relatively resistant to ampicillin, cephalothin, cefotaxime, ciprofloxacin, and gentamicin, but were more susceptible to trimethoprim-sulfamethoxazole. Similar to previous studies rates of susceptibility to imipenem for E. coli were 100%.
Treatment modalities and outcomes
All 56 patients were treated with antibiotics irrespective of other interventions. Twenty patients (35.7%) were treated with antibiotics alone. Among the 30 patients who received percutaneous interventions, 11 patients (19.6%) were subjected to percutaneous needle aspiration of the abscess, and 19 patients (33.9%) underwent percutaneous catheter drainage of the abscess. Surgical drainage was performed in two patients (3.6%); one patient had a renal abscess diagnosed after laparotomy and the other had a perirenal abscess combined with extensive peritonitis. Nephrectomy was performed in four patients (7.2%) for the following reasons, excluding one case for which the operative cause was not described: an extensive perirenal abscess due to staghorn calculus, a perirenal abscess combined with a renal pelvic stone, and an extensive perirenal abscess that had no response to initial percutaneous drainage. Of the 56 patients, 53 patients (94.6%) showed clinical improvement and were discharged from the hospital, but three patients (5.4%) died due to refractory sepsis.
Comparisons between the renal and perirenal abscess groups
1) Patients' characteristics and laboratory findings (Table 1)
There were no differences in the patients' age, gender, and predisposing conditions between the renal and perirenal abscess groups. The perirenal abscess group had a longer duration of symptoms prior to admission than the renal abscess group (14.4±8.2 days vs. 9.3±9.1 days, p=0.004). Fever and chills were more commonly observed in the renal abscess group than in the perirenal abscess group (p=0.019, p=0.002, respectively). The perirenal abscess group had a higher leukocyte count (17,734±8,971/L vs. 13,392±8,179/L, p=0.014) and a lower serum albumin level (2.4±0.6 mg/dL vs. 2.8±0.7 mg/dL, p=0.017). A significant difference in the platelet count was found between the two groups, but both groups had platelet count within the normal range.
2) Culture results (Table 4)
In the renal abscess group, 13 organisms were isolated. These included E. coli (n=9, 69.2%), K. pneumoniae (n=3, 23.1%), and S. saprophyticus (n=1, 7.7%). In the perirenal abscess group, the 12 causative organisms mainly consisted of S. aureus (n=4, 33.3%), K. pneumoniae (n=3, 33.3%), and E. coli (n=2, 16.8%). The infection rate by gram-negative organisms was higher in the renal abscess group(p=0.04). Especially, infections by E. coli were more common in the renal abscess group (p=0.03).
Comparisons between the two groups divided according to treatment modality (Tables 7, 8)
Table 7.
Patient characteristics and abscess features according to treatment modality
WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CVA, costovertebral angle; NS, not significant
*Indicates statistically significant differences between the antibiotics-only group and the intervention group.
Table 8.
Size of renal abscess according to treatment modality
*Indicates statistically significant differences between the antibiotics-only group and the intervention group.
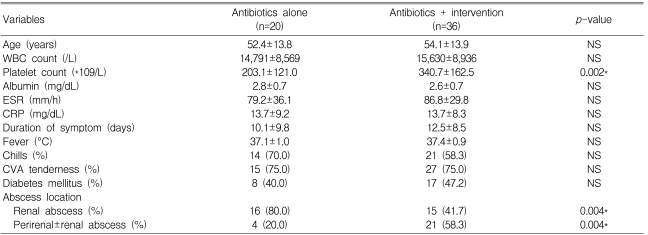
All 56 patients were divided according to their treatment modality into two groups: the antibiotics-only group (n=20) and the antibiotics plus intervention group (n=36). In the perirenal abscess group, four patients (20%) were treated with only antibiotics, and 21 patients (58.3%) underwent additional percutaneous interventions or surgery, which revealed that more invasive treatment was needed in the perirenal abscess group compared to the renal abscess group (p=0.004). A higher platelet count was noted in the antibiotics plus intervention group, but both groups had a normal platelet count. Other differences were not found between the two groups.
