Abstract
AIM: To investigate whether adding ecabet sodium to the standard triple therapy for H pylori infection improve eradication rate.
METHODS: Two hundred and fifty-seven H pylori-infected patients were randomly assigned to standard triple therapy (group A, n = 129) or triple therapy plus ecabet sodium (group B, n = 128). Successful eradication was defined as a negative 13C-urea breath test 6-8 wk after completion of treatment.
RESULTS: After completion of therapy, 194/257 patients showed negative 13C-urea breath test results. According to intention-to-treat analysis, the infection was eradicated in 93/129 (72.1%) patients in group A and 101/128 (78.9%) in group B (P = 0.204). Per-protocol analysis showed successful eradication in 93/118 (78.8%) patients from group A and 101/114 (88.6%) from group B (P = 0.044). There were no significant differences in the side effects experienced by the patients in the two treatment groups.
CONCLUSION: Our results suggest that the addition of ecabet sodium improves the efficacy of the standard triple therapy for H pylori.
Keywords: H pylori, Eradication, Triple therapy, Ecabet sodium
INTRODUCTION
H pylori is a gram-negative spiral-shaped bacterium that colonizes the human stomach and it is associated with several gastroduodenal diseases, including gastritis, peptic ulcer disease and low-grade gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Eradication of H pylori is the recommended treatment for these conditions[1]. The most widely endorsed treatment is triple therapy with amoxicillin, clarithromycin and a proton pump inhibitor two times daily for 7 d, and the reported efficacy of this protocol is 74%-76%[2]. The reasons for its occasional failure are unclear, although bacterial resistance and poor patient compliance are believed to be the primary factors[3]. Ecabet sodium is a dehydroabietic acid derivative that was originally purified from pine resin[4], which is now widely used for the treatment of gastric ulcer and gastritis in East Asia. This agent exhibits a bactericidal effect against H pylori by inhibiting bacterial urease activity[5], or by its direct bactericidal effect under acidic conditions[6,7]. Its bactericidal effect on clarithromycin- and metronidazole-resistant clinical isolates of H pylori has been reported[7]. Therefore, ecabet sodium has been suggested to improve the efficacy of antibiotic therapy for H pylori infection in patients with peptic ulcer[8–12]. To the best of our knowledge, the efficacy and safety of standard triple therapy plus ecabet sodium has not yet been investigated. Therefore, the aim of this study was to compare the efficacy and side effects of standard triple therapy versus triple therapy plus ecabet sodium for the eradication of H pylori.
MATERIALS AND METHODS
Patients
This randomized prospective study was performed at three medical centers (Pusan National University Hospital, Bongseng Memorial Hospital and Maryknoll Hospital, Busan, Korea). The subjects consisted of 257 consecutive H pylori-positive patients who had undergone eradication therapy from May 2006 to April 2007. The criteria for exclusion included: (1) previous H pylori eradication therapy; (2) ingestion of antibiotics, bismuth, or proton pump inhibitors within the prior 4 wk; (3) patients with a history of allergy to the medications used; (4) patients with previous gastric surgery; (5) coexistence of serious concomitant illness (for example, decompensated liver cirrhosis or uremia); and (6) pregnant women.
All the participants were advised to undergo H pylori eradication therapy and they all agreed. There were 148 men and 109 women (mean age 53.9 ± 12.8 years), and these included: 128 patients with gastric ulcer (or ulcer scar); 63 with duodenal ulcer (or ulcer scar); 14 with gastroduodenal ulcer (or ulcer scar); 12 who had received endoscopic treatment for early gastric cancer or adenoma; one with gastric low-grade MALT lymphoma; and 39 with gastritis accompanied by dyspepsia or a family history of gastric cancer (Table 1).
Table 1.
Demographic data and endoscopic findings of 257 patients with H pylori infection who were randomly assigned to standard triple therapy (group A) or triple therapy plus ecabet sodium (group B)
| Group A | Group B | P value | |
| Patients | 129 | 128 | |
| Age (yr) (mean ± SD) | 54.3 ± 13.2 | 53.4 ± 12.4 | 0.562 |
| Gender (male/female) | 68/61 | 80/48 | 0.112 |
| BMI (kg/m2) (mean ± SD) | 23.3 ± 2.9 | 23.2 ± 3.0 | 0.768 |
| Smoking | 32 (24.8%) | 32 (25.0%) | 0.971 |
| Alcohol consumption | 40 (31.0%) | 56 (43.8%) | 0.035 |
| Endoscopic findings | 0.333 | ||
| Gastric ulcer | 62 | 66 | |
| Duodenal ulcer | 37 | 26 | |
| Gastroduodenal ulcer | 8 | 6 | |
| Gastric cancer1 | 7 | 5 | |
| Gastric MALT lymphoma | 0 | 1 | |
| Gastritis | 15 | 24 |
Patients who had received endoscopic treatment for early gastric cancer or adenoma.
