Abstract
Colorectal cancer (CRC) remains a leading cancer killer worldwide. But the disease is both curable and preventable at an early stage. Regular CRC cancer screening has been shown to reduce the risk of dying from CRC. However, the importance of large-scale screening is only now starting to be appreciated. This article reviews a variety of imaging procedures available for detecting ulcerative colitis (UC) and Crohn’s disease (CD), polyps and CRC in their early stage and also presents details on various screening options. Detecting, staging and re-staging of patients with CRC also require multimodality, multistep imaging approaches. Staging and re-staging with conventional colonoscopy (CC), computer tomography colonography (CTC), magnetic resonance colonography (MRC) and positron emission tomography/computer tomography colonography (PET/CTC) are of paramount importance in determining the most appropriate therapeutic method and in predicting the risk of tumor recurrence and overall prognosis. The advantages and limitations of these modalities are also discussed.
Keywords: Colon polyps, Colorectal cancer, Conventional Colonoscopy, Virtual colonoscopy, Computer tomography colonography, Magnetic resonance colonography, Positron emission tomography/computer tomography colonography
INTRODUCTION
Over 55 000 Americans are expected to die of Colorectal cancer (CRC) in 2006[1]. It is estimated that there is over a 5% chance that an American will develop CRC in their lifetime and over a 2% chance that an American will die from CRC. In some Asian countries, the incidence of colorectal cancer rises rapidly[2]. Compared to Westerners, Chinese patients have a slightly lower prevalence of colon neoplasia, more distal distribution of neoplasia, and higher likelihood of concomitant proximal advanced neoplasia and distal neoplasia[3].
When used appropriately, screening for CRC can reduce disease-related morbidity and mortality[4]. Current methods include fecal occult blood testing (FOBT), flexible sigmoidoscopy, double contrast barium enema (DCBE), and conventional colonoscopy (CC); all are cost-effective techniques. Unfortunately, offering an array of options has not increased screening utilization, which continues to lag behind that of other common cancers[5]. Newer techniques, particularly virtual colonoscopy (VC), including CTC, MRC and PET/CTC may offer attractive alternatives for healthcare provider recommendation and patient use[6].
COLORECTAL CANCER RISK FACTORS
The exact causes of CRC are not known. However, studies show that the following risk factors increase a person’s chances of developing CRC: over the age of 50, high fat and calories and low fiber diets, family history of colorectal cancer, previous history of CRC or adenomas, a cholecystectomy was performed ten years or more ago, severe ulcerative colitis (UC) and Crohn’s disease (CD) for over ten years, etc[7–9].
There is substantial evidence that most CRC arise from preexisting adenomas. A large body of clinical evidence supports the belief that over 95% of CRC arise from benign adenomatous polyps that develop and grow very slowly over many years[10]. Consequently, polypectomy of colorectal adenomas was shown to reduce the incidence of CRC by nearly 80%. Progression of an adenoma into cancer can be predicted by its size, villous histology, degree of dysplasia, and inherited or environmental factors. Advanced adenomas are the primary target in colorectal screening. Advanced adenomas were generally large (> or = 10 mm in size); only a small percentage were medium sized (6-9 mm). There was a very low prevalence of high-grade dysplasia and invasive carcinoma in the medium-sized group of lesions[11]. So, there is a firm consensus that larger (> or = 10 mm) colonic polyps should be removed; however, the importance of removing smaller polyps (< 10 mm) is more controversial.
ULCERATIVE COLITIS OR CROHN’S DISEASE AND COLORECTAL CANCER
People with UC or CD have a high risk of developing CRC, even if the disease is in remission[12]. Regular screenings and early detection are critical. In people with UC or CD, CRC may be preceded by polyps or may arise from flat mucus membranes, thus requiring more intensive and more frequent colonoscopies[13]. CRC is more often multiple and uniformly distributed throughout the colon and often occurs in younger people with UC or CD[14]. CRC is one of the most serious potential results of IBD and explains up to one sixth of all deaths in UC patients and one twelfth of all deaths in CD[15]. Longer duration of the disease, extensive colitis, primary sclerosing cholangitis and family history of CRC are the main risk factors. Relative risk of CRC is 2.5 and small bowel cancer risk is 31.2 in CD[16]. A recent meta-analysis estimated that one in five patients with UC will develop CRC over 30 years, with the risk of CRC being greatest in patients with extensive UC of long duration. Patients with CD also have an increased risk of CRC[17]. Given this fact, it is necessary to early examine UC or CD by current and future approaches to CRC prevention in IBD patients[18].
COLORECTAL CANCER PROACTIVE SCREENING
CRC is generally more treatable when it is found early. CRC screening is used to detect cancer, precancerous polyps, or other abnormal conditions. If screening detects an abnormality, diagnosis and treatment can occur promptly. In addition, finding and treating polyps may be one of the most effective ways to prevent the development of cancer altogether. Because often there are no symptoms and symptoms do occur depend on the location of the cancer in the gastrointestinal tract, regular screening and early detection are important for people who are at average risk and have a higher risk, such as those with UC or CD. If CRC is detected at an early stage, the 5-year survival rate is 90%. Unfortunately, less than 40% of CRC are detected at an early stage[19]. Once the cancer has spread regionally and involves adjacent organs or lymph nodes, this rate drops to 40%-65%; 5-year survival is less than 10% for patients with distant metastases[20].
Many existing screening tools, invasive or not, are often debated such as FOBT, sigmoidoscopy and complete colonoscopy. New tools are in development and have to be evaluated in current practice: VC, new endoscopic technologies, DNA on faeces or proteomics with markers in serum. When used appropriately, screening for CRC can reduce disease-related morbidity and mortality[21]. Recent studies stress the fact that finding and resecting advanced adenomatous polyps, and thereby preventing cancer, is becoming a primary objective of screening programs. Several papers also show the potential of emerging new methods of screening by imaging the colon with VC[22]. The sensitivity of VC for large adenomas and CRC appears to be high, although results vary by center and there is a steep learning curve. (Table 1).
Table 1.
Characteristics of medical images in detecting CRC
| Modalities | Characteristics |
| DCBE | Cost-effective, efficient, safe, could be performed and interpreted by GI radiographers to reduce waiting |
| Colonoscopy | Cost-effective, endoscopic resection of polyps, endomicroscopy opening the way of in vivo molecular imaging |
| CTC | Less uncomfortable, minimally invasive, with radiation exposure, 3D virtual dissection of colon, |
| MRC | Limited bowel preparation, unpleasant rectal tube, filling colon with saline and contrast, no radiation |
| PET/CTC | Accurate whole-body tumor staging, with integrated morphological and functional images, more irradiation |
BACKGROUND OF VIRTUAL CONLONOSCOPY
VC is a new method for studying the colon. It consists in acquisition of CT, MR and PET/CT images and could elaborate them with a workstation, creating endoluminal vision as likely as traditional colonoscopy does, permitting the complete exploration of colonic lumen, and of tumoral stenosis[23]. The analysis of the differences between CT, MR and PET/CT colonography shows that these techniques present both advantages and disadvantages, such as the impossibility to perform MR in patients with pace-maker or in claustrophobic patients and the impossibility to perform CT with iodated agents in patients with renal failure or with a story of adverse reactions. The increased use of these techniques is due to the high sensitivity of last-generation CT, MR and PET/CT machine, to the increased spatial resolution, to specific softwares for digital cleaning of colon, to the introduction of high-end workstations and to the possibility of computer assisted diagnosis (CAD)[24]. So, it is desirable that the increasing spread of multidetector CT devices and the future technical innovations should have the effect to increase culture and experience in various diagnostic centers about VC, making possible the spreading of VC as a screening tool (Table 2)[25].
Table 2.
Comparison of medical image modalities’ values in colonography
| Modalities | Specificity | Sensitivity | Accuracy |
| DCBE | + | + | - |
| Colonoscopy | +++ | ++ | +++ |
| CTC | + | ++ | _ |
| MRC | ++ | + | + |
| ET/CTC | ++ | ++ | +++ |
Legend: _- poor; + fair; +++ good; ++++ excellent.
