Abstract
AIM: To investigate the interrelationship between H pylori and Epstein-Barr virus (EBV) infection in the gastric carcinogenesis having in focus the p53 mutation and the c-Myc, Bcl-2 and Bax expression.
METHODS: seventy-one gastric carcinoma tissues were assessed by polymerase chain reaction (PCR) for H pylori and in situ hybridization for EBV. c-Myc, Bcl-2 and Bax expression were detected by immunohistochemistry and single-stranded conformational polymorphism (SSCP) for p53 mutation.
RESULTS: The positivity rates for H pylori and EBV were 94.4% and 8.45%, respectively. The majority of the cases displayed only the H pylori presence. All EBV positive cases were also H pylori positive. None infectious agent was observed in 5.55% of the cases. The intestinal type tumor was more frequent in the co-infected and non-infected groups. The female predominated in the non-infected group showing statistical significance (70.4% vs 29.6%, P = 0.039). The Bcl-2 was only detected in the group exclusively infected by H pylori. However, c-Myc and Bax were detected in the three groups but with a low frequency in the co-infected group. Mutation of p53 was present in all groups, with the highest frequencies in the H pylori positive groups.
CONCLUSION: The frequency of H pylori infection in gastric carcinomas was high. The presented data indicated that gastric carcinogenesis has different pathways depending of the presence of the two investigated infectious agents, suggesting a possible involvement of H pylori with apoptotic process. The low expression of c-Myc and Bax in the EBV-positive groups suggests that EBV may inhibit the expression of these proteins. Nevertheless, p53 mutation shows to be a relevant alteration, independent of both infectious agents.
Keywords: Gastric carcinoma; Helicobacter pylori; Epstein-Barr virus, p53, Bax, Bcl-2, c-Myc
INTRODUCTION
Gastric cancer is the fourth most common cancer and the second cause of cancer-related death worldwide[1]. There is substantial international variation in the gastric cancer incidence with the highest rates reported from Korea, Japan and Eastern Asia. Other high incidence areas include Eastern Europe and parts of Latin America, while Western Europe and the United States of America generally have low incidence rates. The global burden of gastric cancer is shifting rapidly from the developed world to the developing world[2].
Over 95% of stomach tumors are adenocarcinomas which are subdivided into two main histological types: intestinal type and diffuse type. The intestinal type is related to corpus-dominant gastritis with gastric atrophy and intestinal metaplasia, whereas the diffuse type usually originates in pangastritis without atrophy[1,2].
The two main anatomic sites of gastric adenocarcinoma are proximal (cardia) and distal (noncardia). The frequency of the theses tumor sites varies in populations from different geographic locations, racial and socio economic groups. They may differ in genetic susceptibility, pathologic profile, clinical presentation and prognosis. The observed differences between gastric cancers by anatomic sites suggest that they are distinct diseases with different etiologies. Although the distal cancer is usually more frequent, a decreased incidence has been observed in this site while the incidence of the proximal site tumors have increased since the 1970’s especially among males in the Western countries[1,3,4].
There are several etiologic factors involved in gastric cancer such as low socioeconomic status, diet, hereditary factors and H pylori infection, as the most cited in studies about this issue. Besides the H pylori, Epstein-Barr virus (EBV) infection is also associated with development of gastric cancer[1,2,6].
The discovery of H pylori infection in the early eighties proved a turning point in the understanding of the gastric cancer pathogenesis. While the link between H pylori and peptic ulcer was established soon after the successful culture of the bacteria, the association with gastric cancer remained doubtful for almost a decade before credible evidence could be presented. The major reason for this delay was the inability to demonstrate the presence of active infection in gastric cancer tissue[1,2]. Despite the recognition of the association between H pylori and gastric cancer the pathogenic mechanism involved in this process is still not understood.
In spite of the wide bibliography regarding to the other tumors, the first report about the association of the EBV with gastric carcinomas was in a case of lymphoepithelial-like gastric carcinoma[7]. Soon after, it was identified in common gastric carcinomas[8]. EBV infection is found in approximately 10% of ordinary gastric adenocarcinoma cases; however most of the mechanisms used by the virus to control this process are still unknown. Recent studies have shown that the expression pattern of EBV-encoded genes in gastric carcinoma is different from that in Burkitt’s lymphoma and nasopharyngeal carcinoma (NPC), suggesting that the oncogenic mechanism of the EBV in gastric carcinoma may be unique[6,9,10].
In attempt to understand the carcinogenic process triggered by these two infectious agents, some works have looked for alteration in genes/proteins that these agents can target, and therefore, pointing possible pathogenic pathways. In this sense, proteins involved with cell cycle or apoptosis process emerge as a candidate for having a crucial role in the development of the neoplasms. Some of these, like c-Myc, p53 and the apoptotic family members such as Bcl-2 and Bax, deserve attention for playing a key function in the cell proliferation and cell fate, besides they already have shown involvement in the tumorigenesis process in a variety of tumors, including gastric cancer[11–13].
