Abstract
Pneumonia as a secondary infection after influenza is a major cause of excess morbidity and mortality despite the availability and use of antibiotics active against Streptococcus pneumoniae. We hypothesized that use of a bacteriostatic protein synthesis inhibitor would improve outcomes by reducing the inflammatory response. BALB/c mice infected with influenza virus and super-infected with Streptococcus pneumoniae were treated with either the cell wall active antibiotic ampicillin or protein synthesis inhibitors clindamycin or azithromycin. Ampicillin therapy performed significantly worse (56% survival) in the model compared to clindamycin therapy either alone (82%) or in combination with ampicillin (80%) and to azithromycin (92%). Improved survival appeared to be mediated by decreased inflammation manifested as lower levels of inflammatory cells and pro-inflammatory cytokines in the lungs, and less severe histopathology. These data suggest that beta-lactam therapy may not be optimal as first line treatment of community acquired pneumonia when it follows influenza.
Keywords: influenza, Streptococcus pneumoniae, pneumonia, beta-lactam, guidelines
Over the last decade, influenza and pneumonia have ranked as the 7th leading cause of death in the United States for all persons and the 5th leading cause of death for children [1]. Two bacteria are most commonly associated with influenza virus: Streptococcus pneumoniae and Staphylococcus aureus. The pneumococcus primarily affects young children, causing otitis media, sinusitis, and pneumonia, and the elderly, causing pneumonia [2]. During annual epidemics, pneumococcal disease following influenza is a particularly common cause of mortality in elderly persons with co-morbidities such as lung or heart disease. During the influenza pandemics of 1918, 1957, and 1968, a bacterial etiology was found more commonly than in seasonal epidemics -in as many as 50–95% of patients with fatal or life-threatening pneumonia [3–10]. Thus, it can be predicted that highly pathogenic influenza virus strains that are most likely to cause the next pandemic, or to be utilized as agents of bioterrorism, would achieve much of their impact through secondary bacterial infections.
Secondary bacterial pneumonia as a complication of influenza has historically been viewed by clinicians as more difficult to treat than primary pneumonia [7;8;11–13]. Several reasons for this have been inferred from case series. In many instances, the infection may occur while the host is still dealing with the viral infection itself or its aftermath, and the additive effects of the two diseases may predispose to a worse outcome [5;7]. It has also been suggested that bacterial pneumonia following influenza is more likely to involve complex features such as pleural effusions and bacteremia, and to present at a more advanced stage of disease with multiple lobes of the lung involved [4;11]. Host factors are undoubtedly also involved, since the usual population afflicted with influenza and pneumococcus during seasonal influenza epidemics, the frail elderly, have little reserve and would be expected to succumb more easily to severe infections.
We have developed relevant animal models of secondary bacterial pneumonia to facilitate study of mechanisms that underlie this synergistic interaction [3;14;15]. In mice, pre-infection with influenza virus increases the incidence of pneumococcal pneumonia, promotes bacteremia and systemic spread, hastens the tempo of progression of pneumonia, and predisposes to multi-lobar disease [4;14;16]. In addition, viral titers from the lungs are enhanced and the character of the pneumonia is altered, featuring more airway necrosis, fibrin deposition, and inflammatory changes [14;17;18]. Two specific mechanisms have been implicated by these studies thus far, improved adherence through the sialidase activity of the viral neuraminidase [4;17;19;20], and enhanced inflammation mediated by expression of the newly discovered viral accessory protein PB1-F2 [18]. This latter mechanism is of particular interest, since it may help explain the striking pathogenicity of the 1918 “Spanish Flu” pandemic strain. A laboratory virus engineered to express the PB1-F2 protein from the 1918 strain A/Brevig Mission/1/18 (H1N1) was more virulent in mice than the wildtype parent, more strongly stimulated an inflammatory response in the lung, and more efficiently supported secondary bacterial pneumonia [18].