The patients were then randomized into two groups: group A (n = 129) underwent standard triple therapy for 7 d (lansoprazole 30 mg b.i.d., clarithromycin 500 mg b.i.d. and amoxicillin 1 g b.i.d.), while group B (n = 128) patients were given treatment consisting of the same triple therapy plus ecabet sodium (1.0 g b.i.d.) for a period of 7 d. Patients were instructed to take all the drugs 30 min after breakfast and dinner. This study was performed in accordance with good clinical practice and the Declaration of Helsinki guidelines. The Institutional Review Board of Pusan National University Hospital approved this study, and informed consent was obtained from all the patients.
Methods
Before receiving therapy, all the patients underwent upper gastrointestinal endoscopy to confirm the diagnosis and the presence of H pylori infection. Positive H pylori status was confirmed by the rapid urease test, histology, serology (serum IgG antibody) and/or the 13C-urea breath test. The patients were diagnosed as H pylori-positive when they showed positive results by at least two methods.
Possible eradication of H pylori was assessed by the 13C-urea breath test at 6-8 wk after completion of treatment. Proton pump inhibitors and antimicrobial agents that might affect the 13C-urea breath test were not given to the patients after completion of therapy. The 13C-urea breath test was performed as described previously, with capsule-based modification[13]. In brief, the patients fasted for over 12 h before examination and then a gelatin capsule containing 38 mg 13C-urea was ingested with 50 mL water. Breath samples before and 20 min after administration of 13C-urea were collected after a mouthwash. The 13C/12C ratio in the breath samples was measured by mass spectrometry (Heliview; MediChems, Seoul, Korea). The changes in the 13C value over baseline were expressed as Δ13C. A positive result was defined as an increase of > 2‰.
The eradication rate was evaluated for each drug regimen in a per-protocol (PP) analysis based on the number of patients who completed the study, and in an intention-to-treat (ITT) analysis that took into account all the patients, including those who dropped out because of severe side effects, those who showed poor drug compliance, and those who were lost to follow-up. The patients were interviewed in their first visit to the clinic after completion of therapy to clarify their compliance with the therapy and any side effects. Poor compliance was defined as taking less than 70% of the total medication.
Statistical analysis
χ2 or Fisher’s exact tests were used to compare the characteristics of the patients and the results of two regimens. Independent t tests were also employed to compare the possible differences in the age and body mass index of the patients who were treated with the two regimens. P < 0.05 was considered statistically significant. Statistical calculations were performed using SPSS version 12.0 for Windows (SPSS, Chicago, IL, USA).
RESULTS
Group A consisted of 129 patients (68 men and 61 women, mean age 54.3 ± 13.2 years) and group B consisted of 128 patients (80 men and 48 women, mean age 53.4 ± 12.4 years). Data regarding the clinical characteristics of the patients at entry are summarized in Table 1. The two groups had a comparable age, gender and history of smoking. Alcohol consumption was more common in group B than in group A. There were no significant differences in the endoscopic findings between the two groups.
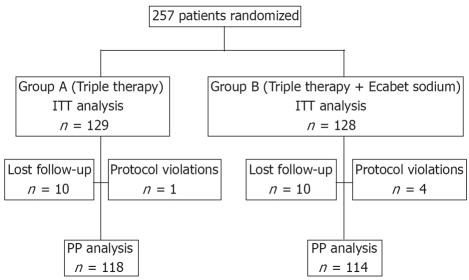
Five patients dropped out due to non-compliance before the final evaluation: three patients due to side effects (one from group A and two from group B) and two patients due to personal reasons. A further 10 patients from each group did not appear at the first visit after completion of therapy or for the 13C-urea breath test; therefore, they were lost to follow-up (Figure 1).
Figure 1.
CONSORT flow diaphragm of the subjects’ progress through the phases of the study.