Although the proteins mentioned above have different levels of alteration in gastric carcinoma, the association with H pylori or EBV is controversial[14–16]. Additionally, there is just one paper related H pylori and EBV infection with concomitant analysis, in gastric carcinomas, in which, proteins involved in cell cycle and apoptosis process were also evaluated[10]. So, the aim of the present study was to investigate the interrelationship between H pylori and EBV infection in the gastric carcinogenesis having in focus the p53 mutation, and the expression of c-Myc, Bcl-2 and Bax.
MATERIALS AND METHODS
Clinical specimens
The present study was approved by the ethic committee from Federal University of Ceará and all subjects signed informed consent prior to inclusion. Samples from eighty-two patients with gastric carcinoma were collected from two hospitals of the Ceará State, Brazil: Walter Cantídeo Hospital at Federal University of Ceará and Saint House of Mercy in Fortaleza, both located in Fortaleza, the capital of the State. A representative formalin-fixed tumor specimens embedded in paraffin blocks was selected and histological sections (5 μm) were subjected to immunohistochemistry and in situ hybridization (ISH). Clinico-epidemiological data were collected from the medical reports. Upon gastrectomy, fragments of tumor were collected and subjected to DNA extraction and Polymerase Chain Reaction (PCR) to detect urease C gene of H pylori and p53 mutation.
DNA extraction
DNA was extracted from frozen tumor tissue just when the fragment presented more than 80% of tumor cells. The genomic DNA was extracted using cetyltrimethyl ammonium bromide (CTAB) adapted from the method of Gary[17]. Although we have collected samples from 82 patients, DNA extraction was done in 71 samples because the others did not reach the established percentage of tumor cells.
Detection of H pylori and the presence of cagA gene
The PCR was done to detected H pylori infection because this technique is sensitive and specific and it was used by many studies[6,18,38].
The H pylori infection was detected by amplification of urease C gene using primers for PCR, described by Lage et al[18]. For the H pylori-positive samples, the presence of the cagA gene was identified using the primers described by Domingo et al[19]. PCR mixtures for amplification of both genes were prepared in a volume of 25 μL containing 0.4 μmol/L of each primer; 1.5 μmol/L MgCl2; 0.8% Tween 20 and 1 μL of DNA sample. PCR products were analyzed by 1% agarose gel electrophoresis with ethidium bromide staining. Sample was considered H pylori positive when an ureC fragment of 294bp was amplified while cagA gene was positive when a fragment of 297bp was detected. As positive control we used DNA known H pylori positive and as negative control we used distillated water.
RNA in situ hybridization
The presence of the EBV was identified by the expression of EBV-encode small RNA-1 (EBER1), the most abundant viral product in latently infected cells. RNA in situ hybridization was performed using a 30bp biotinylated probe complementary to the RNA EBER1 described by Shibata and Weiss[8]. Briefly, after the deparaffinization and rehydration, endogenous peroxidase was blocked with 3% H2O2 solution. Enzymatic digestion was performed with proteinase K (0.02 μg/μL final concentration) for 13 min. Prehybridization was done with pre-hybridization solution (Denhardt’s solution [3.5X]; SSC [4.5X]; EDTA [0.0075 mol/L]; SDS [0.35%]; NaH2PO4 [0.75 mol/L]; dextran sulfate [10%]) for 60 min at 37°C. Soon after, the slides were incubated overnight at 37°C with hybridization solution containing 0.3 ng/μL of the probe. After washing with 2X SSC buffer, the signal was amplified using anti-biotin antibody (clone BK, mouse, dilution 1:20) and biotinylated anti-immunoglobulin antibody (polyclonal, rabbit, dilution 1:100). Detection was accomplished using streptavidin-biotin-peroxidase method and the reaction was developed with 3, 3’-diaminobenzidine chromogen. All antibodies were purchased from DakoCytomation®. The slides were counterstained with Harris hematoxylin. A case of nasopharyngeal carcinoma was used as positive control in each reaction, as well as a negative control by omitting the probe. Nuclei with a brown or black staining were considered positive.
Immunohistochemistry
The expression of the proteins Bcl-2, Bax and c-Myc was detected according to the method described by Hsu et al[20]. After the deparaffinization and rehydration, antigen retrieval was carried out by microwave-treatment of the slides for 15 min in 10 mmol/L citrate buffer solution (pH 6.0). Endogenous peroxidase activity was blocked with 3% H2O2 solution. Primary antibodies were incubated for 16 h at 4°C-8°C in a humid chamber. All antibodies were purchased from DakoCytomation®. The reaction was detected with LSAB + system (DakoCytomation®) according to the manufacturer’s recommendation.
Histopathological and staining analysis
The histological classification (Laurén) was obtained from the medical reporters and was confirmed by a professional pathologist of the team. The slides were evaluated by three experienced technicians independently using light microscopy at a magnification of 400X. In the in situ hybridization and immunohistochemistry techniques, the results were expressed as percentage of positive cases. In the immunohistochemistry analysis, only the cases with ≥ 5% of stained tumor cells were considered as positive while in the in situ hybridization, any nuclear staining in tumor cells were considered. For all analyses, at least 1000 tumor cells were counted in high power fields.