Since Austrian and Gold first demonstrated the efficacy of penicillin in the treatment of adults with pneumococcal pneumonia more than 40 years ago [21], penicillin or other beta-lactam agents have been considered to be the treatment of choice for most patients with pneumococcal pneumonia. The general goal of treatment has been to eliminate pathogens as rapidly as possible, so bactericidal agents have been preferred. The Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) specifically recommend beta-lactam agents as the first line of therapy for bacterial pneumonia following influenza [22]. Since Mycoplasma and Chlamydia are unlikely co-pathogens with influenza, macrolides are generally not recommended for this situation. In patients suspected to have infections with highly pathogenic H5N1 strains, the IDSA / ATS guidelines suggest use of both antiviral therapy as well as antimicrobials targeting S. pneumoniae and S. aureus. In adults in whom influenza is not suspected, macrolides, fluoroquinolones, or a combination of a beta-lactam and a macrolide can be used for outpatient therapy, while for inpatients a fluoroquinolone or combination beta-lactam / macrolide therapy is recommended. The more ill the patient, the greater the emphasis on the beta-lactam component of therapy to cover S. pneumoniae [22]. In children, however, macrolides and fluoroquinolones are not commonly utilized so beta-lactams are the mainstay of therapy unless Mycoplasma infection is documented or strongly suspected [23].
In this report we examine the question of whether beta-lactams are the most appropriate therapy for secondary bacterial pneumonia when it follows influenza. In a previous study of pneumococcal co-infection with influenza virus, ampicillin treatment cleared bacteria from the lungs but did not improve mortality [4]. Antiviral therapy to remove the contribution of the virus to the interaction was necessary to effect cure with a beta-lactam agent. We hypothesize that the robust inflammatory response that occurs during severe influenza virus infections, which is magnified by subsequent bacterial super-infections [16], is further exacerbated by beta-lactam mediated lysis of bacteria. If this hypothesis is correct, use of alternate antimicrobials with different mechanisms of action may treat the infection without the adverse effects. We present data suggesting that treatment of secondary pneumococcal pneumonia with protein synthesis inhibitor antibiotics, either alone or in combination with a beta-lactam, may result in better outcomes by lessening the inflammatory response engendered by lysis of the bacteria.
Methods
Infectious agents
The St. Jude strain of mouse adapted influenza virus A/Puerto Rico/8/34 (H1N1; PR8), generated by reverse genetics [18], was grown in Madin-Darby canine kidney (MDCK) cells. S. pneumoniae strain A66.1 (type 3) was transformed with the lux operon (Xenogen Corp., Alameda, CA) [17]. The minimum inhibitory concentrations (MICs) for this strain were 0.023 µg/ml for ampicillin, 0.047 for clindamycin, and 0.064 for azithromycin.
Measurement of TNFα from macrophages
After growth to an OD600 of 0.6 in Todd-Hewitt broth, pneumococci were exposed to ampicillin or clindamycin for 2 hours at 37°C. The resulting cultures were centrifuged for 5 min at 10,000Xg and 100µl of supernatant was added to wells containing 100µl of infection media (D-MEM supplemented with 3% bovine serum albumin) overlaying 95% confluent J774 macrophages. Supernatants from exposed macrophages were assayed for TNFα production using the BD optEIA TNF ELISA kit (BD Biosciences, San Diego, CA) according to the manufacturer’s instructions.
Mice
Seven- to eight-week old female Balb/c mice were used in biosafety level 2 facilities in these studies in a manner in accordance with the guidelines of the Committee on Care and Use of Laboratory Animals. All experimental procedures were done under general anesthesia with inhaled isoflurane 2.5% (Baxter Healthcare Corporation, Deerfield, IL).
Infectious model
Infectious agents were administered intranasally in a volume of 100 µl (50 µl per nostril) to anesthetized mice. Influenza virus was given at a dose of 37 TCID50, which is equivalent to 0.03 MLD50 for this age and strain of mice. S. pneumoniae was given 7 days later at a dose of 200 CFU (0.02 MLD50). Following secondary bacterial infection, bioluminescent imaging was used to follow development and progression of pneumonia as described, using defined parameters to insure experimental groups were balanced with all mice at an early stage of pneumonia [17;18]. Bacterial counts and cytokine levels in the lungs were determined from lung homogenates as previously described [4;17;18]. Cell counts and differentials from broncho-alveolar lavage fluid were determined by flow cytometry as previously described [18].
Antibiotic treatment
Treatment was started upon identification of pneumonia by bioluminescent imaging. Mice were given antibiotics (Sigma-Aldrich Co., St. Louis, MO) intraperitoneally twice daily in divided doses (ampicillin 200 mg/kg/d; clindamycin 30 mg/kg/d or 120 mg/kg/d) or once daily (azithromycin 10 mg/kg/d first dose, 5 mg/kg/d thereafter) for a total of 7 days or were mock treated with the diluent, PBS.