After the completion of therapy, 194/257 (75.5%) patients tested negative for H pylori on the 13C-urea breath test. ITT analysis showed that H pylori was eradicated in 93/129 (72.1%) patients from group A and in 101/128 (78.9%) patients from group B (P = 0.204). PP analysis showed successful eradication in 93/118 (78.8%) patients from group A and 101/114 (88.6%) patients from group B (Table 2, P = 0.044).
Table 2.
ITT and PP analysis of H pylori eradication in patients who were randomly assigned to standard triple therapy (group A) or triple therapy plus ecabet sodium (group B)
| Group A | Group B | P value | |
| Eradication rate | |||
| ITT1 | 72.1% (93/129) | 78.9% (101/128) | 0.204 |
| PP | 78.8% (93/118) | 88.6% (101/114) | 0.044 |
In this analysis, the patients with unknown outcome were counted as treatment failures.
There were no significant differences in the side effects experienced by the patients in the two treatment groups: 26/119 (21.8%) patients in group A and 24/118 (20.3%) patients in group B reported adverse effects due to therapy (Table 3, P = 0.776). More patients in group A complained of a metallic taste than in group B (11.8% vs 4.2%, P = 0.033). All the side effects were self-limiting and disappeared once therapy was terminated.
Table 3.
Side effects during standard triple therapy (group A) and triple therapy plus ecabet sodium (group B)
| Side effects | Group A | Group B | P value |
| Metallic taste | 14 | 5 | 0.033 |
| Nausea/vomiting | 1 | 4 | 0.213 |
| Abdominal pain | 2 | 2 | 1.000 |
| Constipation | 1 | 0 | 1.000 |
| Diarrhea | 7 | 3 | 0.333 |
| Dizziness | 1 | 6 | 0.066 |
| Skin rash | 0 | 3 | 0.122 |
| Oral mucositis | 2 | 1 | 1.000 |
| Others | 4 | 7 | 0.347 |
DISCUSSION
Reports about failed H pylori eradication therapy have been increasing[14,15]. A recent meta-analysis has demonstrated that a 1-wk course of triple therapy with amoxicillin, clarithromycin and a proton pump inhibitor had a pooled eradication rate of 74%-76% or less[2,16]. A disappointing cure rate of < 80% after 7 d triple therapy was confirmed in the present study.
Various studies are under way to evaluate new antibiotics (e.g., levofloxacin, moxifloxacin, furazolidone and rifabutin) and different therapeutic schedules to increase the efficacy of H pylori eradication therapy[17–20]. Data suggest that antibiotic resistance is frequent and clinicians are arriving at the conclusion that concurrent therapy with another agent may sometimes be necessary.
Ecabet sodium has local cytoprotective activity for the gastric mucosa, but is not absorbed into the systemic circulation[21]. Recent studies have demonstrated that ecabet sodium has anti-H pylori activity. In vitro studies have shown that ecabet sodium inhibits the urease activity of H pylori and its adhesion to the gastric epithelial cells[5,22]. In addition, ecabet sodium is reported to have a strong concentration-dependent bactericidal effect on H. pylori under acidic conditions[7]. Although proton pump inhibitors potently suppress acid secretion, the acid milieu (pH 3-5) is especially maintained during most of the day and night[23]. Therefore, several studies have evaluated the usefulness of ecabet sodium for eradication therapy in combination with proton-pump-inhibitor-based therapy[8,10,12].
A comparative study demonstrated an eradication rate of 26% with lansoprazole-based dual therapy with clarithromycin or amoxicillin, whereas the eradication rate achieved by adding ecabet sodium to the same regimen was 79%[12]. Another study confirmed the additive effect of ecabet sodium on proton-pump-inhibitor-based dual therapy[11]. In addition, a dose-dependent additive effect of ecabet sodium in combination with lansoprazole and amoxicillin has been demonstrated[10].
On the basis of this evidence, we thought it likely that the addition of ecabet sodium to triple therapy contributes to improving the rate of H pylori eradication. In fact, in this study, PP analysis showed that triple therapy plus ecabet sodium was more effective than standard triple therapy, although there was no significant difference according to ITT analysis. It is possible that triple therapy plus ecabet sodium provokes a synergistic effect by launching attacks from different directions against H pylori, and this may lead to complete clearance of the bacterial infection. The bactericidal effect of ecabet sodium on clarithromycin- and metronidazole-resistant clinical isolates of H pylori[7] might also explain the increased eradication rate.