Analysis of p53 mutation
To detection of mutation in the p53 gene was used Single-Stranded Conformational Polymorphism (SSCP) test. Exons 5-9 of p53 gene were amplified by PCR using four pairs of the primes described by Murakami et al[21]. Each PCR reaction for a total of 25 μL final volume consisted of 0.2 mmol/L of the four deoxynucleotide triphosphate, 1.5 mmol/L MgCl2, 0.4 μmol/L of each primer and 0.5 U of Taq DNA polymerase (Invitrogen®). The cycling temperature included an initial temperature at 95°C for 5 min, followed by 35 cycles of 95°C for 1 min, 1 min for annealing temperature (55°C for exons 5 and 8/9; 63°C for exons 6 and 7), and extension at 72°C for 1 min. For SSCP analysis, five or six microliters of each PCR products were mixed with a equal volume of stop solution (95% of formamide, 20 mmol/L EDTA, 0.05% of xylene cyanol and 0.05% of bromophenol blue), heated at 95°C for 5 min and immediately placed on ice and loaded onto a gel containing 12.5% acrylamide (GenePhorTM, Amersham Pharmacia Biotech). Electrophoresis was performed using Electrophoresis Unit GenePhor (GE-Healthcare), at 4°C for 3 h. The patterns of the bands were then visualized by a DNA Silver Staining Kit (Amersham Pharmacia Biotech).
Statistical analysis
The analyses were carried out using the statistical programs EPINFO® 6.04 d version and SPSS® 12.0. Statistically significant differences were evaluated by Chi square test (χ2). The results were considered as statistically significant when P-values were less than 0.05.
RESULTS
Among the analyzed cases, 57 were males and 25 were females. The mean age was 56.5 years (range, 23 to 90 years). Among 71 selected patients, males were a majority [70.4% (50/71)] and 49.3% (35/71) were more than 65 years old. Regard to the Lauren’s classification, the intestinal type tumors demonstrated a slightly higher frequency (59.2%).
Detection of H pylori, EBV and markers
The H pylori infection was detected by PCR in 67 out of 71 (94.4%) gastric carcinomas. The correlation with sex showed a statistically significant difference P = 0.041 (Mantel-Haenszel), in this analysis, since almost all males were H pylori positive [98% (49/50)] while the females had a slightly lower prevalence [85.7% (18/21)]. Concerning the gastric regions, the rate of H pylori infection in the antrum was higher [55.22% (37/67)] than that of the cardia [28.35 (19/67)] and body [14.92% (10/67)]. The pathogenic genotype of H pylori (cagA +) was found in 62.7% (42/67) of the cases. Among these cases the intestinal type tumors was predominant [61.9% (26/42)].
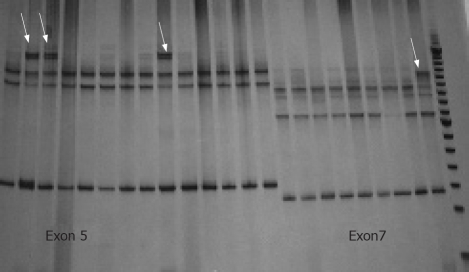
The frequency of EBV infection was 8.45% (6/71). All EBV-positive cases were males and half of them were located in the gastric antrum [50% (3/6)]. Concerning the markers, the frequency of Bcl-2, Bax, c-Myc and of the p53 mutation was 5.63% (4/71), 54.92% (39/71), 42.25% (30/71) and 73.23% (52/71), respectively. An EBV positive case is shown in Figure 1A, while the Figure 1B-D show the Bcl-2, Bax and c-Myc immunodetection, respectively. The Figure 2 is a SSCP gel showing the migration pattern of exons 5 and 7 of p53 gene.
Figure 1.
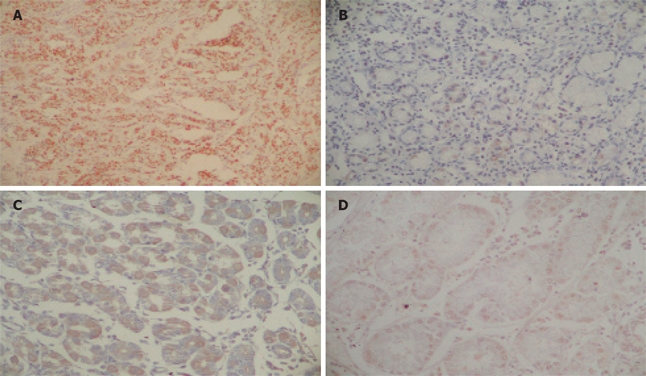
A: In situ hybridization detection of EBV RNA in gastric cancer (X400); B: Bcl-2 expression in gastric carcinoma (× 400); C: Bax expression in gastric carcinoma (×400); D: c-Myc expression in gastric carcinoma (× 400).
Figure 2.
SSCP gel of p53 gene. The arrows show the different migration patterns of exon 5 (lines 1 to 14) and 7 (lines 15 to 23).
Relationship between H pylori, markers and p53 mutation
The cagA gene was detected in 62.7% (42/67) of the cases with H pylori infection. The relationship between Bcl-2, Bax and c-Myc was statistically not significant. The relationship of the cagA(+) with the p53 mutation reached statistical significance (P = 0.035).
