Abstract
Graft-verse-host disease (GVHD) is an uncommon fatal complication following liver transplantation (LTx). In mainland China, only six cases have been reported with a morbidity rate up to 1%-2%. Definitive diagnosis was achieved by molecular techniques (HLA typing or PCR-STR) in only two cases and the remaining cases were diagnosed based on typical clinical features with exclusion of other possible causes. All patients died of septic shock or multiple organ failure even after administration of increased corticosteroids and supportive therapy, and reduced immunosuppressive agents. In our center, two cases of GVHD were found among 128 (1.56%) patients. One case was diagnosed by detecting lymphocyte macrochimerism through DNA-STR. Both of them died even after aggressive treatment. In China, the incidence of GVHD is similar to that reported by foreign centers except for an extremely bad prognosis. Rapid diagnosis is crucial for a better prognosis. In China, only 37.5% of cases are diagnosed by molecular methods. We recommend detecting lymphocyte macrochimerism through DNA-STR to get a rapid diagnosis, and interleukin 2-receptor antibody (basiliximab or daclizumab) therapy seems to be a good choice for the disease.
Keywords: Graft-versus-host disease, Liver transp-lantation, Prognosis, Diagnosis, Treatment
INTRODUCTION
The rapid increase of liver transplantation (LTx) and the development of immunosuppressive agents are followed by an increased incidence of graft-verse-host disease (GVHD) after liver transplantation. GVHD is a rare but extremely severe complication from LTx. The incidence is about 1%-2% and the mortality rate over 75%[1,2]. Although the total number of LTx performed in 2005 reached 3500 in mainland China, less than 10 cases of GVHD after LTx have been reported and many cases were under-diagnosed because of poor clinical awareness and no qualified diagnostic methods. We review in this article the literature on the disease in China, including the incidence, clinical features, diagnostic methods, treatment and prognosis and report about our own experience on GVHD after LTx. This study was approved by the ethical committee of our hospital and conforms to the ethical guidelines of Helsinki Declaration.
CASE REPORT
We reviewed the records of 124 adults and 4 pediatric LTx in our organ transplantation center from December 2005 to December 2006, and found two patients with OLT associated GVHD (an incidence rate of 1.56%).
Case 1
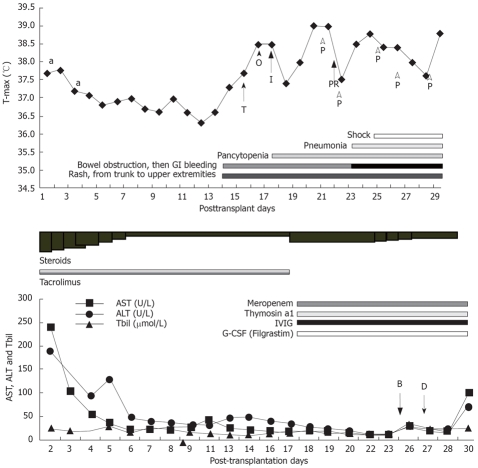
Case one is a 50-year-old man admitted for liver cirrhosis secondary to hepatitis B and hepatocellular carcinoma (HCC). Before admission, the patient received regional therapy for HCC, including ethanol injection and radio-frequency ablation. And he also received chemotherapy (arsenious acid injection 10mg QD for 4 d) just before the operation because his waiting time was longer than expected. He received a modified Piggy-back procedure and two doses of daclizumab on post-transplantation d 1 and d 4 for induction. Donor’s blood type was identical to the recipient’s. Postoperative immunosuppressant regimen consisted of tacrolimus and methylprednisolone. FK506 trough level ranged from 2.9 μg/L to 21.2 μg/L (mean value 11.7 μg/L) (Figure 1). The patient had an uneventful early recovery and could take care of himself. Liver function tests (LFTs), electrolytes, blood film and ultrasonography were all normal. Discharge would be made if he had not complained of fever and headache. Remittent fever persisted until the patient died. Rash appeared one day after fever, so did the constipation. Eruption was maculopapular, accentuated the abdomen at the very beginning and then developed into the back and neck but not palms and toes (Figure 2), subsequently oral ulcer and sore throat occurred one week later. Considering the possibility of drug rash, we discontinued all suspected drugs and gave an injection of dexamethasone. A biopsy of skin showed no classical pathological change of GVHD. The patient also presented with nausea and vomiting without gas and stool passing. The abdominal film revealed lower incomplete intestinal obstruction (Figure 3). Nasogastric suction was used without improvement. On post-transplantation d 40, upper gastrointestinal bleeding developed with severe nausea and vomiting. Pancytopenia appeared suddenly with white blood cell count dropping from 4500/μL to 330/μL within one day, platelet 170 000/μL and hemoglobin 77 g/L. Bone marrow cellular study showed aplastic anemia. We suspected GVHD and a blood sample was sent to laboratory for DNA PCR-short tandem repeat (PCR-STR) which confirmed the diagnosis (Table 1 and Figure 4).We initiated G-CSF injection (Filgrastim 300 μg BID) and increased steroids dosage (methylprednisolone 100 mg QD for 6 d, then tapered gradually). Broad-spectrum antibiotics, anti-fungals and IVIG were also used to prevent infection. Unfortunately, the patient died of sepsis and multiple organ failure 19 d after the first onset of disease. Figure 5 shows the post-transplantation clinical course.