The majority of the recently recommended eradication therapy regimens are 1 wk in duration because these have advantages with respect to compliance and medical cost, with a similar eradication rate[24]. A good eradication rate was observed in this study after a 1-wk-long triple therapy plus ecabet sodium.
Because ecabet sodium does not have any systemic activity, it was expected that ecabet sodium-based therapy would be associated with a low occurrence of adverse events. In a previous report, the use of ecabet sodium combined with lansoprazole and amoxicillin reduced the incidence of diarrhea during therapy[11]. However, there was no significant difference of adverse events between standard triple therapy and triple therapy plus ecabet sodium in this study. The use of triple therapy plus ecabet sodium reduced the incidence of metallic taste during therapy, and this might have been due to the sweet taste of ecabet sodium.
In recent years, many studies have compared the effectiveness of triple and bismuth quadruple therapy as a primary therapy for H pylori[25–28]. A recent meta-analysis including five randomized trials has reported slightly higher, but not statistically significant, eradication rates with quadruple therapy: ITT and PP eradication rates were 79% and 85% for clarithromycin triple therapy and 80% and 87% for bismuth quadruple therapy, respectively[29]. However, quadruple therapy is complex to take (q.i.d. dosing regimen and high pill count) and has a perceived high frequency of side effects[30].
In conclusion, this randomized prospective study showed (based on PP analysis) that the addition of ecabet sodium improved the efficacy of standard triple therapy for H pylori infection. These results need to be confirmed, but triple therapy plus ecabet sodium may represent a valid alternative regimen to the standard H pylori eradication protocol.
COMMENTS
Background
The eradication rate of H pylori by standard triple therapy is only 74%-76%. Ecabet sodium has been reported to exhibit a bactericidal effect against H pylori by inhibiting bacterial urease activity or by its direct bactericidal effect. This study compared the efficacy and side effects of standard triple therapy versus triple therapy plus ecabet sodium for the eradication of H pylori.
Research frontiers
Research in this area is focused on developing more effective eradication regimens for H. pylori infection. Ecabet sodium is widely used for the treatment of gastric ulcer and gastritis in East Asia. Its bactericidal effect on clarithromycin- and metronidazole-resistant clinical isolates of H pylori has been reported. Therefore, it is presumed that adding ecabet sodium to standard triple therapy is more efficient for the eradication of H pylori. In our randomized prospective study, the addition of ecabet sodium may have improved the efficacy of standard triple therapy for H pylori.
Innovations and breakthroughs
Some reports about the efficacy of ecabet sodium for H pylori infection have been reported. But the efficacy and side effects of standard triple therapy plus ecabet sodium have not yet been investigated. By PP analysis, the rate of successful eradication was 78.8% for standard triple therapy and 88.6% for triple therapy plus ecabet sodium. There were no differences in the side effects in the two treatment groups. These results need to be confirmed, but triple therapy plus ecabet sodium may improve the eradication rate of H pylori.
Applications
This study demonstrated the efficacy of standard triple therapy plus ecabet sodium. Therefore, this may represent a valid alternative regimen to the standard H pylori eradication protocol.
Terminology
Ecabet sodium is a dehydroabietic acid derivative purified from pine resin and one of the local cytoprotective agents for the gastric mucosa. It is now widely used for the treatment of gastric ulcer and gastritis in East Asia. The eradication rate was evaluated for each drug regimen by PP analysis, based on the number of patients who completed the study, and in an ITT analysis that took into account all patients.
Peer review
This study compared two therapeutic regimens for the cure of H pylori infection: a classic triple PPI based therapy vs the same regimen plus ecabet sodium (a natural bactericidal compound). The study was well designed and conducted.