Relationship between EBV and H pylori
To investigate a possible relation between H pylori and EBV infection in the gastric tumorigenesis, the cases were divided in three groups: Hp(+)/EBV(+); Hp(+)/EBV(-) and Hp(-)/EBV(-). These groups accounted for 8.45% (6/71), 86% (61/71) and 5.55% (4/71) of the total, respectively. No EBV-positive case was found without H pylori infection.
The Table 1 summarizes the clinico-pathological data of the groups. The difference in gender distribution was statistically significant (P = 0.039), and, although overall the males predominated, in the Hp(-)/EBV(-) group the females were more frequent (75%). Furthermore, this group was represented by the most advanced ages. The intestinal type was more frequent in the Hp(+)/EBV(+) and Hp(-)/EBV(-) groups, although the distribution failed to reach statistical significance (P = 0.339), while in the group with only H pylori infection both histological types, intestinal and diffuse, occurred at the same rate.
Table 1.
Correlation between clinico-pathological data, detection of EBV and H pylori and the pathogenic genotype (cagA) of H pylori n (%)
| Clinico-pathological data | Total |
Groups |
P-value | ||
| Hp(+)/EBV(+) (n = 6) | Hp(+)/EBV(-) (n = 61) | Hp(-)/EBV(-) (n = 4) | |||
| Sex | P = 0.039 | ||||
| Males | 50 (70.4) | 6 (100) | 43 (70.5) | 1 (25.0) | |
| Females | 21 (29.6) | 0 (0) | 18 (29.5) | 3 (75.0) | |
| Age (yr) | P = 0.585 | ||||
| 0-44 | 6 (8.5) | 1 (16.6) | 4 (6.56) | 1 (25.0) | |
| 45-54 | 12 (16.9) | 1 (16.6) | 11 (18.0) | 0 (0.0) | |
| 55-64 | 18 (25.4) | 1 (16.6) | 17 (27.8) | 0 (0.0) | |
| > 65 | 35 (49.3) | 3 (50) | 29 (47.5) | 3 (75.0) | |
| Histological types | P = 0.339 | ||||
| Intestinal | 42 (59.2) | 5 (83.4) | 34 (55.7) | 3 (75.0) | |
| Diffuse | 29 (40.8) | 1 (16.6) | 27 (44.2) | 1 (25.0) | |
| Anatomic site | P = 0.799 | ||||
| Cardia | 19 (26.8) | 2 (33.4) | 17 (27.8) | 0 (0.0) | |
| Body | 11 (15.5) | 1 (16.6) | 9 (14.8) | 1 (25.0) | |
| Antrum | 40 (56.3) | 3 (50) | 34 (55.7) | 3 (75.0) | |
| Multiple sites | 1 (1.4) | 1 (1.7) | |||
| EBV | |||||
| ISH (+) | 6 (8.4) | - | - | - | |
| ISH (-) | 65 (91.5) | - | - | - | |
| H pylori | |||||
| PCR (+) | 67 (94.4) | - | - | - | |
| PCR (-) | 4 (5.6) | - | - | - | |
| H pylori PCR (+) | P = 0.833 | ||||
| cagA (+) | 42 (62.7) | 4 (66.6) | 38 (62.3) | - | |
| cagA (-) | 25 (37.3) | 2 (33.3) | 23 (37.7) | - | |
In the two groups in which the H pylori were present, the cagA genotype predominated in tumors of the intestinal type. In the Hp(+)/EBV(+) group, 2 out of 3 tumors of IV stage were cagA(-), on the other hand, in the group with only H pylori infection, most of the tumors (6/9) in the same stage were cagA(+).
The diffuse type tumors, notably in the Hp(+)/EBV(+) and Hp(-)/EBV(-) groups, were in advanced stages (IIIB and IV), while this was not the case in the intestinal type tumors. However, the correlation of the Lauren’s classification with tumor stage was never significant. The diffuse type tumors of the Hp(+)/EBV(-) group had similar prevalence of cagA (+) and cagA (-) strains of H pylori. There was not any significant association between diffuse type and the tumor sites.
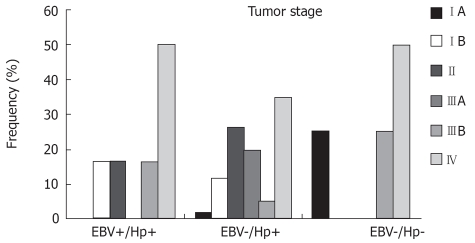
The Figure 3 shows the tumor stage distribution among the groups. Despite the small size of the groups with both or no infectious agents, it was possible to observe that the IV stage was the most represented stage in all groups. Even so, the earlier stage was present in the three groups. Not statistical significance was obtained in this analysis (P = 0.179).
Figure 3.
Distribution of the cases by tumor stage in the three defined groups: Hp(+)/EBV(+); Hp(+)/EBV(-); and Hp(-)/EBV(-). P = 0.179.