In the renal abscess group (n=31), 16 patients were treated with antibiotics only. These patients had abscesses <3 cm (n=11, 68.8%) and 3~5 cm (n=5, 31.2%) in diameter. The 15 patients treated with additional interventions had renal abscesses as follows: <3 cm (n=3, 20.0%), 3~5 cm (n=9, 60.0%), and >5 cm (n=3, 20.0%) in diameter. Hence, interventions were needed in patients with larger-sized renal abscesses (p=0.004).
DISCUSSION
A renal abscess is confined to the renal parenchyma and is known to develop from ascending infections of the lower urinary tract or by hematogenous seeding from primary infected sites. At present, ascending infections account for more than 75% of all renal abscesses, and this usually involve medulla by gram-negative organisms. In contrast, renal abscesses that develop by hematogenous bacterial seeding most often cause cortical abscesses, and this is most commonly associated with S. aureus1, 6, 16, 17). A perirenal abscess is a pocket of pus in the perinephric space between the renal capsule and Gerota's fascia that results from either a rupture of a renal abscess or the hematogenous spread of infection. Aerobic gram-negative organisms are the common cause of perirenal abscesses resulting from ruptured renal abscesses, whereas gram-positive organisms such as S. aureus are the common pathogens of the hematogenous spread of infection11, 12).
The mean age of the patients in this study was 53.5±13.8 years, which was higher than that of previous Korean studies before 2000 in which the mean ages were between 27 and 34 years9, 13, 14). However, a recent study by Choi et al. in 200215) reported that the mean age of the patients in their study was 52.9 years, which is consistent with our current study. In Brazil, Coelho et al. reported that the mean patients' age in their study was 41.1 years18). A similar male-to-female ratio or a slight female predominance of the disease has been noted, but this current study showed a marked female predominance with a male-to-female ratio of 1:31, 6).
In previous foreign studies, urinary obstructions and renal stones have been documented as common underlying conditions, with an incidence of 21~50%1, 3, 4) and 24~54%19-24), respectively. However, this study showed that systemic diseases such as diabetes mellitus (44.6%) and liver cirrhosis (12.5%) were much more common than renal or urologic disorders (16.1%). This is similar to results obtained by Choi et al.15), in which urologic disorders had an incidence of 14.5%.
Vague symptoms and nonspecific physical findings have been documented that make the early diagnosis of renal and perirenal abscesses difficult1-5); the mean duration of symptoms prior to diagnosis was 11.6±9.0 days (range: 3-45 days) in this study. Similar to previous studies9, 13-15, 18), fever (75%) was the most common symptom at the time of admission, but abdominal pain (46.4%), including flank pain, had a relatively low incidence. Chills (62.5%) were the second most frequent symptom, whereas the incidence of chills was 9% in the report by Coelho et al18). Furthermore, we were unable to find any Korean studies that reported incidence of chills. Knocking tenderness on the CVA was present in 75% of the patients, which was the most common physical finding.
To establish an accurate diagnosis of renal and perirenal abscesses, US, CT, and both were performed for 16.1%, 58%, and 19.6% of the patients, respectively. Of the 56 patients, 78.5% of the patients required CT, and this proportion was higher compared to previous studies9, 13). This suggests that the role of CT to diagnose renal and perirenal abscesses has been increasing. MRI was useful in 5.4% of the patients to differentiate renal or perirenal abscesses from other renal diseases. Moreover, the improved quality of ultrasound examinations and the widespread availability of CT facilitate easy diagnoses of renal or perirenal abscesses. Although US is noninvasive, CT has been documented to be superior to US for diagnosing renal or perirenal abscesses, with an accuracy rate of 90~100%1, 2, 21, 25); CT has the ability to allow detection of small-sized abscesses and helps differentiate abscesses from other mass-like lesions. Nevertheless, MRI is more sensitive and specific than CT for surveying renal lesions. Due to its high cost and low accessibility, however, MRI is not typically used as a first-line diagnostic tool. Hence, MRI is primarily used to rule out renal malignancy in patients that likely have renal or perirenal abscesses. In this study, MRI was needed in two patients to distinguish renal abscesses from renal cell carcinomas.