Histopathology
Microscopic evaluation of lungs was performed by an experienced veterinary pathologist (KLB) blinded to study purpose and design and to group composition. A semi-quantitative grading scheme was utilized to score two parameters, the overall character of the pneumonic process and the pathology specifically observed in the interstitum and terminal airways as previously described [18].
Statistical analyses
Comparison of survival between groups of mice was done with the Log Rank chi-squared test on the Kaplan-Meier survival data. Comparisons of weight loss, bacterial lung titers, BAL cell counts, flux of light through the lung, and cytokine levels between groups were done using analysis of variance (ANOVA). A p-value of < 0.05 was considered significant for these comparisons. SigmaStat for Windows (SysStat Software, Inc., V 3.11) was utilized for all statistical analyses.
Results
Ampicillin-mediated killing elicits a higher TNFα response than killing with clindamycin
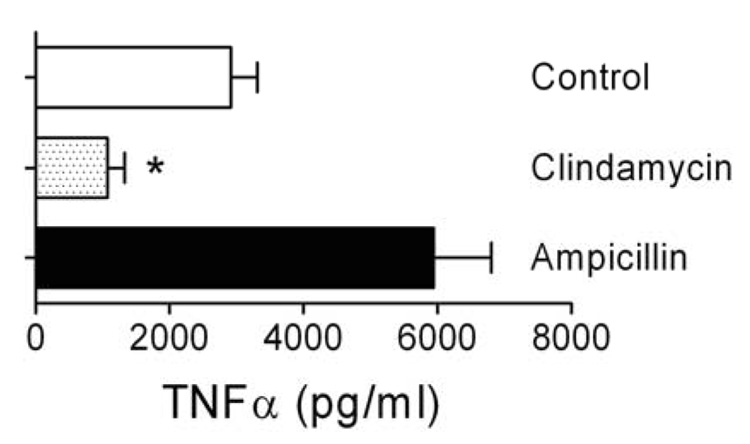
We hypothesized that the poor clinical outcomes observed with secondary pneumococcal pneumonia following influenza could be due to immunopathology engendered by lysis of bacteria by this beta-lactam antibiotic. We elected to first test this possibility in vitro by killing pneumococci with either the cell-wall active agent ampicillin, or a protein synthesis inhibitor, clindamycin. Production of TNFα by macrophages was used as a surrogate for the inflammatory response. Bacterial killing with clindamycin resulted in significantly less (p < 0.001 by ANOVA) TNFα production than killing with ampicillin (Figure 1) or exposure to live pneumococci (Control). Neither antibiotic had an effect on TNFα production by macrophages when they were exposed in the absence of bacteria (mean values of 25.3 and 27.0 pg/ml for ampicillin and clindamycin, respectively, compared to 51.4 pg/ml for media alone). The 6-fold increase in this pro-inflammatory cytokine from ampicillin treatment relative to clindamycin treatment prompted us to compare these two antibiotics in our mouse model of secondary bacterial pneumonia.
Figure 1.
TNFα released due to antibiotic-mediated killing of S. pneumoniae. J774 macrophages were exposed to supernatants from bacteria treated for 2 hours with ampicillin, clinidamycin, or mock treated. TNFα production (mean ± s.d. of 6 wells from 4 consecutive, independent experiments) by the macrophages was determined by ELISA. An asterisk (*) indicates a significant difference (p < 0.05) by ANOVA compared to the other groups. There was no statistical differences between the ampicillin and control data.