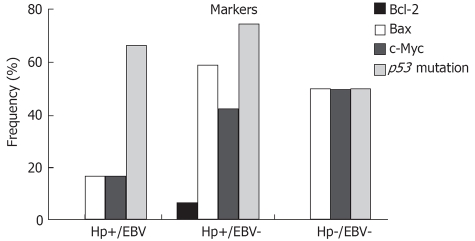
The distribution of the studied markers according to the groups is presented in Figure 4. Although the expression of Bcl-2 was low, it was only observed in tumors with H pylori infection alone. Nevertheless, high expression of Bax and c-Myc was observed in the groups Hp(+)/EBV(-) and Hp(-)/EBV(-), when compared with the coinfected tumors. A high frequency of the p53 mutation was observed in all groups, especially Hp(+)/EBV(+) and Hp(+)/EBV(-). None of the markers significantly correlated with any of the groups.
Figure 4.
Frequencies of positive cases for the oncoproteins in the three defined groups: Hp(+)/EBV(+); Hp(+)/EBV(-); and Hp(-)/EBV(-). Bcl-2, P = 0.706; Bax P = 0.135; c-Myc, P = 0.433; p53, P = 0.501.
DISCUSSION
H pylori is known to be a carcinogen agent of gastric cancer. The major advance in this field came with the recognition that chronic H pylori infection induces physiological and morphological changes within the gastric environment increasing the risk for neoplastic transformation. Now, it is widely accepted that chronic H pylori infection induces hypochlorhydria and gastric atrophy, which are precursors of gastric cancer[1,2]. Recently, it was found that EBV is also linked to the development of a portion of gastric carcinomas. Since then, several studies have been carried out about EBV carcinogenic role in these tumors. The relationship between EBV infection and gastric cancer has been demonstrated by strong pieces of evidence such as the monoclonality of the viral genome and its presence in almost all tumor cells, indicating that the infection had occurred before malignant transformation and that the tumor cells had been originated from an infected primary cell[6,8,9].
In this study, we assessed the status of EBV by ISH and H pylori infection by PCR in 71 cases of gastric carcinomas. The frequency of H pylori infection (94.4%) was higher than previous studies, where it ranged from 34.1% to 92%. These differences can be due to: (a) the sensitivity of the screening technique, since most previous studies used serology, rapid urease test and/or histological evaluation; (b) and the fact that these studies were performed in different world regions and it is known that the H pylori infection is more frequent in developing countries[8,16,22–24]. On the other hand, EBV was detected in 8.45% of our cases. Although low, this prevalence is in agreement with reported results from various world regions, which vary from 2% to 23.6%[6,9,14,24,25]. The epidemiological data are also in accordance with previous studies. The male-female ratio was 2:1 and the majority of the patients were older than fifty five[1,2]. Like in other studies, the intestinal type was more frequent than the diffuse type[1,8].
Despite the known importance of H pylori and EBV in the gastric cancer etiology, few studies have focused on the interrelationship of these two agents in gastric cancer cases. Thus, we investigated the presence of both H pylori and EBV, in parallel with the histopathological features, the status of the tumor suppressor p53, the expression of the c-Myc oncogene and the apoptotic proteins Bcl-2 and Bax, which have a key role in the tumor development. To do so, patients were divided in three groups based on the presence or absence of the infectious agents.
In our analysis, we observed a skewed gender distribution among groups, which reached statistical significance (P = 0.039). The groups with both infectious agents were composed only by males, while in the group without any agent females were predominant (3:1). The association between sex and infection observed in other studies showed that males were more susceptible than females to EBV and H pylori infection[26,27]. Although the gastric antrum was the prevalent site of gastric cancer, some studies have shown that EBV is related to the gastric carcinoma in cardia, middle stomach and gastric stump[8]. In our study, we found a slight increase of incidence of tumors located in the cardia in the group with EBV infection compared with patients with H pylori infection alone. However, due to the absence of tumors with only EBV infection, we could not evaluate the real relevance of this finding. In spite of the fact that previous studies failed to show a significant association between cardia tumors and H pylori infection[6,28], our data point to a possible association between cardia tumors and the presence of either infectious agents, since only the Hp(-)/EBV(-) group did not present tumors located in the cardia[8,29].
The intestinal type was prevalent in all groups, however in the group with H pylori infection alone the prevalence of the diffuse and intestinal type were similar, indicating that H pylori can be involved with the diffuse type. Reports concerning the association of H pylori with histological types of gastric cancers are controversial. Some studies have shown an association between H pylori and intestinal type[2,30], while others have observed a balanced distribution between the two histological types, like in the present work[31,32].
Some works have shown an association between Bcl-2 expression and EBV-positive gastric carcinomas[9]. However, the present study did not detect Bcl-2 in the group with EBV infection. On the other hand, Bax was detected in all groups with the highest expression in the group with H pylori infection alone, which also presented Bcl-2 expression as mentioned above. Although some authors have shown an increased Bcl-2 expression in H pylori-positive cases, in the group (Hp+/EBV-) the Bcl-2 expression was very low[33–37]. Although it was not statistically significant, our findings suggest a possible correlation between H pylori and Bcl-2 expression, unlike with EBV.