CT COLONOGRAPHY
Current CT techniques require meticulous bowel prepara-tion and gas insufflations prior to the examination. The procedure requires a scan time of about 25 to 30 s with new multidetector CT scanners, and sedation is not used. The advantages of CTC over CC include its safety, its ability to demonstrate the entire large bowel in almost all patients, even following incomplete endoscopy, to accurately localize lesions, and to examine the entire colon in patients with obstructing tumors. Additionally, CTC allows simultaneous preoperative tumor staging. There are few reported complications from CTC[26,27].
People with ulcerative colitis or Crohn’s disease
Over the past several years, significant advances have been made in the diagnostic techniques used in the management of UC and CD. Improved radiographic imaging techniques based on CT imaging allow noninvasive means of evaluating the small bowel in patients with known or suspected CD[28]. CTC may help to distinguish between patients with acute and chronic inflammatory bowel disease (IBD). Especially extraintestinal complications, tumorous as well as pseudo-tumorous lesions can be detected with high sensitivity and specificity. CTC is clinically useful for the evaluation of CD, especially those with stenotic lesions[29,30]. Current findings also suggest that although the widespread use of VC in CD is currently not indicated because of possible false-negative findings, this technique may represent an alternative to CD in noncompliant postsurgical patients with a rigid stenosis not allowing passage of the endoscope[31].
People with polyps
Screening for colorectal polyps is a controversially dis-cussed indication for CTC. Sensitivity and specificity range widely and decrease with decreasing polyp size. However, better results can be achieved using multidetector technology. Most frequently, the examination is well tolerated and assessed by patients to be more acceptable than CC[32]. A meta-analysis of 2610 patients, 206 of whom had large polyps, showed high per-patient average sensitivity (93%) and specificity (97%) for colonography; sensitivity and specificity decreased to 86% and 86%, respectively, when the threshold was lowered to include medium polyps. When polyps of all sizes were included, studies were too heterogeneous in sensitivity (range, 45%-97%) and specificity (range, 26%-97%) to allow meaningful meta-analysis. CTC seems sufficiently sensitive and specific in the detection of large and medium polyps[33,34].
The sensitivity of CTC was heterogeneous but im-proved as polyp size increased (48% for detection of polyps < 6 mm, 70% for polyps between 6 and 9 mm, and 85% for polyps > 9 mm). Characteristics of the CTC scanner, including width of collimation, type of detector, and mode of imaging, explained some of this heterogeneity. In contrast, specificity was homogenous (92% for detection of polyps < 6 mm, 93% for polyps from 6 to 9 mm, and 97% for polyps > 9 mm). CTC is highly specific, but the range of reported sensitivities is wide[35]. Patient or scanner characteristics do not fully account for this variability, but collimation, type of scanner, and mode of imaging explain some of the discrepancy. This heterogeneity raises concerns about consistency of performance and about technical variability. These issues must be resolved before CTC can be advocated for generalized screening for CRC[36].
Diagnosis and staging of colorectal cancer
Because CRC has widely varying appearances in both endoscopy and CTC, familiarity with the gamut of morphologic appearances can help improve interpretation of the results[37,38]. The addition of intravenous contrast material to CTC can aid differentiation of true colonic masses from pseudolesions such as residual stool and improves the depiction of enhancing masses that might otherwise be obscured by residual colonic fluid[39]. In contrast to staging of most other tumors, staging of CRC depends more on the depth of tumor invasion than on the size of the primary mass. The diverse appearances of colorectal cancers at two- and three-dimensional CTC include sessile, annular, ulcerated, necrotic, mucinous, invasive, and noninvasive lesions[40]. Imaging pitfalls that can simulate or obscure neoplasms are retained fecal material or fluid, incomplete distention, and advanced diverticulosis[41].
Contrast-enhanced CTC can simultaneously evaluate metastatic disease, local recurrence, and metachronous neoplasia in CRC, in recurrent CRC. Contrast-enhanced CTC has the potential to detect local recurrence, metach-ronous disease, and distant metastases in patients with a history of invasive CRC. Suboptimal sigmoid distention can be seen on contrast-enhanced CTC, predominantly in patients with right hemicolectomies[42]. Contrast-enhanced CTC is a promising method for detecting local recurrence, metachronous disease, and distant metastases in patients with prior invasive CRC. The technique can also serve as a useful adjunct to colonoscopy by detecting local recurrences or metachronous disease that are endosco-pically obscure or by serving as a full structural colonic examination when endoscopy is incomplete[43].
MR COLONOGRAPHY
MRC has gained access into clinical routine as a means for the assessment of the large bowel. There are widely accepted indications for MRC, especially in patients with incomplete CC. Furthermore, virtual MRC is more and more propagated as a screening tool, with advantages especially inherent to the non-invasive character of this procedure and the lack of ionizing radiation exposition. Beyond a sufficiently high diagnostic accuracy, outstanding patient acceptance is a major advantage of MRC as a diagnostic modality[44]. Precondition for establishment of MRC as a diagnostic tool in secondary prevention of CRC is not only high diagnostic accuracy but also a good acceptance amongst patients[45].
People with ulcerative colitis or Crohn’s disease
The degree of the contrast enhancement of the bowel wall may be a criterion for the degree of inflammation in CD[46]. This study provides strong evidence that the combination of MRI enteroclysis and MRC is practicable and supplies additional results regarding the regions which are not seen with ileo-colonoscopy in the work-up of patients with CD[47]. The presented data indicate that ‘fecal tagging MRC’ is not suitable for an adequate quantification of inflammatory diseases of the large bowel. Furthermore, overall acceptance of endoscopic colonoscopy was superior to MRC[45,48]. MRC represents a promising alternative to CC for the assessment of colonic anastomoses in patients with previous colonic resection[49]. In summary, whether or not the survival of endoscopy is under debate, MRI could mark a historic turning point in gastroenterology. So, MRI hardware might interdisciplinarily be used by, e.g., radiologists and gastroenterologists[50].
People with polyps
Dark-lumen MRC has failed to detect all polyps smaller than 5 mm in diameter which are generally not clinically relevant at the moment of their detection and thus can be kept under surveillance. However, MRC as a non-invasive imaging modality is a promising alternative to CC in the detection of clinically relevant polyps larger than 5 mm in diameter[51]. The diagnostic performance of MRC was prospectively evaluated by using limited bowel preparation in patients with polyps of 10 mm or larger in diameter in a population at increased risk for CRC, with CC as the reference standard. Two hundred patients (mean age, 58 years; 128 male patients) were included; 41 patients had coexistent symptoms. At colonoscopy, 12 patients had 22 polyps of 10 mm or larger. Per-patient sensitivity was 58% (seven of 12) for observer 1, 67% (8 of 12) for observer 2, and 75% (9 of 12) for both observers combined for polyps of 10 mm or larger. Per-patient specificity was 95% (178 of 188) for observer 1, 97% (183 of 188) for observer 2, and 93% (175 of 188) for both observers combined. Per-polyp sensitivity was 55% (12 of 22) for observer 1, 50% (11 of 22) for observer 2, and 77% (17 of 22) for both observers combined. Interobserver agreement was 93% for identification of patients with lesions of 10 mm or larger. In patients at increased risk for CRC, specificity of MRC by using limited bowel preparation was high, but sensitivity was modest[52–54].
Diagnosis and staging of colorectal cancer
MRC is useful for detection of colonic pathology and assessment of proximal colon in patients with colonic cancer after incomplete colonoscopy[55,56] Wong et al reported 51 patients with incomplete colonoscopy who were recruited to have MRC performed. Forty-four patients had incomplete colonoscopy because of an obstructing tumor. The other seven patients had incomplete colonoscopy because of excessive bowel looping. Apart from one patient suffering from chronic obstructive airway disease with resulting nondiagnostic MRC, all other patients had MRC successfully performed. Each colon was divided into six bowel segments for analysis. All 300 segments were of diagnostic quality and were assessed by the MRC. MRC correctly identified all 44 obstructing tumors demonstrated by initial CC. Synchronous tumors in proximal colonic segments were identified in two patients by MRC. In addition, MRC identified two colonic tumors located in bowel segments inaccessible by CC because of excessive looping[57]. A meta-analysis had MRC versus colonoscopy as a diagnostic investigation for CRC. This study suggests that MRC is an imaging technique with high discrimination for cases presenting with CRC. The exact diagnostic role of MRC needs to be clarified (e.g. suitable for an elderly person with suspected CRC. Further evaluation is necessary to refine its applicability and diagnostic accuracy in comparison with other imaging methods such as CTC[58]. MRC could be useful in screening programs of patients at high risk for colon cancer. Patients with MRC-detected endoluminal lesions must undergo CC for histologic diagnosis[59].