Supported by An unrestricted grant by JEIL Pharm. Co., Ltd., Korea
Peer reviewers: Reza Malekzadeh, Professor, Director, Digestive Disease Research Center, Tehran University of Medical Sciences, Shariati Hospital, Kargar Shomali Avenue, 19119 Tehran, Iran; Bruno Annibale, Professor, Digestive and Liver Disease Unit, University “La Sapienza” ¢ò School of Medicine, Via di Grottarossa 1035, Roma 00189, Italy; Frank I Tovey, OBE, ChM, FRCS, Honorary Research Fellow, Department of Surgery, University College London, London, United Kingdom
S- Editor Zhu LH L- Editor Kerr C E- Editor Wang HF
References
- 1.Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergara M, Vallve M, Gisbert JP, Calvet X. Meta-analysis: comparative efficacy of different proton-pump inhibitors in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2003;18:647–654. doi: 10.1046/j.1365-2036.2003.01746.x. [DOI] [PubMed] [Google Scholar]
- 3.Hunt RH. Eradication of Helicobacter pylori infection. Am J Med. 1996;100:42S–50S; discussion 50S-51S. doi: 10.1016/s0002-9343(96)80228-2. [DOI] [PubMed] [Google Scholar]
- 4.Onoda Y, Magaribuchi T, Tamaki H. Effects of the new anti-ulcer agent 12-sulfodehydroabietic acid monosodium salt on duodenal alkaline secretion in rats. Arzneimittelforschung. 1990;40:576–578. [PubMed] [Google Scholar]
- 5.Ito Y, Shibata K, Hongo A, Kinoshita M. Ecabet sodium, a locally acting antiulcer drug, inhibits urease activity of Helicobacter pylori. Eur J Pharmacol. 1998;345:193–198. doi: 10.1016/s0014-2999(97)01622-1. [DOI] [PubMed] [Google Scholar]
- 6.Shibata K, Ito Y, Hongo A, Yasoshima A, Endo T, Ohashi M. Bacterial activity of a new antiulcer agent, ecabet sodium, against Helicobacter pylori under acidic conditions. Antimicrob Agents Chemother. 1995;39:1295–1299. doi: 10.1128/aac.39.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata K, Kasuga O, Yasoshima A, Matsushita T, Kawakami Y. Bactericidal effect of ecabet sodium on clarithromycin- and metronidazole-resistant clinical isolates of Helicobacter pylori. Jpn J Antibiot. 1997;50:525–531. [PubMed] [Google Scholar]
- 8.Shimoyama T, Fukuda S, Liu Q, Nakaji S, Munakata A, Sugawara K. Ecabet sodium inhibits the ability of Helicobacter pylori to induce neutrophil production of reactive oxygen species and interleukin-8. J Gastroenterol. 2001;36:153–157. doi: 10.1007/s005350170122. [DOI] [PubMed] [Google Scholar]
- 9.Adachi K, Ishihara S, Hashimoto T, Hirakawa K, Ishimura N, Niigaki M, Kaji T, Kawamura A, Sato H, Fujishiro H, et al. Efficacy of ecabet sodium for Helicobacter pylori eradication triple therapy in comparison with a lansoprazole-based regimen. Aliment Pharmacol Ther. 2001;15:1187–1191. doi: 10.1046/j.1365-2036.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 10.Kagaya H, Kato M, Komatsu Y, Mizushima T, Sukegawa M, Nishikawa K, Hokari K, Takeda H, Sugiyama T, Asaka M. High-dose ecabet sodium improves the eradication rate of helicobacter pylori in dual therapy with lansoprazole and amoxicillin. Aliment Pharmacol Ther. 2000;14:1523–1527. doi: 10.1046/j.1365-2036.2000.00852.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohkusa T, Takashimizu I, Fujiki K, Araki A, Ariake K, Shimoi K, Honda K, Enomoto Y, Sakurazawa T, Horiuchi T, et al. Prospective evaluation of a new anti-ulcer agent, ecabet sodium, for the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1998;12:457–461. doi: 10.1046/j.1365-2036.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 12.Shimoyama T, Fukuda Y, Fukuda S, Munakata A, Yoshida Y, Shimoyama T. Ecabet sodium eradicates Helicobacter pylori infection in gastric ulcer patients. J Gastroenterol. 1996;31 Suppl 9:59–62. [PubMed] [Google Scholar]
- 13.Bielanski W, Konturek SJ. New approach to 13C-urea breath test: capsule-based modification with low-dose of 13C-urea in the diagnosis of Helicobacter pylori infection. J Physiol Pharmacol. 1996;47:545–553. [PubMed] [Google Scholar]
- 14.McLoughlin R, Racz I, Buckley M, O'Connor HJ, O'Morain C. Therapy of Helicobacter pylori. Helicobacter. 2004;9 Suppl 1:42–48. doi: 10.1111/j.1083-4389.2004.00251.x. [DOI] [PubMed] [Google Scholar]