A few in vitro studies have shown that EBV facilitates the tumor development by inducing the expression of the c-Myc protein[39,40]. In present study, the expression of c-Myc was higher in the groups without EBV infection indicating a relationship of c-Myc with gastric carcinogenesis but not with EBV infection[41]. Additionally, some studies point to the involvement of H pylori with c-Myc[38,41]. The biological significance between H pylori and c-Myc is still not understood. A study from Zang et al[38] has linked the c-Myc expression with cell proliferation while a study from Yang et al[40] on a gastric carcinoma cell line has pointed to a relationship with apoptosis. On the other hand, Kim et al[42] have shown a decrease of c-Myc expression after H pylori eradication, but not in its mRNA level, showing the complexity of this process. The data presented here show a high frequency of c-Myc expression in the group with only H pylori, but this was similar to the noninfected group. Therefore, the c-Myc expression may be influenced by the presence of H pylori, but it is also triggered in a H pylori independent way. Nevertheless, it seems that the presence of the EBV may also play a role since the frequency of c-Myc protein was the lowest among the groups, and this may be a disturbing factor when its biologic significance is considered.
The mutation of the p53 gene is among the major alterations of the multi-step process of gastric carcinogenesis, while it has also been reported in pre-malignant lesions of the stomach, such as chronic gastritis, intestinal metaplasia and dyspepsia. Kodama et al[43] have suggested an accumulation of wild-type p53, especially in the H pylori infected mucosa probably due to the H pylori-induced DNA damage. The present study shows the highest frequency of p53 mutation in the groups with H pylori infection, especially among the cagA+ cases, corroborating previous studies[16,3,4]. The high percentage of p53 mutation in EBV-associated and EBV-negative gastric carcinomas was observed in our study which was also demonstrated in other studies, demonstrating that the p53 mutation is a relevant alteration in gastric carcinogenesis independent of the infection[15,16,43].
Finally, this study shows the high prevalence of H pylori infection in gastric carcinomas in Ceará State. The groups indicate that there are different pathways according to the presence of infectious agents, with female being predominant in the group without infection. H pylori seems to influence the expression of Bcl-2, since this protein was observed only in the group infected exclusively by this microorganism. The Bax and c-Myc expression was present in all groups. However, the highest expression in the EBV-negative groups suggests that EBV may inhibit their expression. The mutation of the p53 gene was present in all groups possibly indicating that it was not only a consequence of the infectious agent.
COMMENTS
Background
Helicobacter pylori and Epstein-Barr virus are important agents on gastric carcinogenesis. Proteins involved with cell cycle or apoptosis process emerge as a candidate for having a crucial role in the development of the neoplasms. Some these, like c-Myc, p53 and the apoptotic family members such as Bcl-2 and Bax, deserves attention for having a key function in the cell proliferation and cell fate, besides they already have shown involvement in the tumorigenesis process in a variety of tumors, including gastric cancer. So this article tries to associate H pylori and EBV infection, at the same time, with gastric carcinoma and some proteins of the cell cycle.
Research frontiers
Helicobacter pylori and Epstein-Barr virus infection related with gastric carcinogenesis.
Innovations and breakthroughs
Although H pylori and Epstein-Barr virus were well established as gastric cancer etiological agents, works on gastric cancer never associated an analysis of both agents at the same time, so this article did it.
Applications
Although this work had a small number of cases, it may justify further studies on the issue.
Peer review
This paper demonstrates that H pylori infection is very high (94.4%) in Brazil, associated with EBV infection and c-Myc, Bcl-2, Bax and p53 overexpression in some cases. These results have significant implications on the clinical management and on the research on the pathogenesis of this cancer.
Peer reviewers: Jian-Zhong Zhang, Professor, Department of Pathology and Laboratory Medicine, Beijing 306 Hospital, 9 North Anxiang Road, PO Box 9720, Beijing 100101, China; Harry HX Xia, PhD, MD, Novartis Pharmaceuticals Corporation, One Health Plaza, East Hanover, NJ 07936-1080, United States; Yusuf Bayraktar, Professor, Department of Gastroenterology, School of Medicine, Hacettepe University, Ankara 06100, Turkey
S- Editor Zhu LH L- Editor Negro F E- Editor Wang HF
References