PET/CT COLONOGRAPHY
Endoscopic and radiologic studies are frequently required in IBD to determine disease activity, extent of disease, and delineating disease type. PET and PET/CT using fluorine-18-fluoro-deoxyglucose to identify metabolically active tissues may offer a simple noninvasive alternative to conventional studies in identification and localization of active intestinal inflammation[60]. Today, PET imaging has been shown to be superior to conventional CT staging when assessing patients with CRC for local and distant metastases. PET/CT scanners provide accurately fused morphological and functional imaging within a single examination. Thus a combined whole body PET/CTC approach may serve as an attractive alternative to a multistep multimodality workup comprising CT, PET/CT, and CC[61–63].
Detection of ulcerative colitis or Crohn’s disease
Over the past several years, significant advances have been made in the diagnostic techniques used in the management of UC and CD. Detection of disease activity in IBD is of crucial importance for diagnosis and management of the disease[64]. 18F-fluorodeoxyglucose (18F-FDG) accumulates in malignant tissues but also at the sites of infection and inflammation and in autoimmune and granulomatous diseases by the overexpression of distinct facultative glucose transporter (GLUT) isotypes (mainly GLUT-1 and GLUT-3) and by an overproduction of glycolytic enzymes in cancer cells and inflammatory cells[65]. It is confirmed that PET activity correlated well with active inflammation in both UC and CD, suggesting that this may be a noninvasive method of identifying disease activity in patients with IBD[66]. Neurath et al suggested that FDG-PET appear to be a reliable noninvasive tool for simultaneous detection of inflamed areas in the small and large bowel of patients with CD and can be used to detect disease activity in the terminal ileum and colon of CD patients with high sensitivity and specificity[67].
PET or PET/CT in pediatric inflammatory bowel disease
Diagnosis of chronic IBD in children requires noninvasive, atraumatic diagnostic tools that depict localization and acuity of inflammation and yield only a low radiation dose. Loffler et al reported a retrospective analysis with histology as the standard of reference, FDG-PET showed a sensitivity/specificity/accuracy of 98%/68%/83% as compared to CC (90%/75%/82%) and US (56%/92%/75%). For the small bowel, FDG-PET was even more reliable (100%/86%/90%). Because of its high sensitivity and accuracy, FDG-PET is an excellent, noninvasive diagnostic tool for IBD. Depicting inflammation in the whole bowel, while being not traumatic, it is attractive for use especially in children. FDG-PET is especially reliable for the small bowel and can inform application of topical therapy[68,69]. However, curtailed anatomical information provided by FDG-PET often renders accurate localization of lesions difficult. PET/CT offers a better noninvasive tool for identifying and localizing active intestinal inflammation in patients with IBD. PET and PET/CT may not be able to replace conventional studies; however, it may be useful when conventional studies cannot be performed or fail to be completed[70].
PET or PET/CT in radiation enterocolitis
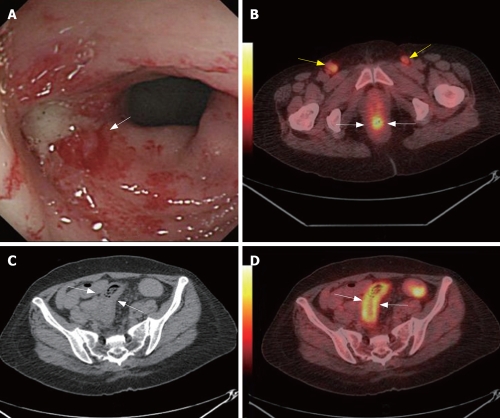
Radiation enteritis is a functional disorder of the large and small bowel that occurs during or after a course of radiation therapy to the abdomen, pelvis, or rectum. The large and small bowels are very sensitive to ionizing radiation. Almost all patients undergoing radiation to the abdomen, pelvis, or rectum will show signs of acute enteritis. Injuries clinically evident during the first course of radiation and up to 8 wk later are considered acute[71]. Chronic radiation enteritis may present months to years after the completion of therapy, or it may begin as acute enteritis and persist after the cessation of treatment. Only 5% to 15% of persons treated with radiation to the abdomen will develop chronic problems[72]. According to our initial experiences, FDG-PET/CT also has a potential value in management of radiation enterocolitis and other radiation complications (Figure 1).
Figure 1.
A 56-year-old woman was suffered from uterine cervix cancer and accepted local radiation and chemotherapy 2-mo ago. CC found enterocolitis of sigmoid colon (white arrow, A). PET/CT detected high metabolism metastasis lymphoid nodes at both sides of the inguina (yellow arrows, B) and rectum wall was high metabolism (white arrows, B). CT showed thickening of sigmoid colon wall (white arrows, C). PET/CT illustrated sigmoid colon wall was high metabolism (white arrows, D). Biopsy followed by PET/CT revealed metastasis lymphoid nodes at both sides of the inguina. The case illustrated the potential value of PET/CTC in radiation enterocolitis.
Whole body staging and restaging of colorectal cancer by PET/CT
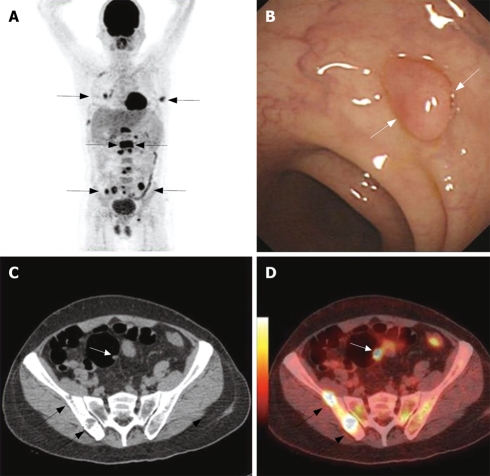
Staging of patients with CRC often requires multimodality, multistep imaging approach. PET/CTC is the latest technique used in CRC screening and staging, which could complete the TNM staging in one-stop examination. Kuehle et al performed a rodent polyp model to assess the feasibility of PET/CTC for the detection of colorectal masses in a rodent polyp model in an intraindividual comparison with dark-lumen MRC. Detection of small tumors with PET/CTC and MRC is possible in a rodent model[73]. PET/CTC is equivalent to CT + PET for tumor staging in patients with CRC. PET/CTC in conjunction with optical colonoscopy may be a suitable concept of tumor staging for patients with CRC[74]. Kinner et al developed and evaluated a combined whole-body PET/CTC protocol for dedicated CRC staging in routine clinical use. The results showed that staging patients with whole-body PET/CTC is technically feasible and accurate and patients with incomplete colonoscopy or potential synchronous bowel lesions might benefit from this approach (Figure 2)[75].
Figure 2.
A 63-year-old man was detected multi metastases in whole body PET/CT scan with unknown original tumor (black arrows, A). CC showed a polyp at the sigmoid colon (white arrows, panel B). CT detected bone destruction at the right ilium (black arrow, C) and a polyp at the sigmoid colon (white arrow, C). PET/CTC localizes the high metabolism bone destruction lesions (black arrows, D) and high metabolism polyps at sigmoid colon (white arrow, D). Histopathology follow by CC revealed a inflame polyp. The case illustrated the potential value of PET/CTC in screening colorectal polyps.