Protein synthesis inhibitors improve outcomes from secondary bacterial pneumonia
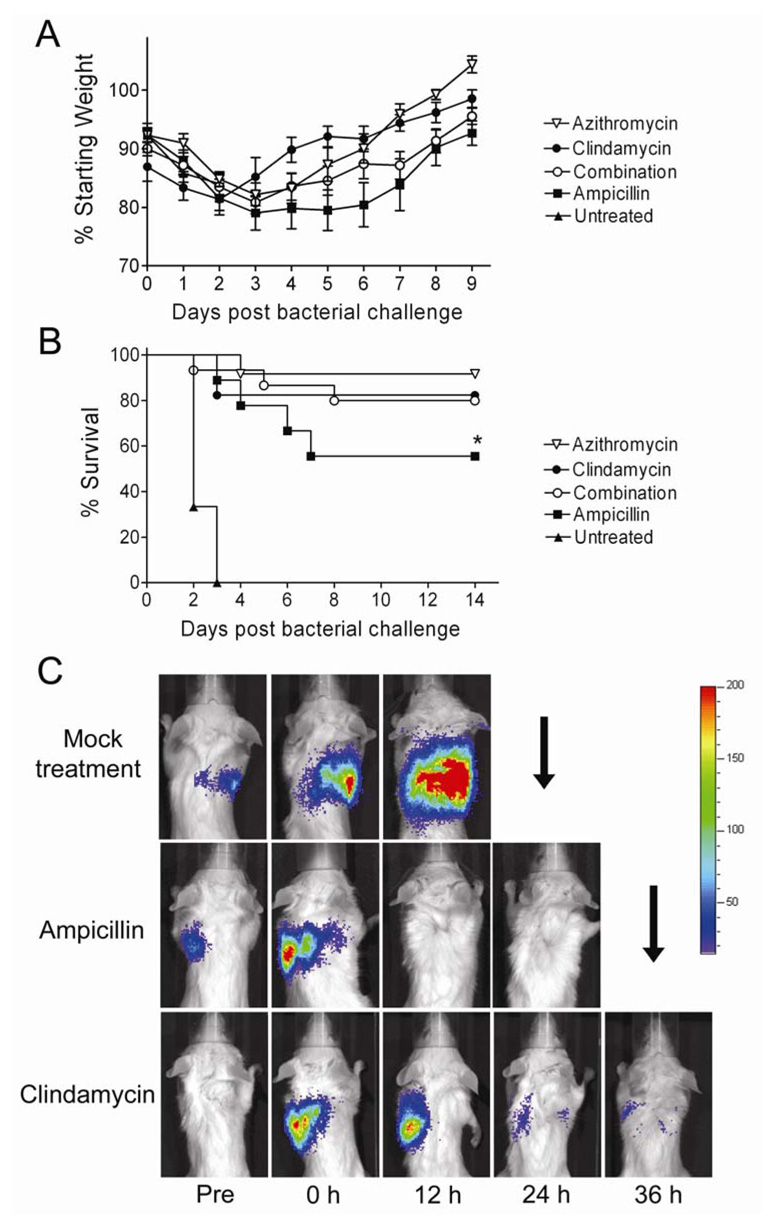
We studied the effect of antibiotic treatment on outcomes by infecting mice with influenza virus and 7 days later challenging them with a type 3 strain of S. pneumoniae [16]. Mice were monitored twice daily for development of pneumonia by bioluminescent imaging and treatment was initiated immediately upon identification of infection. In a preliminary study, all mice treated with ampicillin (8/8) cleared the bacteria from their lungs by bioluminescent imaging, but 3 of the 8 mice (38%) died a mean of 63 hours after clearance. This result was significantly better than with the control untreated group, where no mice (0/7) cleared the infections or survived for more than 72 hours. When treated with clindamycin at a dose of 30 mg/kg/d, only 10/16 mice cleared infection, but all of these mice survived (10/10). Thus, clindamycin at this dose was poorly effective at eliminating bacteria from the lungs of influenza virus and S. pneumoniae co-infected mice, but survival following bacterial clearance was significantly improved (p < 0.05 by log-rank test) compared to the group treated with ampicillin. In addition, mice treated with clindamycin had significantly less weight loss on days 3–9 following secondary infection than mice treated with ampicillin (p < 0.05 by repeated measures ANOVA; data not shown).
We next tested several alternate strategies involving a protein synthesis inhibitor, including a higher dose of clindamycin (120 mg/kg/d), use of a macrolide, azithromycin, or combination therapy with clindamycin and ampicillin. Increasing the dose of clindamycin improved outcomes by improving clearance of pneumonia; 17/17 (100%) of mice treated with the higher dose cleared pneumococci from their lungs within 36 hours and 14/17 (82%) survived (Figure 2). The 3 mice in this group which died had severe diarrhea, which may have contributed to their deaths. Combination therapy consisting of 24 hours of clindamycin therapy begun at identification of pneumonia followed by combined therapy with both ampicillin and clindamycin resulted in a similar outcome; all mice cleared pneumonia and 12/15 (80%) survived. Azithromycin, which shares a mechanism of action with clindamycin but possesses anti-inflammatory qualities as well, performed best in the model, clearing pneumonia from all 12 mice and promoting survival in 11/12 (92%). While ampicillin therapy was superior to no treatment (5/9 (56%) survived vs. 0/6 (0%)), it was inferior to any of the other treatment regimens (Figure 2; p < 0.05 by Log-Rank test for each comparison).