Figure 1.
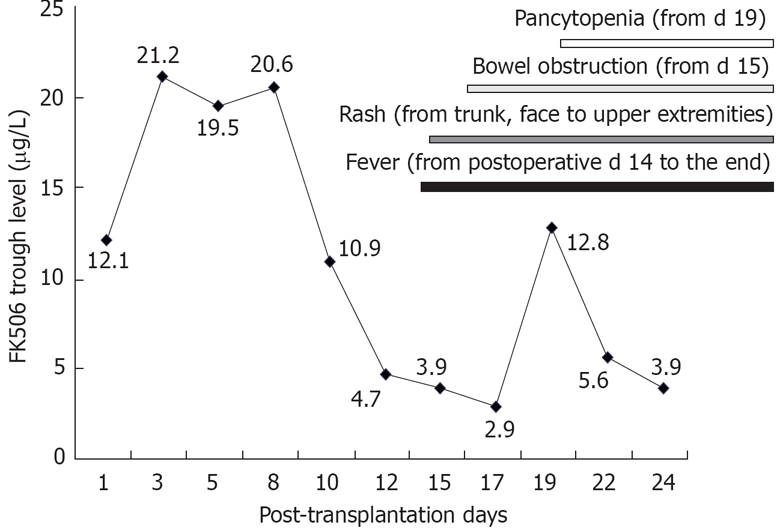
The relationship of FK506 trough level and the symptoms. Before the onset of disease, FK506 trough level was higher than the target level in case 1.
Figure 2.
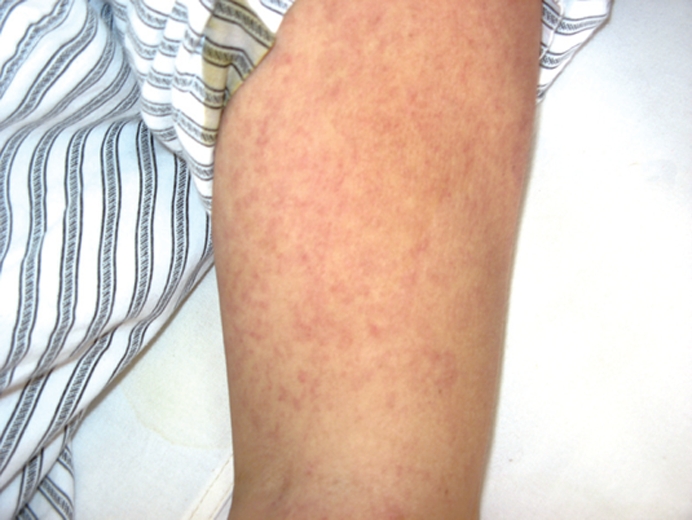
Rash on the right arm.
Figure 3.