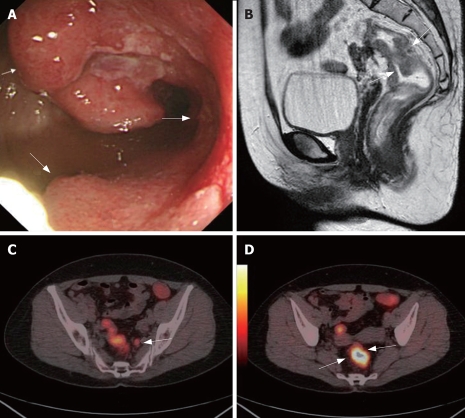
Whole body PET/CT with integrated colonography is technically feasible for whole body staging in patients with CRC. PET/CT has altered management plan in 24% of patients with primary CRC in correct direction. PET/CT should be considered a part of standard workup for preoperative evaluation in a subset of patients with CRC[76]. CT and superparamagnetic iron oxide-enhanced MR imaging are more sensitive but less specific than PET in the detection of liver metastases. PET/CT can detect more patients with extrahepatic tumor than CT alone[77]. The sensitivity, specificity and overall accuracy of PET in detecting intra-abdominal extrahepatic CRC were 82%, 88% and 86%, respectively, compared with 88%, 94% and 92%, respectively, of PET/CT. For detection of extra-abdominal and/or hepatic CRC, these were 74%, 88% and 85% of PET and 95%, 100% and 99% of PET/CT. The sensitivity, specificity and overall accuracy of PET in detecting primary tumors were 80%, 69% and 75%, respectively, compared with 89%, 92% and 90%, respectively, of PET/CT. PET/CT appears to be a very promising method for distinguishing a viable tumor from fibrous changes, thereby avoiding unnecessary laparotomy[78]. This integrated protocol may be of substantial benefit in staging and restaging patients with CRC (Figure 3)[79].
Figure 3.
A 56-year-old women complained about blood stool for about 1 year. CC showed one stenotic tumoral site in the rectum (white arrows, A). Sagittal MRC showed thickened rectum with suspected infiltration of the surrounding tissue (white arrows, B). Axial PET/CTC showed a high metabolism metastasis lymphoid node (white arrow, C). Axial PET/CTC revealed tumor sites with elevated glucose metabolism and clear circumscription (two white arrows, D). PET/CT also indicated no tumorous infiltration of the adjacent tissue and no distant metastases. This was later verified by histopathology. The case illustrated the value of VC in CRC TNM staging.
CT COLONOGRAPHY VS MR COLONOGRAPHY
A meta-regression technique was performed to compare the diagnostic accuracy of CTC and MRC, compared with CC for patients presenting with CRC. Overall sensitivity and specificity of CTC (95%, 95% respectively) and MRC 95%, 95% respectively) in detection of CRC was similar. Meta-regression analysis showed no significant difference in the diagnostic accuracy of both modalities. Both tests showed large area under the summary receiver operating characteristic curve, with high diagnostic odds ratios. Factors that enhanced the overall accuracy of MRC were the use of intravenous contrast, faecal tagging and exclusion of low-quality studies. This meta-analysis suggested that CTC and MRC have similar diagnostic accuracy in detecting CRC. Study quality, size and intravenous/intra-luminal contrast agents affect diagnostic accuracies. For an exact comparison to be made, studies evaluating CTC, MRC and CC in the same patient cohort would be necessary[80–82].
VIRTUAL COLONOSCOPY VS COVENTIONAL COLONOSCOPY
CTC is reliable for detecting lesions 6 mm or larger in size. It permits evaluation of the region proximal to an occlusive growth, which is often impossible with CC[83]. CTC is a good imaging tool for the exclusion of CRC in a population unfit for or unable to complete colonoscopy or barium enema, with reasonable sensitivity and specificity for detection of CRC[84]. MRC is a promising modality with high accuracy for detecting colorectal polyps larger than 5 mm in diameter. In IBD, MRC can be used to assess disease activity, including spreading[85,86]. In detecting colon lesions, MRC achieved a diagnostic accuracy similar to CC. However, MRC is minimally invasive, with no need for sedation or analgesics during investigation. There is a lower percentage of perforation risk, and all colon segments can be evaluated due to multi-sectional imaging availability; intramural, extra-intestinal components of colonic lesions, metastasis and any additional lesions can be evaluated easily[87]. MRC is a feasible and useful method of evaluating the entire colon in patients with incomplete CC. The majority of the patients found MRC less unpleasant than CC and a majority would prefer MRC over CC as a future colon examination. MRC also appears to be less time consuming to the patients and medical personnel than CC with post-procedural monitoring[88,89]. MRC proved reliable in evaluating the majority of colonic segments inaccessible with CC. The identification of additional disease at MRC underscores the need for a second diagnostic step in the setting of incomplete CC[90].
COMPLICATIONS AND LIMITATIONS
Perforation of the colon and rectum is a rare complication of VC. Older age and underlying concomitant colonic disease were present in patients with perforation[91]. The cancer risks associated with the radiation exposure from VC are unlikely to be zero, but they are small. A best estimate for the absolute lifetime cancer risk associated with the radiation exposure using typical current scanner techniques is about 0.14% for paired VC scans for a 50-year-old, and about half that for a 70-year-old. These values probably could be reduced by factors of 5 or 10 with optimized VC protocols. In terms of the radiation exposure, the benefit-risk ratio is potentially large for VC[92].
Many differences between the studies with high sensitivity (94%)[93]. Additional obstacles for implementation in prevention of CRC may be controversial results concerning patient acceptance, the large-scale use of ionizing radiation, difficulties in detecting flat adenomas, and extracolonic findings[94]. Flat lesions and small polyps are the other two main causes for missed lesions at 8 multi-detector rows CTC[95,96]. Currently, CTC is less cost-effective than conventional endoscopy[97]. CTC and MRC present both advantages and disadvantages, such as the impossibility to perform MR in patients with pace-maker or in claustrophobic patients and the impossibility to perform CT with iodated agents in patients with renal failure or with a story of adverse reactions[98,99]. Although application of PET and PET/CT in CRC diagnosis, staging and re-staging has been widely accepted by oncologists, there are no data of PET/CTC in CRC screening. But in the high risk patients and follow up of CRC patients after treatment, the examination is still cost-effective. Radiation dose exposures to the patient who accepts this examination is another main problem which should be further studied in the near future[100,101].
COST-EFFECTIVE ISSUES
CTC is an effective screening test for colorectal neoplasia. However, it is more expensive and generally less effective than CC. CTC can be reasonably cost-effective when its diagnostic accuracy is high, as with primary 3-dimensional technology, as if costs are about 60% of those of CC. Overall, CTC technology will need to improve its accuracy and reliability to be a cost-effective screening option[102]. CRC screening is cost-saving in Italy, irrespective of the technique applied. CTC appeared to be more cost-effective than flexible sigmoidoscopy, and it may also become a valid alternative to CC[103]. VC involves a helical CT or MR scan of the abdomen and pelvis to detect colorectal polyps and cancer. Both modalities have shown promising sensitivity in revealing larger polyps, in comparison with CC. Caution should be exercised in its clinical implementation due to significant interobserver variation and individual learning curves. A Danish study indicates that CTC can be performed cost-effectively compared to CC. CTC is recommended in preference to DCBE after incomplete CC[104].
DERIVATIVE TECHNIQUE FOR CRC SCREENING
Stool DNA testing
Stool DNA testing provides an acceptable noninvasive alternative for CRC screening that can identify early-stage CRC and adenomatous polyps in routine clinical practice[105]. Abbaszadegan[106] et al reported that this test could detect CRC related genetic alterations by analyzing stool DNA with a sensitivity of 64% and 20% and a specificity of 95% and 100% for long DNA and p16 respectively. An abnormal stool DNA test correlated with a colonoscopically demonstrable abnormality in 49% of cases (34 of 69). Abnormal findings, including CRC in 4% of cases (3 patients), single or multiple adenomatous polyps in 23 patients (33%), hyperplastic polyps in 3 patients (4%), and colitis in 5 patients (7%). Colonoscopy was reported as negative in 51% of patients (35 of 69), including 2 cases (3%). A non-invasive molecular stool-based DNA testing can provide a screening strategy in high-risk individuals[107].