Figure 2.
Survival of mice treated for secondary bacterial pneumonia with a cell wall active agent or protein synthesis inhibitors. Groups of mice were infected with influenza virus PR8 then challenged 7 days later with S. pneumoniae and monitored for development of pneumonia by bioluminescent imaging (defined as a flux of light through the thorax of > 22,000 RLU per minute). Treatment with azithromycin (n=12), clindamycin (n=17), a combination of clindamycin for 24 hours followed by clindamycin plus ampicillin (n=15), ampicillin alone (n=9) or mock treatment (n=6) was initiated after development of pneumonia. A) Weight loss and B) survival are plotted. Error bars indicate the s.d. of the mean weights. An asterisk (*) indicates a significant difference (p < 0.05) compared to all other groups by the Log Rank test on the Kaplan-Meier survival data. C) Representative pictures from bioluminescent imaging of mice with pneumonia before (Pre) development of pneumonia, at the initiation of treatment (o h), and 12, 24 and 36 hours after treatment starts. The scale at the right indicates RLU per pixel. A down-going arrow indicates death of the animal prior to that imaging timepoint.
Increased inflammatory response with ampicillin
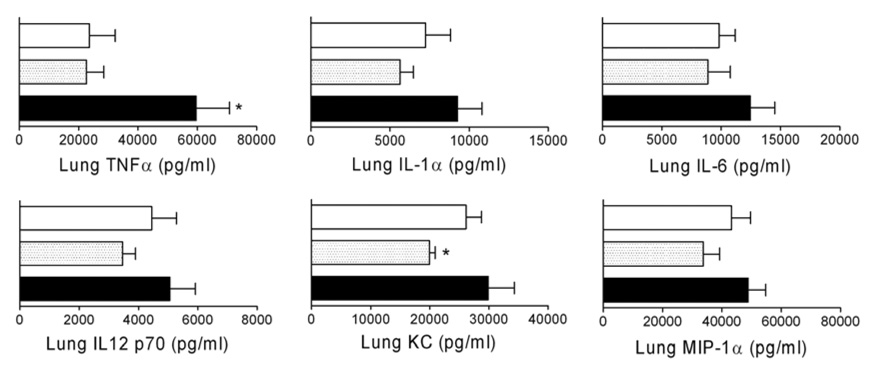
The underlying hypothesis for this work was that the inflammatory response accompanying lysis by beta-lactams was responsible for poor outcomes with ampicillin. As correlates of antibiotic treatment-mediated pulmonary inflammation we measured cytokines and chemokines from lung homogenates and cell counts from BAL fluid. These indices have previously been shown to correlate with poor outcomes in the mouse model of bacterial super-infection following influenza [16;18]. A general increase in pro-inflammatory cytokines and chemokines including IL-1α, , TNFα, IL-6, IL-12p70, KC, and MIP-1α (Figure 3), as well as IL-1β and IL-12p40 (data not shown) was seen in the lungs 4 hours after initiation of treatment with ampicillin compared to untreated animals. Conversely, levels of most of these cytokines and chemokines were lower in clindamycin treated animals, although statistical significance was only achieved for TNFα and KC (Figure 3).
Figure 3.
Cytokines from lung homogenates following treatment for secondary bacterial pneumonia. Groups of mice (n=9) were infected with influenza virus PR8 then challenged 7 days later with S. pneumoniae and monitored for development of pneumonia by bioluminescent imaging. Treatment with clindamycin (gray shaded bars; dose of 120 mg/kg/day), ampicillin (black bars), or mock treatment (white bars) was initiated after development of pneumonia. Four hours after the first antibiotic dose, lungs were homogenized and assayed for cytokines. Error bars indicate the s.d. of the mean cytokine values. An asterisk (*) indicates a significant difference (p < 0.05) by ANOVA compared to the other groups.