- 1.Konturek PC, Konturek SJ, Brzozowski T. Gastric cancer and Helicobacter pylori infection. J Physiol Pharmacol. 2006 Sep;57 Suppl 3:51–65. [PubMed] [Google Scholar]
- 2.Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–256. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. International variation. Oncogene. 2004;23:6329–6340. doi: 10.1038/sj.onc.1207726. [DOI] [PubMed] [Google Scholar]
- 5.Kume T, Oshima K, Shinohara T, Takeo H, Yamashita Y, Shirakusa T, Kikuchi M. Low rate of apoptosis and overexpression of bcl-2 in Epstein-Barr virus-associated gastric carcinoma. Histopathology. 1999;34:502–509. doi: 10.1111/j.1365-2559.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 6.Luo B, Wang Y, Wang XF, Gao Y, Huang BH, Zhao P. Correlation of Epstein-Barr virus and its encoded proteins with Helicobacter pylori and expression of c-met and c-myc in gastric carcinoma. World J Gastroenterol. 2006;12:1842–1848. doi: 10.3748/wjg.v12.i12.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377–380. [PubMed] [Google Scholar]
- 8.Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HS, Chang MS, Yang HK, Lee BL, Kim WH. Epstein-barr virus-positive gastric carcinoma has a distinct protein expression profile in comparison with epstein-barr virus-negative carcinoma. Clin Cancer Res. 2004;10:1698–1705. doi: 10.1158/1078-0432.ccr-1122-3. [DOI] [PubMed] [Google Scholar]
- 10.Lopes LF, Bacchi MM, Elgui-de-Oliveira D, Zanati SG, Alvarenga M, Bacchi CE. Epstein-Barr virus infection and gastric carcinoma in Sao Paulo State, Brazil. Braz J Med Biol Res. 2004;37:1707–1712. doi: 10.1590/s0100-879x2004001100016. [DOI] [PubMed] [Google Scholar]
- 11.Bertram JS. The molecular biology of cancer. Mol Aspects Med. 2000;21:167–223. doi: 10.1016/s0098-2997(00)00007-8. [DOI] [PubMed] [Google Scholar]
- 12.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 13.Calcagno DQ, Leal MF, Seabra AD, Khayat AS, Chen ES, Demachki S, Assumpcao PP, Faria MH, Rabenhorst SH, Ferreira MV, et al. Interrelationship between chromosome 8 aneuploidy, C-MYC amplification and increased expression in individuals from northern Brazil with gastric adenocarcinoma. World J Gastroenterol. 2006;12:6207–6211. doi: 10.3748/wjg.v12.i38.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corvalan A, Akiba S, Valenzuela MT, Cumsille MA, Koriyama C, Argandona J, Backhouse C, Bal M, Mena F, Palma M, et al. Clinical and molecular features of cardial gastric cancer associated to Epstein Barr virus. Rev Med Chil. 2005;133:753–760. doi: 10.4067/s0034-98872005000700001. [DOI] [PubMed] [Google Scholar]
- 15.Szkaradkiewicz A, Majewski W, Wal M, Czyzak M, Majewski P, Bierla J, Kuch A. Epstein-Barr virus (EBV) infection and p53 protein expression in gastric carcinoma. Virus Res. 2006;118:115–119. doi: 10.1016/j.virusres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Shibata A, Parsonnet J, Longacre TA, Garcia MI, Puligandla B, Davis RE, Vogelman JH, Orentreich N, Habel LA. CagA status of Helicobacter pylori infection and p53 gene mutations in gastric adenocarcinoma. Carcinogenesis. 2002;23:419–424. doi: 10.1093/carcin/23.3.419. [DOI] [PubMed] [Google Scholar]
- 17.Gary D. Foster, David. Twell. Plant Gene Isolation: Principles and Practice. Vol. 23. Chichester; New York: Wiley; 1996. [Google Scholar]
- 18.Lage AP, Godfroid E, Fauconnier A, Burette A, Butzler JP, Bollen A, Glupczynski Y. Diagnosis of Helicobacter pylori infection by PCR: comparison with other invasive techniques and detection of cagA gene in gastric biopsy specimens. J Clin Microbiol. 1995;33:2752–2756. doi: 10.1128/jcm.33.10.2752-2756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domingo D, Alarcon T, Prieto N, Sanchez I, Lopez-Brea M. cagA and vacA status of Spanish Helicobacter pylori clinical isolates. J Clin Microbiol. 1999;37:2113–2114. doi: 10.1128/jcm.37.6.2113-2114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu SM, Raine L. Protein A, avidin, and biotin in immunohistochemistry. J Histochem Cytochem. 1981;29:1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- 21.Murakami Y, Suzuki Y, Kishimoto Y, Hirohashi S, Hayashi K, Sekiya T. Detection of DNA aberrations in human cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Tohoku J Exp Med. 1992;168:247–255. doi: 10.1620/tjem.168.247. [DOI] [PubMed] [Google Scholar]
- 22.Araujo-Filho I, Brandao-Neto J, Pinheiro LA, Azevedo IM, Freire FH, Medeiros AC. Prevalence of Helicobacter pylori infection in advanced gastric carcinoma. Arq Gastroenterol. 2006;43:288–292. doi: 10.1590/s0004-28032006000400009. [DOI] [PubMed] [Google Scholar]
- 23.Hsu PI, Lai KH, Hsu PN, Lo GH, Yu HC, Chen WC, Tsay FW, Lin HC, Tseng HH, Ger LP, et al. Helicobacter pylori infection and the risk of gastric malignancy. Am J Gastroenterol. 2007;102:725–730. doi: 10.1111/j.1572-0241.2006.01109.x. [DOI] [PubMed] [Google Scholar]
- 24.Shibata D, Hawes D, Stemmermann GN, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma among Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev. 1993;2:213–217. [PubMed] [Google Scholar]