Computer-aided detection
The latest CAD systems indicate a clinically acceptable high sensitivity and a low false-positive rate, and observer studies have demonstrated the benefits of these systems in improving radiologists’ detection performance[108]. Polyps were classified as small (≤ 5 mm), medium (6-9 mm), and large (≥ 10 mm). A total of 118 polyps (small, 85; medium, 19; large, 14) were found in 56 patients. CAD detected 72 polyps (61%) with an average of 2.2 false-positives. Sensitivity was 51% (43/85) for small, 90% (17/19) for medium, and 86% (12/14) for large polyps. For all polyps, per-patient sensitivity was 89% (50/56) for the radiologist and 73% (41/56) for CAD. For large and medium polyps, per-patient sensitivity was 100% for the radiologist, and 96% for CAD. CAD shows high sensitivity in the detection of clinically significant polyps with acceptable false-positive rates[109] and CAD for CTC significantly increases per-patient and per-polyp detection and significantly reduces interpretation times but cannot substitute adequate training[100].
PET/MRI colonography
Predicting the future is difficult, and any attempt to do so is fundamentally flawed. But the fusion of PET with MRI could compensate for their disadvantages and therefore offers several advantages in comparison to PET or MRI alone. The combination of these two excellent diagnostic imaging modalities into a single scanner improves the diagnostic accuracy by facilitating the accurate registration of molecular aspects and metabolic alterations of the diseases with exact correlation to anatomical findings and morphological information. PET/MR imaging is to become a multimodality platform for molecular imaging and PET/MRI colonography might be a promising diagnostic modality for use in CRC screening in the decades to come due to the considerably lower radiation exposure in contrast to PET/CTC and the high soft tissue resolution of MRI in contrast to CT in the future[].
CONCLUSION
With the exponential development in computer processing power, CT, MR and PET/CT colonography offer numerous advantages over more traditional methods of radiologic diagnosis, and provide essential information not only for initial diagnosis, but also for management, follow-up and detection of potential complications. Will CT, MR and PET/CT colonography replace conventional colonoscopy in the future? We do not believe so at present. However, combined with several derivative techniques on the horizon involving stool DNA testing, computer-aided detection and PET/MRI colonography, these techniques may further improve the specificity and sensitivity of imaging modalities in CRC screening and save the colonoscopy resource for the patients who need treatment.
Peer reviewer: Mitsuhiro Fujishiro, Dr, Department of Gastroenterology, Faculty of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, Japan
S- Editor Liu Y L- Editor Alpini GD E- Editor Wang HF
References
- 1.Kuriki K, Tajima K. The increasing incidence of colorectal cancer and the preventive strategy in Japan. Asian Pac J Cancer Prev. 2006;7:495–501. [PubMed] [Google Scholar]
- 2.Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871–876. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Soon MS, Kozarek RA, Ayub K, Soon A, Lin TY, Lin OS. Screening colonoscopy in Chinese and Western patients: a comparative study. Am J Gastroenterol. 2005;100:2749–2755. doi: 10.1111/j.1572-0241.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 5.Bromer MQ, Weinberg DS. Screening for colorectal cancer now and the near future. Semin Oncol. 2005;32:3–10. doi: 10.1053/j.seminoncol.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Burling D, Moore A, Taylor S, La Porte S, Marshall M. Virtual colonoscopy training and accreditation: a national survey of radiologist experience and attitudes in the UK. Clin Radiol. 2007;62:651–659. doi: 10.1016/j.crad.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs ET, Thompson PA, Martinez ME. Diet, Gender, and Colorectal Neoplasia. J Clin Gastroenterol. 2007;41:731–746. doi: 10.1097/MCG.0b013e3180338e56. [DOI] [PubMed] [Google Scholar]
- 8.Madlensky L, Daftary D, Burnett T, Harmon P, Jenkins M, Maskiell J, Nigon S, Phillips K, Templeton A, Limburg PJ, et al. Accuracy of Colorectal Polyp Self-Reports: Findings from the Colon Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2007;16:1898–1901. doi: 10.1158/1055-9965.EPI-07-0151. [DOI] [PubMed] [Google Scholar]
- 9.Vinikoor LC, Galanko JA, Sandler RS. Cholecystectomy and the risk of colorectal adenomas. Dig Dis Sci. 2008;53:730–735. doi: 10.1007/s10620-007-9912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bond JH. Clinical evidence for the adenoma-carcinoma sequence, and the management of patients with colorectal adenomas. Semin Gastrointest Dis. 2000;11:176–184. [PubMed] [Google Scholar]
- 11.Kim DH, Pickhardt PJ, Taylor AJ. Characteristics of advanced adenomas detected at CT colonographic screening: implications for appropriate polyp size thresholds for polypectomy versus surveillance. AJR Am J Roentgenol. 2007;188:940–944. doi: 10.2214/AJR.06.0764. [DOI] [PubMed] [Google Scholar]
- 12.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18 Suppl 2:1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology. 2004;127:80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Choi PM, Zelig MP. Similarity of colorectal cancer in CD and UC. Implications for carcinogenesis and prevention. Gut. 1994;35:950–954. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin DT, Rothe JA, Hetzel JT, Cohen RD, Hanauer SB. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007;65:998–1004. doi: 10.1016/j.gie.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Behm BW, Bickston SJ. Efficacy of infliximab for luminal and fistulizing Crohn’s disease andin ulcerative colitis. Curr Treat Options Gastroenterol. 2007;10:171–177. doi: 10.1007/s11938-007-0010-6. [DOI] [PubMed] [Google Scholar]
- 17.van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–1578. doi: 10.1136/gut.2005.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munkholm P, Loftus EV, Reinacher-Schick A, Kornbluth A, Mittmann U, Esendal B. Prevention of colorectal cancer in inflammatory bowel disease: value of screening and 5-aminosalicylates. Digestion. 2006;73:11–19. doi: 10.1159/000090763. [DOI] [PubMed] [Google Scholar]
- 19.Cancer statistics 2006. American Cancer Society Inc. Vol. 73. 2006. p. July; 11. [Google Scholar]
- 20.Kang H, O’connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007;22:183–189. doi: 10.1007/s00384-006-0145-2. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman RK, Nowalk MP, Tabbarah M, Grufferman S. Predictors of colorectal cancer screening in diverse primary care practices. BMC Health Serv Res. 2006;6:116. doi: 10.1186/1472-6963-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saliangas K. Screening for colorectal cancer. Tech Coloproctol. 2004;8 Suppl 1:s10–s13. doi: 10.1007/s10151-004-0098-9. [DOI] [PubMed] [Google Scholar]