- 15.Fischbach LA, Goodman KJ, Feldman M, Aragaki C. Sources of variation of Helicobacter pylori treatment success in adults worldwide: a meta-analysis. Int J Epidemiol. 2002;31:128–139. doi: 10.1093/ije/31.1.128. [DOI] [PubMed] [Google Scholar]
- 16.Altintas E, Sezgin O, Ulu O, Aydin O, Camdeviren H. Maastricht II treatment scheme and efficacy of different proton pump inhibitors in eradicating Helicobacter pylori. World J Gastroenterol. 2004;10:1656–1658. doi: 10.3748/wjg.v10.i11.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rispo A, Di Girolamo E, Cozzolino A, Bozzi R, Morante A, Pasquale L. Levofloxacin in first-line treatment of Helicobacter pylori infection. Helicobacter. 2007;12:364–365. doi: 10.1111/j.1523-5378.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 18.Sezgin O, Altintas E, Ucbilek E, Tombak A, Tellioglu B. Low efficacy rate of moxifloxacin-containing Helicobacter pylori eradication treatment: in an observational study in a Turkish population. Helicobacter. 2007;12:518–522. doi: 10.1111/j.1523-5378.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 19.Daghaghzadeh H, Emami MH, Karimi S, Raeisi M. One-week versus two-week furazolidone-based quadruple therapy as the first-line treatment for Helicobacter pylori infection in Iran. J Gastroenterol Hepatol. 2007;22:1399–1403. doi: 10.1111/j.1440-1746.2007.05029.x. [DOI] [PubMed] [Google Scholar]
- 20.Miehlke S, Hansky K, Schneider-Brachert W, Kirsch C, Morgner A, Madisch A, Kuhlisch E, Bastlein E, Jacobs E, Bayerdorffer E, et al. Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment Pharmacol Ther. 2006;24:395–403. doi: 10.1111/j.1365-2036.2006.02993.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Sugawara Y, Takaiti O, Nakamura S. Metabolic fate of a new anti-ulcer drug (+)-(1R,4aS,10aR)-1,2,3,4,4a,9,10,10a- octahydro-1,4a-dimethyl-7-(1-methylethyl)-6-sulfo-1- phenanthrenecarboxylic acid 6-sodium salt pentahydrate (TA-2711). II. Distribution in the rat stomach. J Pharmacobiodyn. 1991;14:547–554. doi: 10.1248/bpb1978.14.547. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda Y, Yamamoto I, Okui M, Tonokatsu Y, Shimoyama T. Combination therapies with a proton pump inhibitor for Helicobacter pylori-infected gastric ulcer patients. J Clin Gastroenterol. 1995;20 Suppl 2:S132–S135. doi: 10.1097/00004836-199506002-00036. [DOI] [PubMed] [Google Scholar]
- 23.Bruley des Varannes S, Levy P, Lartigue S, Dellatolas F, Lemaire M, Galmiche JP. Comparison of lansoprazole with omeprazole on 24-hour intragastric pH, acid secretion and serum gastrin in healthy volunteers. Aliment Pharmacol Ther. 1994;8:309–314. doi: 10.1111/j.1365-2036.1994.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 24.Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553–562. doi: 10.7326/0003-4819-147-8-200710160-00008. [DOI] [PubMed] [Google Scholar]
- 25.Calvet X, Ducons J, Guardiola J, Tito L, Andreu V, Bory F, Guirao R. One-week triple vs. quadruple therapy for Helicobacter pylori infection - a randomized trial. Aliment Pharmacol Ther. 2002;16:1261–1267. doi: 10.1046/j.1365-2036.2002.01278.x. [DOI] [PubMed] [Google Scholar]
- 26.Gomollon F, Valdeperez J, Garuz R, Fuentes J, Barrera F, Malo J, Tirado M, Simon MA. Cost-effectiveness analysis of 2 strategies of Helicobacter pylori eradication: results of a prospective and randomized study in primary care. Med Clin (Barc) 2000;115:1–6. doi: 10.1016/s0025-7753(00)71447-3. [DOI] [PubMed] [Google Scholar]
- 27.Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spenard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562–567. doi: 10.1111/j.1572-0241.2003.t01-1-07288.x. [DOI] [PubMed] [Google Scholar]
- 28.Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002;123:1763–1769. doi: 10.1053/gast.2002.37051. [DOI] [PubMed] [Google Scholar]
- 29.Gene E, Calvet X, Azagra R, Gisbert JP. Triple vs quadruple therapy for treating Helicobacter pylori infection: an updated meta-analysis. Aliment Pharmacol Ther. 2003;18:543–544. doi: 10.1046/j.1365-2036.2003.t01-1-01712.x. [DOI] [PubMed] [Google Scholar]
- 30.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]