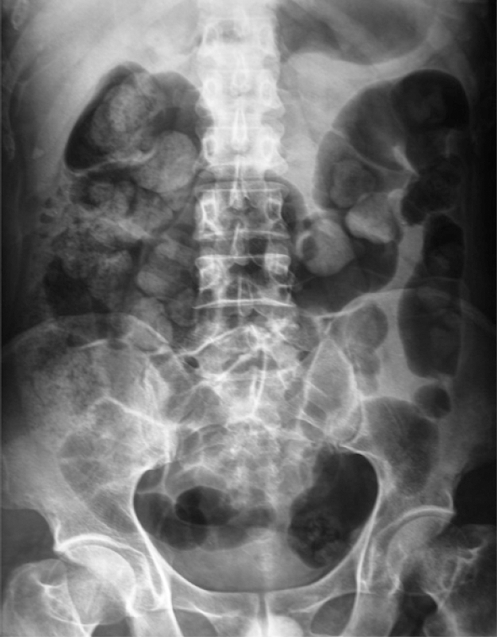
Pneumatosis intestinalis.
Table 1.
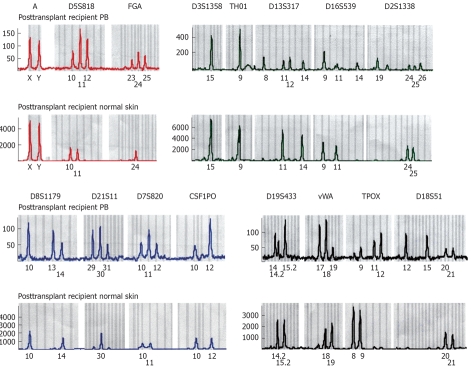
STR system alleles of peripheral blood and normal skin after transplantation
| STR alleles | Skin alleles | Peripheral blood alleles |
| Amelogenin | X, Y | X, Y |
| D5S818 | 10, 11 | 10, 11, 12 |
| FGA | 24, 24 | 23, 24, 25 |
| D3S1358 | 15, 15 | 15, 15 |
| TH01 | 9, 9 | 9, 9 |
| D13S317 | 11, 14 | 8, 11,12, 14 |
| D16S539 | 9, 11 | 9, 11, 14 |
| D2S1338 | 24, 25 | 19, 24, 25, 26 |
| D8S1179 | 10, 14 | 10, 13, 14 |
| D21S11 | 30, 30 | 29, 30, 31 |
| D7S820 | 10, 11 | 10, 11, 12 |
| CSF1PO | 10, 12 | 10, 12 |
| D19S433 | 14.2, 15.2 | 14, 14.2, 15.2 |
| vWA | 18, 19 | 17, 18, 19 |
| TPOX | 8, 9 | 9, 11, 12 |
| D18S51 | 20, 21 | 12, 13, 20, 21 |
STR: Short tandem repeat.
Figure 4.
DNA-STR results of peripheral blood and normal skin in the transplanted recipients. The X axis corresponds to the size of amplified products as noted in base pairs. The Y axis illustrates the amount of fluorescent PCR products. Donor alleles are visible on makers D5S818, FGA D13S317, D16S539, D2S1338, D8S1179, D21S11, D7S820, D19S433, vWA, TPOX, D18S51.
Figure 5.
The posttransplant clinical course of case 1. a: Daclizumab, T: Tazime; O: Ornidazole; I: Isepamicin; P: Platelet; PR: Platelet and packed red blood cell; B: Biopsy of skin and bone marrow tapping; D: DNA-STR test of peripheral blood.
Case 2
Case 2 is a 56-year-old man with end-stage liver disease secondary to HBV infection. He received chemotherapy preoperatively for tuberculosis infection of chest wall for 6 mo. Modified Piggy-back liver transplantation was performed and he was treated with the immunosuppressive agents tacrolimus plus corticosteroids. The patient had an initial uneventful recovery in post-transplantation first week with LFTs dropping to the normal range. From postoperative d 8, the patient suffered from acute rejection which rapidly recovered in 10 d after MMF and corticosteroid impulse therapy was added. The patient experienced unidentified remittent fever without chill, accompanied by severe diarrhea, and maculopapular rash in face and neck. Since the patient had a history of tuberculosis infection, disseminated tuberculosis was suspected and he was started on chemotherapy. Multiple TB culture of urine and sputum (over 10 times respectively) and repeated bacterial culture and fungal culture of urine and peripheral blood showed no pathogen. Both CMV-Ag and EB-IgA were negative. And pancytopenia developed one week later. Then drug reaction and GVHD were suspected. The disease progressed even after withdrawing all suspected drug, corticosteroids therapy and supportive therapy were added, including broad-spectrum antibiotics, anti-fungals, G-CSF (Filgrastim 300 μg bid) and IVIG. Gastrointestinal bleeding occurred on post-transplantation d 59 and the patient died of pneumonia and multiple organ failure 5 d later. No definitive diagnosis was made at the end, however, GVHD was highly suspected because: (1) the clinical features were characteristic; (2) All symptoms started from post-transplantation wk 3, and no pathogen was found in the clinical course; (3) the disease progressed after withdrawing all suspected drugs; and (4) LFTs were within normal range when the disease developed and deteriorated at the end of clinical course.