- 23.Ajaj W, Goyen M. MR imaging of the colon: “technique, indications, results and limitations”. Eur J Radiol. 2007;61:415–423. doi: 10.1016/j.ejrad.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Luo MY, Shan H, Yao LQ, Zhou KR, Liang WW. Postprocessing techniques of CT colonography in detection of colorectal carcinoma. World J Gastroenterol. 2004;10:1574–1577. doi: 10.3748/wjg.v10.i11.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gollub MJ, Schwartz LH, Akhurst T. Update on colorectal cancer imaging. Radiol Clin North Am. 2007;45:85–118. doi: 10.1016/j.rcl.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Limburg PJ, Fletcher JG. Making sense of CT colonography-related complication rates. Gastroenterology. 2006;131:2023–2024. doi: 10.1053/j.gastro.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 27.Sosna J, Sella T, Bar-Ziv J, Libson E. Perforation of the colon and rectum--a newly recognized complication of CT colonography. Semin Ultrasound CT MR. 2006;27:161–165. doi: 10.1053/j.sult.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Leighton JA, Loftus EV. Evolving diagnostic modalities in inflammatory bowel disease. Curr Gastroenterol Rep. 2005;7:467–474. doi: 10.1007/s11894-005-0078-x. [DOI] [PubMed] [Google Scholar]
- 29.Andersen K, Vogt C, Blondin D, Beck A, Heinen W, Aurich V, Haussinger D, Modder U, Cohnen M. Multi-detector CT-colonography in inflammatory bowel disease: prospective analysis of CT-findings to high-resolution video colonoscopy. Eur J Radiol. 2006;58:140–146. doi: 10.1016/j.ejrad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Ota Y, Matsui T, Ono H, Uno H, Matake H, Tsuda S, Sakurai T, Yao T. Value of virtual computed tomographic colonography for Crohn’s colitis: comparison with endoscopy and barium enema. Abdom Imaging. 2003;28:778–783. doi: 10.1007/s00261-003-0023-0. [DOI] [PubMed] [Google Scholar]
- 31.Biancone L, Fiori R, Tosti C, Marinetti A, Catarinacci M, De Nigris F, Simonetti G, Pallone F. Virtual colonoscopy compared with conventional colonoscopy for stricturing postoperative recurrence in Crohn’s disease. Inflamm Bowel Dis. 2003;9:343–350. doi: 10.1097/00054725-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Brambs , Juchems Virtual endoscopy using CT scan. Minim Invasive Ther Allied Technol. 2003;12:207–216. doi: 10.1080/13645700310015572. [DOI] [PubMed] [Google Scholar]
- 33.Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartram CI, Atkin W. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology. 2005;237:893–904. doi: 10.1148/radiol.2373050176. [DOI] [PubMed] [Google Scholar]
- 34.Kim DH, Pickhardt PJ, Hoff G, Kay CL. Computed tomographic colonography for colorectal screening. Endoscopy. 2007;39:545–549. doi: 10.1055/s-2007-966240. [DOI] [PubMed] [Google Scholar]
- 35.Bazensky I, Shoobridge-Moran C, Yoder LH. Colorectal cancer: an overview of the epidemiology, risk factors, symptoms, and screening guidelines. Medsurg Nurs. 2007;16:46–51; quiz 52. [PubMed] [Google Scholar]
- 36.Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005;142:635–650. doi: 10.7326/0003-4819-142-8-200504190-00013. [DOI] [PubMed] [Google Scholar]
- 37.Silva AC, Hara AK, Leighton JA, Heppell JP. CT colonography with intravenous contrast material: varied appearances of colorectal carcinoma. Radiographics. 2005;25:1321–1334. doi: 10.1148/rg.255045184. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H, Nappi J. CAD in CT colonography without and with oral contrast agents: progress and challenges. Comput Med Imaging Graph. 2007;31:267–284. doi: 10.1016/j.compmedimag.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Hjern F, Jonas E, Holmstrom B, Josephson T, Mellgren A, Johansson C. CT colonography versus colonoscopy in the follow-up of patients after diverticulitis - A prospective, comparative study. Clin Radiol. 2007;62:645–650. doi: 10.1016/j.crad.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Mang T, Graser A, Schima W, Maier A. CT colonography: techniques, indications, findings. Eur J Radiol. 2007;61:388–399. doi: 10.1016/j.ejrad.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Dachman AH, Dawson DO, Lefere P, Yoshida H, Khan NU, Cipriani N, Rubin DT. Comparison of routine and unprepped CT colonography augmented by low fiber diet and stool tagging: a pilot study. Abdom Imaging. 2007;32:96–104. doi: 10.1007/s00261-006-9044-9. [DOI] [PubMed] [Google Scholar]
- 42.Dachman AH, Lefere P, Gryspeerdt S, Morin M. CT Colonography: Visualization Methods, Interpretation, and Pitfalls. Radiol Clin North Am. 2007;45:347–359. doi: 10.1016/j.rcl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Fletcher JG, Johnson CD, Krueger WR, Ahlquist DA, Nelson H, Ilstrup D, Harmsen WS, Corcoran KE. Contrast-enhanced CT colonography in recurrent colorectal carcinoma: feasibility of simultaneous evaluation for metastatic disease, local recurrence, and metachronous neoplasia in colorectal carcinoma. AJR Am J Roentgenol. 2002;178:283–290. doi: 10.2214/ajr.178.2.1780283. [DOI] [PubMed] [Google Scholar]
- 44.Lauenstein TC. MR colonography: current status. Eur Radiol. 2006;16:1519–1526. doi: 10.1007/s00330-006-0260-z. [DOI] [PubMed] [Google Scholar]
- 45.Kinner S, Lauenstein TC. MR Colonography. Radiol Clin North Am. 2007;45:377–387. doi: 10.1016/j.rcl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Rottgen R, Herzog H, Lopez-Haninnen E, Felix R. Bowel wall enhancement in magnetic resonance colonography for assessing activity in Crohn’s disease. Clin Imaging. 2006;30:27–31. doi: 10.1016/j.clinimag.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 47.Schreyer AG, Golder S, Scheibl K, Volk M, Lenhart M, Timmer A, Scholmerich J, Feuerbach S, Rogler G, Herfarth H, et al. Dark lumen magnetic resonance enteroclysis in combination with MRI colonography for whole bowel assessment in patients with Crohn’s disease: first clinical experience. Inflamm Bowel Dis. 2005;11:388–394. doi: 10.1097/01.mib.0000164022.72729.06. [DOI] [PubMed] [Google Scholar]
- 48.Lauenstein TC, Goehde SC, Debatin JF. Fecal tagging: MR colonography without colonic cleansing. Abdom Imaging. 2002;27:410–417. doi: 10.1007/s00261-001-0121-9. [DOI] [PubMed] [Google Scholar]
- 49.Ajaj W, Goyen M, Langhorst J, Ruehm SG, Gerken G, Lauenstein TC. MR colonography for the assessment of colonic anastomoses. J Magn Reson Imaging. 2006;24:101–107. doi: 10.1002/jmri.20606. [DOI] [PubMed] [Google Scholar]
- 50.Florie J, Jensch S, Nievelstein RA, Bartelsman JF, Baak LC, van Gelder RE, Haberkorn B, van Randen A, van der Ham MM, Snel P, et al. MR colonography with limited bowel preparation compared with optical colonoscopy in patients at increased risk for colorectal cancer. Radiology. 2007;243:122–131. doi: 10.1148/radiol.2431052088. [DOI] [PubMed] [Google Scholar]
- 51.Ajaj W, Ruehm SG, Gerken G, Goyen M. Strengths and weaknesses of dark-lumen MR colonography: clinical relevance of polyps smaller than 5 mm in diameter at the moment of their detection. J Magn Reson Imaging. 2006;24:1088–1094. doi: 10.1002/jmri.20734. [DOI] [PubMed] [Google Scholar]
- 52.Bielen DJ, Bosmans HT, De Wever LL, Maes F, Tejpar S, Vanbeckevoort D, Marchal GJ. Clinical validation of high-resolution fast spin-echo MR colonography after colon distention with air. J Magn Reson Imaging. 2005;22:400–405. doi: 10.1002/jmri.20397. [DOI] [PubMed] [Google Scholar]
- 53.Haykir R, Karakose S, Karabacakoglu A, Kayacetin E, Sahin M. Detection of colonic masses with MR colonography. Turk J Gastroenterol. 2006;17:191–197. [PubMed] [Google Scholar]
- 54.Luboldt W, Bauerfeind P, Wildermuth S, Marincek B, Fried M, Debatin JF. Colonic masses: detection with MR colonography. Radiology. 2000;216:383–388. doi: 10.1148/radiology.216.2.r00au11383. [DOI] [PubMed] [Google Scholar]