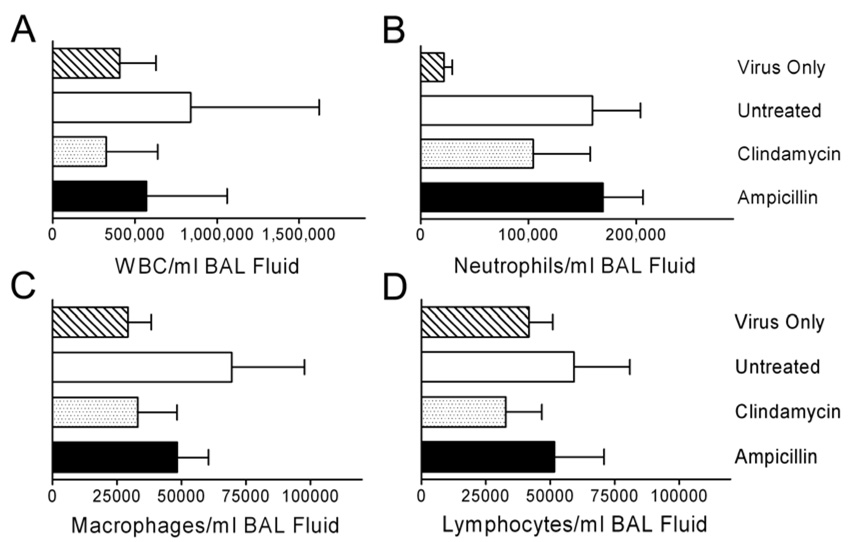
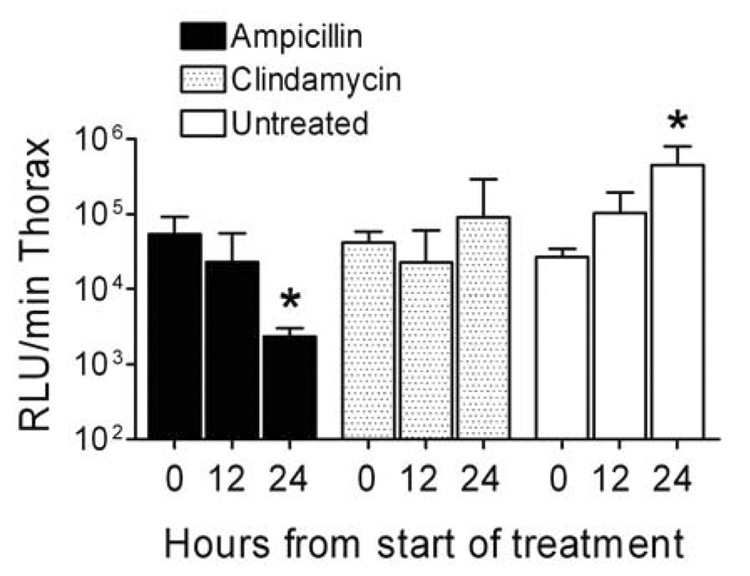
The relevance of these findings is supported by examination of the infiltration of effector cells into the lungs as determined by examination of the BAL fluid 24 hours after start of treatment. The overall numbers of white cells, as well as the relative numbers of neutrophils, macrophages, and lymphocytes were 46% – 76% higher in mice treated with ampicillin than in mice treated with clindamycin (Figure 4). While these comparisons lack the power necessary to achieve statistical significance due to the number of mice used (6–9 per group), the trend is clear. These observed differences in cytokines and cell counts were not due to increases in bacterial counts in the lungs of ampicillin-treated mice compared to those treated with clindamycin. The amount of bacteria in the lungs as quantitated by bioluminescence decreased significantly (more than 23-fold; p < 0.05 by ANOVA) in the 24 hours after treatment with ampicillin, increased more than 2-fold in untreated mice (p < 0.05), and remained stable in mice treated with clindamycin (Figure 5). Bacterial counts from homogenized lungs 4 hours after detection of pneumonia and onset of treatment (at the time cytokines were determined) were not different between the ampicillin and clindamycin groups (mean of 5.3×106 CFU/ml lung homogenate for ampicillin-treated mice, 3.0×106 CFU/ml for clindamycin, and 2.8×107 CFU/ml without treatment). Thus, increased killing and more rapid clearance of bacteria from the lungs resulted in worse cytokinemia, more infiltration of inflammatory cells, and poorer outcomes.
Figure 4.
Cell counts from broncho-alveolar lavage fluid following treatment for secondary bacterial pneumonia. Groups of mice were infected with influenza virus PR8 then challenged 7 days later with S. pneumoniae or mock challenged (n=6) and monitored for development of pneumonia by bioluminescent imaging. Treatment with clindamycin (n=6), ampicillin (n=9), or mock treatment (n=6) was initiated after development of pneumonia. 24 hours after the first antibiotic dose, lungs were lavaged and assayed for cell counts by flow cytometry. Error bars indicate the s.d. of the mean values. The differences are not statistically significant.