Before 1960, most renal and perirenal abscesses originated via hematogenous bacteremia by S. aureus, but the widespread use of antimicrobial agents has decreased the incidence of hematogenous dissemination. Recently, gram-negative organisms have emerged as the most common pathogens promoting renal and perirenal abscesses11, 12). Our results also reflect the recent bacteriologic trend in E. coli and K. pneumoniae as major abscess-causing pathogenic organisms. We further examined the antimicrobial susceptibilities of E. coli, K. pneumonia, and S. aureus, and the resistance rates of E. coli against ampicillin, cephalothin, cefotaxime, ciprofloxacin, and gentamicin were higher than those in CA-APN26-28). However, no E. coli strains were imipenem-resistant. The distinct primary sites of infection in the four patients infected with S. aureus could not be detected. These isolates were resistant to penicillin, but they were all susceptible to methicillin.
No differences were observed in the clinical characteristics or the treatment modalities between the diabetic and nondiabetic groups (data not shown). The laboratory findings showed that the serum blood urea nitrogen level in the diabetic group was higher than in nondiabetics (34.3±43.1 mg/dL vs. 15.1±15.2 mg/dL, respectively, p=0.004). Serum creatinine was also higher in the diabetic group (2.0±2.0 mg/dL vs. 1.1±0.7 mg/dL, respectively, p=0.107). After comparing the causative organisms between the diabetic and nondiabetic groups, the following results were obtained. E. coli caused abscesses in 36.4% of the patients with diabetes (n=4) and 50% of the nondiabetic patients (n=7); K. pneumoniae caused abscesses in 27.3% of the patients with diabetes (n=3) and 28.6% of the nondiabetic patients (n=4); and S. aureus caused abscesses in 27.3% of the patients with diabetes (n=3) and 7.1% of the nondiabetic patients (n=1). Gram-negative organisms were also the main pathogens that promoted abscesses in the diabetic group, but no significant difference was detected in the S. aureus infection rates between the diabetic and nondiabetic groups (p=0.399).
The mortality rates for renal and perirenal abscesses ranged from 0 to 7% in previous Korean studies9, 12-15). The mortality rate in this study was 5.4%. Two patients died of combined pneumonia, and one patient died of sepsis and complicated disseminated intravascular coagulation. Among the dead patients, two patients had mixed abscesses and one patient had a perirenal abscess. All patients who died suffered from diabetes mellitus and were male; however, these data should not be extrapolated to larger populations because of the relatively small number of those that died in the study. Hence, a large-scale study is needed to investigate the factors that influence the prognosis of renal and perirenal abscesses.
Comparisons between the renal abscess and perirenal abscess groups were also performed. In 2002, Choi et al.15) reported that the abscess size was larger in the perirenal abscess group, but no other significant differences were noted in their study. But in our study, an earlier visit to the hospital was noted in the renal abscess group (9.3±9.1 days vs. 14.4±8.2 days), and the reason for this might have been due to symptoms such as fever and chills, which were more common in the renal abscess group. Leukocytosis and hypoalbuminemia were more remarkable in the perirenal abscess group than in the renal abscess group. Moreover, the identified organisms involved in the infection differed between the groups. Gram-negative organisms were commonly isolated in the renal abscess group, whereas gram-positive organisms were common in the perirenal abscess group. Infection by E. coli was markedly frequent in the renal abscess group. In this study, accurately measuring the size of the perirenal abscesses was difficult because most cases of perirenal abscesses originated from ruptured renal abscesses, and they were commonly combined with peritonitis. Because of this, comparisons of abscess sizes were not performed between the renal and perirenal abscess groups.
The development of antibiotics, advances in diagnostic modalities, and the introduction of nonsurgical intervention methods such as percutaneous drainage and aspiration have all contributed to the improved outcome of renal and perirenal abscesses. With these changes, the rate of complete recovery from renal and perirenal abscesses without surgery has increased, and reduced mortality has been documented in several studies6, 7, 29, 30).
To investigate the factors associated with treatment modalities, the patients were divided into two groups according to treatment modality, and the baseline characteristics, clinical and laboratory findings, and character of the abscesses in each group were analyzed. As noted in the Results section, patients with perirenal abscesses or large renal abscesses required more invasive treatments such as percutaneous drainage and surgery. Coelho et al.18) reported that 95.3% of the patients with perirenal abscesses underwent percutaneous drainage or surgery, and surgery was performed for 56.2% of the perirenal abscess group. In this study, percutaneous drainage or surgery was needed in 84% of the patients in the perirenal abscess group, and surgery was performed on 24% of these patients. Choi et al.15) previously reported that the mean abscess size in the group treated with percutaneous drainage was significantly larger than that of the antibiotics and surgery groups. Renal and perirenal abscesses have been safely and effectively managed with proper antibiotics and additional percutaneous abscess drainage without surgical treatment, and some studies suggest that percutaneous abscess drainage should be considered as an initial treatment for patients with an abscess larger than 3 cm, those with systemic diseases such as diabetes mellitus, and patients with urologic disorders including urinary obstructions31). Based on these results, large-scale prospective studies are needed to establish a more reasonable guideline for the treatment of renal or perirenal abscesses.