- 55.Kinner S, Kuehle CA, Langhorst J, Ladd SC, Nuefer M, Barkhausen J, Lauenstein TC. MR colonography with fecal tagging: do individual patient characteristics influence image quality? J Magn Reson Imaging. 2007;25:1007–1012. doi: 10.1002/jmri.20907. [DOI] [PubMed] [Google Scholar]
- 56.Ajaj W, Lauenstein TC, Pelster G, Holtmann G, Ruehm SG, Debatin JF, Goehde SC. MR colonography in patients with incomplete conventional colonoscopy. Radiology. 2005;234:452–459. doi: 10.1148/radiol.2342032001. [DOI] [PubMed] [Google Scholar]
- 57.Wong TY, Lam WW, So NM, Lee JF, Leung KL. Air-inflated magnetic resonance colonography in patients with incomplete conventional colonoscopy: Comparison with intraoperative findings, pathology specimens, and follow-up conventional colonoscopy. Am J Gastroenterol. 2007;102:56–63. doi: 10.1111/j.1572-0241.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 58.Purkayastha S, Tekkis PP, Athanasiou T, Aziz O, Negus R, Gedroyc W, Darzi AW. Magnetic resonance colonography versus colonoscopy as a diagnostic investigation for colorectal cancer: a meta-analysis. Clin Radiol. 2005;60:980–989. doi: 10.1016/j.crad.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Pappalardo G, Polettini E, Frattaroli FM, Casciani E, D’Orta C, D’Amato M, Gualdi GF. Magnetic resonance colonography versus conventional colonoscopy for the detection of colonic endoluminal lesions. Gastroenterology. 2000;119:300–304. doi: 10.1053/gast.2000.9353. [DOI] [PubMed] [Google Scholar]
- 60.Loffler M, Weckesser M, Franzius C, Schober O, Zimmer KP. High diagnostic value of 18F-FDG-PET in pediatric patients with chronic inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:379–385. doi: 10.1196/annals.1326.014. [DOI] [PubMed] [Google Scholar]
- 61.Reinhardt MJ, Joe AY, Jaeger U, Huber A, Matthies A, Bucerius J, Roedel R, Strunk H, Bieber T, Biersack HJ, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol. 2006;24:1178–1187. doi: 10.1200/JCO.2005.03.5634. [DOI] [PubMed] [Google Scholar]
- 62.Veit-Haibach P, Kuehle CA, Beyer T, Stergar H, Kuehl H, Schmidt J, Borsch G, Dahmen G, Barkhausen J, Bockisch A, et al. Diagnostic accuracy of colorectal cancer staging with whole-body PET/CT colonography. JAMA. 2006;296:2590–2600. doi: 10.1001/jama.296.21.2590. [DOI] [PubMed] [Google Scholar]
- 63.Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, Dahmen G, Bockisch A, Debatin JF, Ruehm SG. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290:3199–3206. doi: 10.1001/jama.290.24.3199. [DOI] [PubMed] [Google Scholar]
- 64.Charron M. Inflammatory bowel disease activity assessment with biologic markers and 99mTc-WBC scintigraphy: are there different trends in ileitis versus colitis? J Nucl Med. 2003;44:1586–1591. [PubMed] [Google Scholar]
- 65.Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007;48:35–45. [PubMed] [Google Scholar]
- 66.Prabhakar HB, Sahani DV, Fischman AJ, Mueller PR, Blake MA. Bowel hot spots at PET-CT. Radiographics. 2007;27:145–159. doi: 10.1148/rg.271065080. [DOI] [PubMed] [Google Scholar]
- 67.Neurath MF, Vehling D, Schunk K, Holtmann M, Brockmann H, Helisch A, Orth T, Schreckenberger M, Galle PR, Bartenstein P. Noninvasive assessment of Crohn’s disease activity: a comparison of 18F-fluorodeoxyglucose positron emission tomography, hydromagnetic resonance imaging, and granulocyte scintigraphy with labeled antibodies. Am J Gastroenterol. 2002;97:1978–1985. doi: 10.1111/j.1572-0241.2002.05836.x. [DOI] [PubMed] [Google Scholar]
- 68.Loffler M, Weckesser M, Franzius C, Schober O, Zimmer KP. High diagnostic value of 18F-FDG-PET in pediatric patients with chronic inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:379–385. doi: 10.1196/annals.1326.014. [DOI] [PubMed] [Google Scholar]
- 69.Israel O, Yefremov N, Bar-Shalom R, Kagana O, Frenkel A, Keidar Z, Fischer D. PET/CT detection of unexpected gastrointestinal foci of 18F-FDG uptake: incidence, localization patterns, and clinical significance. J Nucl Med. 2005;46:758–762. [PubMed] [Google Scholar]
- 70.Lemberg DA, Issenman RM, Cawdron R, Green T, Mernagh J, Skehan SJ, Nahmias C, Jacobson K. Positron emission tomography in the investigation of pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:733–738. doi: 10.1097/01.mib.0000172810.49619.cb. [DOI] [PubMed] [Google Scholar]
- 71.O’Brien PH, Jenrette JM 3rd, Garvin AJ. Radiation enteritis. Am Surg. 1987;53:501–504. [PubMed] [Google Scholar]
- 72.Yeoh EK, Horowitz M: Radiation enteritis. Surg Gynecol Obstet. 1987;165:373–379. [PubMed] [Google Scholar]
- 73.Kuehle CA, Veit P, Antoch G, Grabellus F, Robert P, Beyer T, Herborn CU. Contrast-enhanced dark lumen PET/CT and MR colonography in a rodent polyp model: initial results with histopathologic correlation. AJR Am J Roentgenol. 2005;185:1045–1047. doi: 10.2214/AJR.04.1337. [DOI] [PubMed] [Google Scholar]
- 74.Kamel EM, Thumshirn M, Truninger K, Schiesser M, Fried M, Padberg B, Schneiter D, Stoeckli SJ, von Schulthess GK, Stumpe KD. Significance of incidental 18F-FDG accumulations in the gastrointestinal tract in PET/CT: correlation with endoscopic and histopathologic results. J Nucl Med. 2004;45:1804–1810. [PubMed] [Google Scholar]
- 75.Kantorova I, Lipska L, Belohlavek O, Visokai V, Trubac M, Schneiderova M. Routine (18)F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003;44:1784–1788. [PubMed] [Google Scholar]
- 76.Park IJ, Kim HC, Yu CS, Ryu MH, Chang HM, Kim JH, Ryu JS, Yeo JS, Kim JC. Efficacy of PET/CT in the accurate evaluation of primary colorectal carcinoma. Eur J Surg Oncol. 2006;32:941–947. doi: 10.1016/j.ejso.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 77.Rappeport ED, Loft A, Berthelsen AK, von der Recke P, Larsen PN, Mogensen AM, Wettergren A, Rasmussen A, Hillingsoe J, Kirkegaard P, et al. Contrast-enhanced FDG-PET/CT vs SPIO-enhanced MRI vs FDG-PET vs CT in patients with liver metastases from colorectal cancer: a prospective study with intraoperative confirmation. Acta Radiol. 2007;48:369–378. doi: 10.1080/02841850701294560. [DOI] [PubMed] [Google Scholar]
- 78.Votrubova J, Belohlavek O, Jaruskova M, Oliverius M, Lohynska R, Trskova K, Sedlackova E, Lipska L, Stahalova V. The role of FDG-PET/CT in the detection of recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2006;33:779–784. doi: 10.1007/s00259-006-0072-z. [DOI] [PubMed] [Google Scholar]
- 79.Veit P, Kuhle C, Beyer T, Kuehl H, Herborn CU, Borsch G, Stergar H, Barkhausen J, Bockisch A, Antoch G. Whole body positron emission tomography/computed tomography (PET/CT) tumour staging with integrated PET/CT colonography: technical feasibility and first experiences in patients with colorectal cancer. Gut. 2006;55:68–73. doi: 10.1136/gut.2005.064170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Purkayastha S, Athanasiou T, Tekkis PP, Constantinides V, Teare J, Darzi AW. Magnetic resonance colonography vs computed tomography colonography for the diagnosis of colorectal cancer: an indirect comparison. Colorectal Dis. 2007;9:100–111. doi: 10.1111/j.1463-1318.2006.01126.x. [DOI] [PubMed] [Google Scholar]
- 81.Op de Beeck B, Van Cutsem E. Computed tomographic colonography. Acta Gastroenterol Belg. 2005;68:258–260. [PubMed] [Google Scholar]
- 82.Macari M, Bini EJ. CT colonography: where have we been and where are we going? Radiology. 2005;237:819–833. doi: 10.1148/radiol.2373041717. [DOI] [PubMed] [Google Scholar]