DISCUSSION
GVHD is an increasing common fatal complication after LTx. The reported incidence of this complication varied from 0.1% to 2% with a high mortality rate of over 75%[1,2]. Its mechanism is still unclear although there are three known essential requirements[3]: (1) the graft must contain immunocompetent cells, (2) the recipient must be recognized as foreigner by the graft, and (3) the recipient must be unable to reject the graft before it mounts an effective immune response. A three-phase model is useful as a framework for understanding the pathogenesis of GVHD following LTx[4]. Phase 1 comprises the preparative regimen (chemotherapy and/or radiotherapy); phase 2 is the activation and proliferation of alloreactive donor T cells; and phase 3 is the effector phase. To date more and more research focuses on the cytokines network in the disease course. The clinical features consist of fever, rash, intestinal symptoms and neutropenia or pancytopenia which is undistinguished from drug reaction and viral infections. The diagnosis is based on the characteristic clinical presentations, histological proof of target tissue damage and molecular manifestation of invasion of recipient target tissue by donor T cells. The latter is most important because it is specific for GVHD and the remaining two may also be found in other diseases. The treatment regimen is generally disappointing, including corticosteroid therapy, reduced or increased immunosuppressant, prevention of infection and supportive treatment, although interleukin-2 receptor antibodies (basiliximab and daclizumab) are recommended as an important treatment choice[5–7].
In a thorough search of Chinese language medical literatures and English language literatures by Chinese organ transplant institutions, we could find only two case reports by two different organ transplantation centers in mainland China (up to January 2007)[8,9]. Six cases with GVHD after LTx are summarized in Tables 2 and 3. Four cases were diagnosed by characteristic clinical presentation, the remaining two by PCR-SSP for detecting the peripheral lymphocyte macrochimerism. None of the six cases survived after empirical treatment.
Table 2.
General data of the patients, onset of GVHD and diagnostic methods
| Case | Gender | Age (yr) | Indication for LTx | Onset of GVHD | Diagnostic method |
| 1 | M | 47 | HCC | PD28 (rash) | Clinical diagnosis |
| 2 | M | 58 | HCC | PD27 (fever) | Clinical diagnosis |
| 3 | F | 58 | LC | PD25 (fever) | HLA typing by PCR-SSP |
| 4 | M | 48 | LC | PD21 (fever) | Clinical diagnosis |
| 5 | M | 69 | LC | PD21 (fever) | HLA typing by PCR-SSP |
| 6 | M | 43 | LC and HCC | PD27 (fever) | Clinical diagnosis |
HCC: Hepatocellular carcinoma; LC: Lliver cirrhosis; HLA: Human leukocyte antigens; PCR-SSP: PCR-sequence specific primer; PD: Post-transplantation day.
Table 3.
Treatment, time point of treatment and patient outcome
| Case | Treatment | Time point of treatment after onset (d) | Outcome |
| 1 | Decreased immunosuppressant; G-CSF; IVIG | 16 | Death, infection |
| 2 | Decreased immunosuppressant; Methylprednisolone 80 mg × 10 d, then taper rapidly; G-CSF; IVIG | 6 | Death, gastrointestinal bleeding, infection, MOF |
| 3 | Decreased immunosuppressant; Methylprednisolone 80 mg × 10 d, then taper rapidly; G-CSF; ATG; IVIG | 16 | Death, gastrointestinal bleeding, infection, ARDS |
| 4 | Decreased immunosuppressant; Methylpredprednisolone impulse treatment, then taper; G-CSF | 5 | Death, gastrointestinal bleeding, infection, MOF |
| 5 | Discontinued immunosuppressant; Methylprednisolone impulse 480 mg/d, then taper; G-CSF; IVIG | 6 | Death, infection, gastrointestinal bleeding, respiratory failure |
| 6 | Non-specific treatment | ? | Death, infection |
G-CSF: Granulocyte colony-stimulating factor; IVIG: Intravenous immunoglobin therapy; MOF: Multiple organ failure; ARDS: Acute respiratory distress syndrome.