In conclusion, this study revealed somewhat different results compared to those of previous studies. Clinical and microbial differences were noted between the renal and perirenal abscess groups. And abscess location and the size of renal abscess were the factors associated with treatment modalities.
Footnotes
This study was supported by a clinical research grant from Pusan National University Hospital 2008.
References
- 1.Fowler JE, Jr, Perkins T. Presentation, diagnosis and treatment of renal abscesses: 1972-1988. J Urol. 1994;151:847–851. doi: 10.1016/s0022-5347(17)35103-0. [DOI] [PubMed] [Google Scholar]
- 2.Capitan Manjon C, Tejido Sanchez A, Piedra Lara JD, Martinez Silva V, Cruceyra Betriu G, Rosino Sanchez A, Garcia Penalver C, Leiva Galvis O. Retroperitoneal abscesses: analysis of a series of 66 cases. Scand J Urol Nephrol. 2003;37:139–144. doi: 10.1080/00365590310008884. [DOI] [PubMed] [Google Scholar]
- 3.Thorley JD, Jones SR, Sanford JP. Perinephric abscess. Medicine. 1974;53:441–451. doi: 10.1097/00005792-197411000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Salvatierra O, Jr, Bucklew WB, Morrow JW. Perinephric abscess:a report of 71 cases. J Urol. 1967;98:296–302. doi: 10.1016/S0022-5347(17)62874-X. [DOI] [PubMed] [Google Scholar]
- 5.Adachi RT, Carter R. Perinephric abscess: current concepts in diagnosis and management. Am Surg. 1969;35:72–75. [PubMed] [Google Scholar]
- 6.Hoverman IV, Gentry LO, Jones DW, Guerriero WG. Intrarenal abscess: report of 14 cases. Arch Intern Med. 1980;140:914–916. doi: 10.1001/archinte.140.7.914. [DOI] [PubMed] [Google Scholar]
- 7.Finn DJ, Palestrant AM, Dewolf WC. Successful percutaneous management of renal abscess. J Urol. 1982;127:425–426. doi: 10.1016/s0022-5347(17)53844-6. [DOI] [PubMed] [Google Scholar]
- 8.Rives RK, Harty JI, Amin M. Renal abscess: emerging concepts of diagnosis and treatment. J Urol. 1980;124:446–447. doi: 10.1016/s0022-5347(17)55489-0. [DOI] [PubMed] [Google Scholar]
- 9.Jin WY, Lee JH, Lee YJ, Jang IC, Jo DH. A clinical review of 16 cases of renal of perirenal abscess. Korean J Urol. 1988;29:761–765. [Google Scholar]
- 10.Hwang YJ, Woo YN. Percutaneous management of the renal and perirenal abscess. Korean J Urol. 1994;35:261–264. [Google Scholar]
- 11.Schrier RW, Gottschalk CW. Diseases of the kidney. 6th ed. Boston: Little brown and company; 1997. pp. 947–959. [Google Scholar]
- 12.Jung UY, Kim DH. A clinical survey on perinephric abscess. Korean J Urol. 1985;26:7–12. [Google Scholar]
- 13.Lee JH, Earm JH, Han JS, Kim SK, Lee JS. Clinical features of renal and perirenal abscess. Korean J Nephrol. 1990;9:357–363. [Google Scholar]
- 14.Kim YS, Hong SJ. A clinical survey on renal and perinephric abscess. Korean J Urol. 1994;35:43–47. [Google Scholar]
- 15.Choi JY, Kim MS, Kim YK, Lee KS, Chang KH, Huh AJ, Yeom JS, Song YG, Kim JM. Recent clinical trend of renal and perirenal abscess. J Korean Soc Chemother. 2002;20:91–100. [Google Scholar]
- 16.Hutchison FN, Kaysen GA. Perinephric abscess: the missed diagnosis. Med Clin North Am. 1988;72:993–1014. doi: 10.1016/s0025-7125(16)30726-x. [DOI] [PubMed] [Google Scholar]