- 83.Kalra N, Suri S, Bhasin DK, Sinha SK, Saravanan N, Kour T, Vaiphei K, Wig JD. Comparison of multidetector computed tomographic colonography and conventional colonoscopy for detection of colorectal polyps and cancer. Indian J Gastroenterol. 2006;25:229–232. [PubMed] [Google Scholar]
- 84.Gallo TM, Galatola G, Laudi C, Regge D. CT colonography: screening in individuals at high risk for colorectal cancer. Abdom Imaging. 2006;31:297–301. doi: 10.1007/s00261-005-0368-7. [DOI] [PubMed] [Google Scholar]
- 85.Hartmann D, Bassler B, Schilling D, Adamek HE, Jakobs R, Pfeifer B, Eickhoff A, Zindel C, Riemann JF, Layer G. Colorectal polyps: detection with dark-lumen MR colonography versus conventional colonoscopy. Radiology. 2006;238:143–149. doi: 10.1148/radiol.2381041756. [DOI] [PubMed] [Google Scholar]
- 86.Debatin JF, Lauenstein TC. Virtual magnetic resonance colonography. Gut. 2003;52 Suppl 4:iv17–iv22. doi: 10.1136/gut.52.suppl_4.iv17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haykir R, Karakose S, Karabacakoglu A, Sahin M, Kayacetin E. Three-dimensional MR and axial CT colonography versus conventional colonoscopy for detection of colon pathologies. World J Gastroenterol. 2006;12:2345–2348. doi: 10.3748/wjg.v12.i15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartmann D, Bassler B, Schilling D, Pfeiffer B, Jakobs R, Eickhoff A, Riemann JF, Layer G. Incomplete conventional colonoscopy: magnetic resonance colonography in the evaluation of the proximal colon. Endoscopy. 2005;37:816–820. doi: 10.1055/s-2005-870309. [DOI] [PubMed] [Google Scholar]
- 89.Lauenstein TC, Goehde SC, Ruehm SG, Holtmann G, Debatin JF. MR colonography with barium-based fecal tagging: initial clinical experience. Radiology. 2002;223:248–254. doi: 10.1148/radiol.2231010887. [DOI] [PubMed] [Google Scholar]
- 90.Rosman AS, Korsten MA. Meta-analysis comparing CT colonography, air contrast barium enema, and colonoscopy. Am J Med. 2007;120:203–210. doi: 10.1016/j.amjmed.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 91.Sosna J, Blachar A, Amitai M, Barmeir E, Peled N, Goldberg SN, Bar-Ziv J. Colonic perforation at CT colonography: assessment of risk in a multicenter large cohort. Radiology. 2006;239:457–458. doi: 10.1148/radiol.2392050287. [DOI] [PubMed] [Google Scholar]
- 92.Brenner DJ, Georgsson MA. Mass screening with CT colonography: should the radiation exposure be of concern? Gastroenterology. 2005;129:328–337. doi: 10.1053/j.gastro.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 93.Yoshida H, Nappi J. CAD in CT colonography without and with oral contrast agents: Progress and challenges. Comput Med Imaging Graph. 2007;31:267–284. doi: 10.1016/j.compmedimag.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 94.Nio Y, Van Gelder RE, Stoker J. Computed tomography colonography: current issues. Scand J Gastroenterol Suppl. 2006;243:139–145. doi: 10.1080/00365520600664482. [DOI] [PubMed] [Google Scholar]
- 95.Park SH, Ha HK, Kim MJ, Kim KW, Kim AY, Yang DH, Lee MG, Kim PN, Shin YM, Yang SK, et al. False-negative results at multi-detector row CT colonography: multivariate analysis of causes for missed lesions. Radiology. 2005;235:495–502. doi: 10.1148/radiol.2352040606. [DOI] [PubMed] [Google Scholar]
- 96.Park SH, Lee SS, Choi EK, Kim SY, Yang SK, Kim JH, Ha HK. Flat colorectal neoplasms: definition, importance, and visualization on CT colonography. AJR Am J Roentgenol. 2007;188:953–959. doi: 10.2214/AJR.06.0436. [DOI] [PubMed] [Google Scholar]
- 97.Vogt C, Cohnen M, Beck A, vom Dahl S, Aurich V, Modder U, Haussinger D. Detection of colorectal polyps by multislice CT colonography with ultra-low-dose technique: comparison with high-resolution videocolonoscopy. Gastrointest Endosc. 2004;60:201–209. doi: 10.1016/s0016-5107(04)01684-0. [DOI] [PubMed] [Google Scholar]
- 98.Achiam MP, Bulow S, Rosenberg J. CT- and MR colonography. Scand J Surg. 2002;91:322–327. doi: 10.1177/145749690209100402. [DOI] [PubMed] [Google Scholar]
- 99.Hoppe H, Netzer P, Spreng A, Quattropani C, Mattich J, Dinkel HP. Prospective comparison of contrast enhanced CT colonography and conventional colonoscopy for detection of colorectal neoplasms in a single institutional study using second-look colonoscopy with discrepant results. Am J Gastroenterol. 2004;99:1924–1935. doi: 10.1111/j.1572-0241.2004.40238.x. [DOI] [PubMed] [Google Scholar]
- 100.Nakamoto Y, Sakamoto S, Okada T, Senda M, Higashi T, Saga T, Togashi K. Clinical value of manual fusion of PET and CT images in patients with suspected recurrent colorectal cancer. AJR Am J Roentgenol. 2007;188:257–267. doi: 10.2214/AJR.05.0708. [DOI] [PubMed] [Google Scholar]
- 101.Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, Altman H, Keidar Z, Israel O. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200–1209. [PubMed] [Google Scholar]
- 102.Vijan S, Hwang I, Inadomi J, Wong RK, Choi JR, Napierkowski J, Koff JM, Pickhardt PJ. The cost-effectiveness of CT colonography in screening for colorectal neoplasia. Am J Gastroenterol. 2007;102:380–390. doi: 10.1111/j.1572-0241.2006.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hassan C, Zullo A, Laghi A, Reitano I, Taggi F, Cerro P, Iafrate F, Giustini M, Winn S, Morini S. Colon cancer prevention in Italy: cost-effectiveness analysis with CT colonography and endoscopy. Dig Liver Dis. 2007;39:242–350. doi: 10.1016/j.dld.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 104.Pedersen BG, Achiam MP, Arnesen RB. Virtual colonoscopy is now reality. Ugeskr Laeger. 2005;167:4175–4179. [PubMed] [Google Scholar]
- 105.Berger BM, Schroy PC, Rosenberg JL, Lai-Goldman M, Eisenberg M, Brown T, Rochelle RB, Billings PR. CRCscreening using stool DNA analysis in clinical practice: early clinical experience with respect to patient acceptance and colonoscopic follow-up of abnormal tests. Clin Colorectal Cancer. 2006;5:338–343. doi: 10.3816/CCC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 106.Abbaszadegan MR, Tavasoli A, Velayati A, Sima HR, Vosooghinia H, Farzadnia M, Asadzedeh H, Gholamin M, Dadkhah E, Aarabi A. Stool-based DNA testing, a new noninvasive method for colorectal cancer screening, the first report from Iran. World J Gastroenterol. 2007;13:1528–1533. doi: 10.3748/wjg.v13.i10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC, Sontag S, Johnson D, Skoletsky J, Durkee K, Markowitz S, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 108.Summers RM, Jerebko AK, Franaszek M, Malley JD, Johnson CD. Colonic polyps: complementary role of computer-aided detection in CT colonography. Radiology. 2002;225:391–399. doi: 10.1148/radiol.2252011619. [DOI] [PubMed] [Google Scholar]
- 109.Graser A, Kolligs FT, Mang T, Schaefer C, Geisbusch S, Reiser MF, Becker CR. Computer-aided detection in CT colonography: initial clinical experience using a prototype system. Eur Radiol. 2007;17:2608–2615. doi: 10.1007/s00330-007-0579-0. [DOI] [PubMed] [Google Scholar]
- 110.Seemann MD. Whole-body PET/MRI: the future in oncological imaging. Technol Cancer Res Treat. 2005;4:577–582. doi: 10.1177/153303460500400512. [DOI] [PubMed] [Google Scholar]
- 111.Zalis M, Singh AK. Imaging of inflammatory bowel disease: CT and MR. Dig Dis. 2004;22:56–62. doi: 10.1159/000078735. [DOI] [PubMed] [Google Scholar]