- 25.Wang Y, Luo B, Zhao P, Huang BH. Expression of Epstein-Barr virus genes in EBV-associated gastric carcinoma. Ai zheng. 2004;23:782–787. [PubMed] [Google Scholar]
- 26.Alipov G, Nakayama T, Nakashima M, Wen CY, Niino D, Kondo H, Pruglo Y, Sekine I. Epstein-Barr virus-associated gastric carcinoma in Kazakhstan. World J Gastroenterol. 2005;11:27–30. doi: 10.3748/wjg.v11.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trimeche M, Bonnet C, Korbi S, Boniver J, de Leval L. Association between Epstein-Barr virus and Hodgkin’s lymphoma in Belgium: A pathological and virological study. Leuk Lymphoma. 2007;48:1323–1331. doi: 10.1080/10428190701411177. [DOI] [PubMed] [Google Scholar]
- 28.Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, Wang HP, Lin JT. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology. 2000;118:1031–1038. doi: 10.1016/s0016-5085(00)70355-6. [DOI] [PubMed] [Google Scholar]
- 29.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Buruk F, Berberoglu U, Pak I, Aksaz E, Celen O. Gastric cancer and Helicobacter pylori infection. Br J Surg. 1993;80:378–379. doi: 10.1002/bjs.1800800339. [DOI] [PubMed] [Google Scholar]
- 31.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 32.Hansson LE, Engstrand L, Nyrén O, Evans DJ, Lindgren A, Bergström R, Andersson B, Athlin L, Bendtsen O, Tracz P. Helicobacter pylori infection: independent risk indicator of gastric adenocarcinoma. Gastroenterology. 1993;105:1098–1103. doi: 10.1016/0016-5085(93)90954-b. [DOI] [PubMed] [Google Scholar]
- 33.Konturek PC, Pierzchalski P, Konturek SJ, Meixner H, Faller G, Kirchner T, Hahn EG. Helicobacter pylori induces apoptosis in gastric mucosa through an upregulation of Bax expression in humans. Scand J Gastroenterol. 1999;34:375–383. doi: 10.1080/003655299750026380. [DOI] [PubMed] [Google Scholar]
- 34.Jorge O, Cuello Carrion FD, Jorge A, Ciocca DR. Helicobacter pylori infection affects the expression of PCNA, p53, cerbB- 2 and Bcl-2 in the human gastric mucosa. Rev Esp Enferm Dig. 2003;95:97–104, 89-96. [PubMed] [Google Scholar]
- 35.Choi IJ, Kim JS, Kim JM, Jung HC, Song IS. Effect of inhibition of extracellular signal-regulated kinase 1 and 2 pathway on apoptosis and bcl-2 expression in Helicobacter pylori-infected AGS cells. Infect Immun. 2003;71:830–837. doi: 10.1128/IAI.71.2.830-837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Deng CS, Peng JZ, Wong BC, Lam SK, Xia HH. Effect of Helicobacter pylori on apoptosis and apoptosis related genes in gastric cancer cells. Mol Pathol. 2003;56:19–24. doi: 10.1136/mp.56.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhan N, Xiong YY, Lan J, Wang BC, Tian SF, Yu SP. Relationship between Helicobacter pylori infection and expression of c-myc, Bcl-2, and Bax protein in different gastric mucosa lesions. Ai zheng. 2003;22:1034–1037. [PubMed] [Google Scholar]
- 38.Zhang H, Fang DC, Wang RQ, Yang SM, Liu HF, Luo YH. Effect of Helicobacter pylori infection on expression of Bcl-2 family members in gastric adenocarcinoma. World J Gastroenterol. 2004;10:227–230. doi: 10.3748/wjg.v10.i2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niller HH, Salamon D, Ilg K, Koroknai A, Banati F, Schwarzmann F, Wolf H, Minarovits J. EBV-associated neoplasms: alternative pathogenetic pathways. Med Hypotheses. 2004;62:387–391. doi: 10.1016/j.mehy.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Deng X, Deng L, Gu H, Fan W, Cao Y. Telomerase activation by Epstein-Barr virus latent membrane protein 1 is associated with c-Myc expression in human nasopharyngeal epithelial cells. J Exp Clin Cancer Res. 2004;23:495–506. [PubMed] [Google Scholar]
- 41.Nardone G, Staibano S, Rocco A, Mezza E, Balzano T, Salvatore G, Staiano A, Donofrio V, Grazioli B, De Rosa G, et al. Effect of Helicobacter pylori infection on gastric cell proliferation and genomic instability in a paediatric population of southern Italy. Dig Liver Dis. 2001;33:743–749. doi: 10.1016/s1590-8658(01)80690-3. [DOI] [PubMed] [Google Scholar]
- 42.Kim SH, Nakagawa H, Navaraj A, Naomoto Y, Klein-Szanto AJ, Rustgi AK, El-Deiry WS. Tumorigenic conversion of primary human esophageal epithelial cells using oncogene combinations in the absence of exogenous Ras. Cancer Res. 2006;66:10415–10424. doi: 10.1158/0008-5472.CAN-06-2104. [DOI] [PubMed] [Google Scholar]
- 43.Kodama M, Murakami K, Okimoto T, Sato R, Watanabe K, Fujioka T. Expression of mutant type-p53 products in H pylori-associated chronic gastritis. World J Gastroenterol. 2007;13:1541–1546. doi: 10.3748/wjg.v13.i10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taguchi A, Ohmiya N, Itoh A, Hirooka Y, Niwa Y, Mori N, Goto H. Severity of atrophic gastritis related to antiparietal cell antibody and gastric carcinogenesis, including p53 mutations. J Gastroenterol Hepatol. 2006;21:545–51. doi: 10.1111/j.1440-1746.2005.03983.x. [DOI] [PubMed] [Google Scholar]