- 17.Dembry LM, Andriole VT. Renal and perirenal abscesses. Infect Dis Clin North Am. 1997;11:663–680. doi: 10.1016/s0891-5520(05)70379-2. [DOI] [PubMed] [Google Scholar]
- 18.Coelho RF, Schneider-Monterio ED, Mesquita JL, Mazzucchi E, Marmo Lucon A, Srougi M. Renal and perinephric abscesses: analysis of 65 consecutive cases. World J Surg. 2007;31:431–436. doi: 10.1007/s00268-006-0162-x. [DOI] [PubMed] [Google Scholar]
- 19.Siegel JF, Smith A, Moldwin R. Minimally invasive treatment of renal abscess. J Urol. 1996;155:52–55. [PubMed] [Google Scholar]
- 20.Anderson KA, McAninch JW. Renal abscesses: classification and review of 40 cases. Urology. 1980;16:333–338. doi: 10.1016/0090-4295(80)90132-6. [DOI] [PubMed] [Google Scholar]
- 21.Meng MV, Mario LA, McAninch JW. Current treatment and outcomes of perinephric abscesses. J Urol. 2002;168:1337–1340. doi: 10.1016/S0022-5347(05)64443-6. [DOI] [PubMed] [Google Scholar]
- 22.Angel C, Shu T, Green J, Orihuela E, Rodriquez G, Hendrick E. Renal and peri-renal abscesses in children: proposed physio-pathologic mechanisms and treatment algorhithm. Pediatr Surg Int. 2003;19:35–39. doi: 10.1007/s00383-002-0888-y. [DOI] [PubMed] [Google Scholar]
- 23.Bova JG, Potter JL, Arevalos E, Hopens T, Goldstein HM, Radwin HM. Renal and perirenal infection: the role of computed tomography. J Urol. 1985;133:375–378. doi: 10.1016/s0022-5347(17)48982-8. [DOI] [PubMed] [Google Scholar]
- 24.Sheinfeld J, Erturk E, Spataro RF, Cockett AT. Perinephric abscess: current concepts. J Urol. 1987;137:191–194. doi: 10.1016/s0022-5347(17)43946-2. [DOI] [PubMed] [Google Scholar]
- 25.Sundaram M, Wolverson MK, Heiberg E, Pilla T, Vas WG, Shields JB. Utility of CT-guided abdominal aspiration procedures. AJR Am J Roentgenol. 1982;139:1111–1115. doi: 10.2214/ajr.139.6.1111. [DOI] [PubMed] [Google Scholar]
- 26.Wie SH, Chang UI, Kim HW, Kim YS, Kim SY, Hur J, Kim SI, Kim YR, Kang MW. Clinical features and antimicrobial resistance among clinical isolates of women with community-acquired acute pyelonephritis in 2001-2006. Infect Chemother. 2007;39:9–16. [Google Scholar]
- 27.Kim JH, Lee CS, Choi NW, Park SK, Lee CH, Kim GH, Kang CM. Quinolone resistance in community-acquired acute pyelonephritis. Korean J Nephrol. 2006;25:571–578. [Google Scholar]
- 28.Lee JS, Rho SH, Kim SE, Nam TM, Kim JS, Kim SG, Lee YK, Noh JW, Chae DW, Oh KH. A study on the clinical and microbiologic features of community-acquired acute pyelonephritis for the recent 5 years in a university hospital. Korean J Nephrol. 2002;21:905–913. [Google Scholar]
- 29.Lyons RW, Long JM, Lytton B, Andriole VT. Arteriography and antibiotic therapy of a renal carbuncle. J Urol. 1972;107:524–526. doi: 10.1016/s0022-5347(17)61068-1. [DOI] [PubMed] [Google Scholar]
- 30.Wright FW. Percutaneous diagnosis and treatment of intra-renal abscess. Br J Urol. 1977;49:22. doi: 10.1111/j.1464-410x.1977.tb04516.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Park YI. Conservative management of the renal and perirenal abscesses. Korean J Urol. 2001;42:185–188. [Google Scholar]