The incidence and clinical presentations resemble that reported by others. We must take GVHD into consideration when a recipient presents with fever, rash, diarrhea and neutropenia or pancytopenia without known causes since the incidence of this disease is not so low as expected and the outcome is terribly bad. The diagnosis is difficult in the beginning of the clinical course, which poses a great challenge in treatment. In China, only 3/8 cases are diagnosed with specific molecular techniques which detect the lymphocyte macrochimerism or skin invasion by donor T cells, and 5/8 cases are diagnosed based on the classical clinical features with or without histology of rash skin biopsy and other causes. Without rapid molecular methods to confirm the diagnosis, early initiation of specific treatment is impossible and the poor outcome is unavoidable. No percentage of donor lymphocyte has been reported by other Chinese authors except our article, the so-called confirmed diagnosis may not be accurate because microchimerism is a common phenomenon within 3-4 wk after LTx[10]. The diagnosis needs to be supported by lymphocyte macrochimerism usually detected by HLA typing through PCR-SSP or by DNA PCR-STR with or without flow cytometric tests[11,12]. Fluorescent in situ hybridization (FISH) can also be used to confirm the diagnosis with limited function because unmatched gender is needed for this test. R.Domiati-Saad et al[13] compared different molecular techniques used for confirming the lymphocyte macrochimerism. They recommended: (1) Repeat HLA classItyping by serologic methods using purified T cells and/or class II typing by PCR-SSP; (2) since PCR-SSP is too sensitive to normal levels, a level above 10% should be detected by serologic method, while PCR-SSP may detect levels around 1%; (3) STR method can be used to quantify the contribution of donor T cells when donor HLA antigens are detected, CD3 donor fraction above 20% is highly suggestive of GVHD; and 4) STR testing of CD3 or CD8 populations should be performed twice a week to monitor the patients’ response to treatment. In our case 1, DNA-STR was used to detect lymphocyte macrochimerism. Lymphocytes of donor origin occupied over 60% of lymphocyte population and the diagnosis was confirmed although the patient did not have diarrhea and the histology of skin biopsy was not so clear. In our study, no patient survived after aggressive treatment. The extremely poor prognosis was attributed mostly to the failure in confirming early diagnosis and lack of specific treatment. The common treatment includes reducing or even withdrawing immunosuppressant, increasing corticosteroids and supportive therapy (broad-spectrum antibiotics, anti-fungals, antivirus, IVIG and G-CSF if neutropenia or pancytopenia develops). The dosage and duration of corticosteroids varied among different centers. No interleukin-2 receptor antibody (basiliximab or daclizumab) has been used as a treatment choice.
In summary, GVHD following OLT is under-diagnosed in China with a 100% mortality rate due to the poor awareness of the disease and lack of specific molecular techniques for diagnosis, and this is why we could only select the cases from 2005 to 2006. It is reported that HLA and age may increase the chance of GVHD development after OLT[1]. We believe that immunocompromise status of the recipients is important in the development of this disease because both of our patients received chemotherapy preoperatively. For the recipients suspected of having GVHD, we would suggest that: (1) when a recipient is presented with unidentified fever, rash, gastrointestinal symptoms and bone marrow failure, GVHD must be taken into consideration as the failed early diagnosis would endanger the patient with the edge of death; (2) biopsy of rash skin or damaged gut should be done as soon as possible, which can support the diagnosis of GVHD even though it is not specific for this disease. And HLA typing of both recipients and donors should be performed preoperatively so that lymphocyte macrochimerism can be detected by HLA typing through PCR-SSP or serologic methods. Quantified the contribution of donor T cells is crucial for establishing the diagnosis since lymphocyte microchimerism is a common phenomenon in the early stage after OLT. STR is a valid and rapid technique for this purpose; (3) when GVHD is highly suspected or confirmed and infection is ruled out, specific treatment should be initiated as soon as possible which includes corticosteroids, reduced immunosuppressive agents, prevention of infection and supportive therapy. Interleukin-2 receptor antibody (basiliximab or daclizumab) should also be tried since it has been reported to be successful in several cases.
Supported by China Medical Board of New York Inc., No. 06-837 and Program for New Century Excellent Talents in Universities, No. NCET-04-0794
Peer reviewer: Xupeng Ge, MD, PhD, Transplant Biology Research Center, Massachusetts General Hospital, Harvard Medical School, MGH East, Building 149-5102, 13th street, Boston MA 02129, United States
S- Editor Liu Y L- Editor Ma JY E- Editor Wang HF
References
- 1.Smith DM, Agura E, Netto G, Collins R, Levy M, Goldstein R, Christensen L, Baker J, Altrabulsi B, Osowski L, et al. Liver transplant-associated graft-versus-host disease. Transplantation. 2003;75:118–126. doi: 10.1097/00007890-200301150-00022. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AL, Gibbs P, Sudhindran S, Key T, Goodman RS, Morgan CH, Watson CJ, Delriviere L, Alexander GJ, Jamieson NV, et al. Monitoring systemic donor lymphocyte macrochimerism to aid the diagnosis of graft-versus-host disease after liver transplantation. Transplantation. 2004;77:441–446. doi: 10.1097/01.TP.0000103721.29729.FE. [DOI] [PubMed] [Google Scholar]
- 3.Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. [PubMed] [Google Scholar]
- 4.Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. doi: 10.1146/annurev.med.54.101601.152339. [DOI] [PubMed] [Google Scholar]
- 5.Sudhindran S, Taylor A, Delriviere L, Collins VP, Liu L, Taylor CJ, Alexander GJ, Gimson AE, Jamieson NV, Watson CJ, et al. Treatment of graft-versus-host disease after liver transplantation with basiliximab followed by bowel resection. Am J Transplant. 2003;3:1024–1029. doi: 10.1034/j.1600-6143.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 6.Riñón M, Maruri N, Arrieta A, Fernández JR, Ortiz de Urbina J, García Masdevall MD. Selective immunosuppression with daclizumab in liver transplantation with graft-versus-host disease. Transplant Proc. 2002;34:109–110. doi: 10.1016/s0041-1345(01)02828-7. [DOI] [PubMed] [Google Scholar]
- 7.Przepiorka D, Kernan NA, Ippoliti C, Papadopoulos EB, Giralt S, Khouri I, Lu JG, Gajewski J, Durett A, Cleary K, et al. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood. 2000;95:83–89. [PubMed] [Google Scholar]
- 8.Liang TB, Tang XF, Zheng SS, Lu AW, Wang WL, Shen Y, Zhang M, Meng XQ. Graft versus host disease after liver transplantation: a report of 3 cases. Zhonghua Yixue Zazhi. 2004;84:826–829. [PubMed] [Google Scholar]
- 9.He Q, Han DD, Chen DZ, Lang R, Li N, Qing JM, Fan H. Preliminary clinical experience of liver transplant-associated graft-versus- host disease. J Hepatobiliary Surgery. 2006;14:91–94. [Google Scholar]
- 10.Schlitt HJ, Kanehiro H, Raddatz G, Steinhoff G, Richter N, Nashan B, Ringe B, Wonigeit K, Pichlmayr R. Persistence of donor lymphocytes in liver allograft recipients. Transplantation. 1993;56:1001–1007. doi: 10.1097/00007890-199310000-00042. [DOI] [PubMed] [Google Scholar]
- 11.Hahn AB, Baliga P. Rapid method for the analysis of peripheral chimerism in suspected graft-versus-host disease after liver transplantation. Liver Transpl. 2000;6:180–184. doi: 10.1002/lt.500060214. [DOI] [PubMed] [Google Scholar]
- 12.Schrager JJ, Vnencak-Jones CL, Graber SE, Neff AT, Chari RS, Wright KJ Jr, Pinson CW, Stewart JH, Gorden DL. Use of short tandem repeats for DNA fingerprinting to rapidly diagnose graft-versus-host disease in solid organ transplant patients. Transplantation. 2006;81:21–25. doi: 10.1097/01.tp.0000190431.94252.3f. [DOI] [PubMed] [Google Scholar]
- 13.Domiati-Saad R, Klintmalm GB, Netto G, Agura ED, Chinnakotla S, Smith DM. Acute graft versus host disease after liver transplantation: patterns of lymphocyte chimerism. Am J Transplant. 2005;5:2968–2973. doi: 10.1111/j.1600-6143.2005.01110.x. [DOI] [PubMed] [Google Scholar]