Figure 5.
Bacterial load in the lungs during treatment of secondary bacterial pneumonia. Groups of mice were infected with influenza virus PR8 then challenged 7 days later with S. pneumoniae and monitored for development of pneumonia by bioluminescent imaging. Treatment with clindamycin (n=6), ampicillin (n=9), or mock treatment (n=6) was initiated after development of pneumonia. Bacterial lung load was assessed in live, anesthetized animals 0, 12, and 24 hours from the start of treatment using bioluminescent imaging and is reported as mean ± s.d. of the flux of light from the thorax (relative light units (RLU) per minute). An asterisk (*) indicates a significant difference (p < 0.05) by ANOVA compared to the zero hour timepoint for that group..
Histopathologic findings are worse with ampicillin
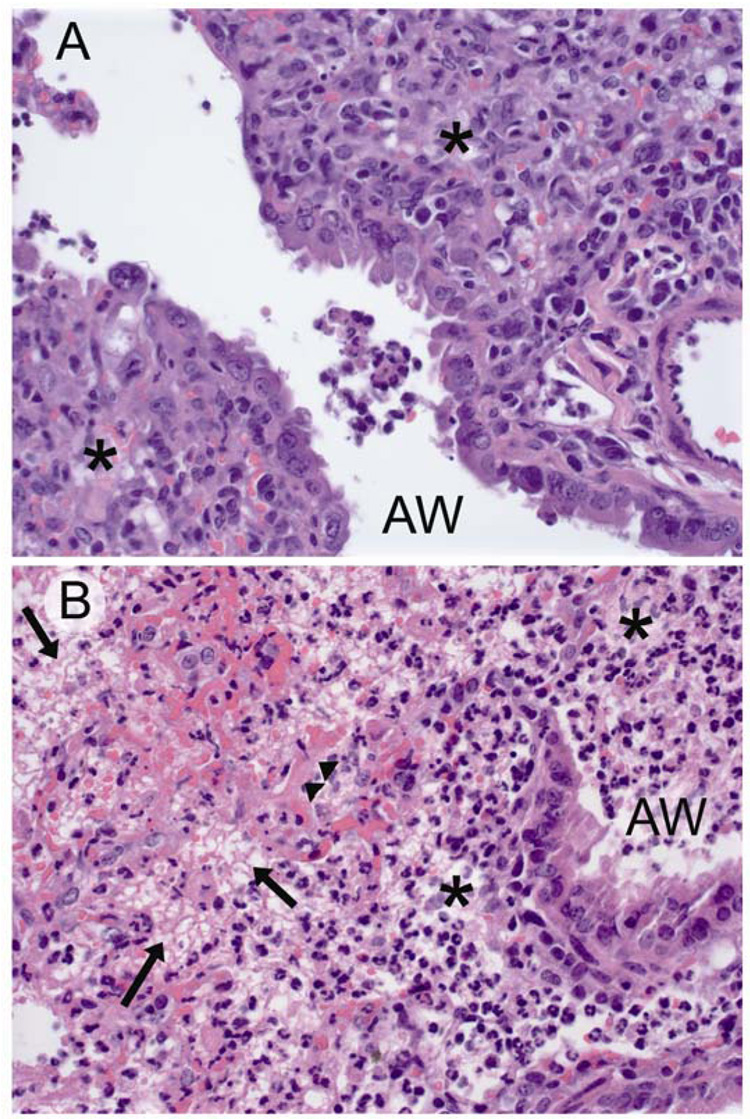
We next performed microscopic examination of lungs from mice treated for pneumonia both 4 and 24 hours after development of pneumonia and the start of treatment. In mice infected only with virus, minimal to mild changes in the airways and interstitium were noted in all animals with mild airway hyperplasia and an inflammatory infiltrate consisting of lymphocytes and plasma cells (Table 1). All mice secondarily infected with pneumococcus had extensive bronchointerstitial pneumonia (Table 1; similar values for airway and interstitial involvement between groups that were infected with bacteria). However, there was significant variation in the character and severity of the pneumonic process comparing mice treated with clindamycin to those treated with ampicillin or left untreated (Figure 6). Untreated mice and those treated with ampicillin had similar findings (Figure 6A), including extensive epithelial necrosis throughout the bronchial tree and lobar fibrinonecrosis, characterized by acute coagulative necrosis of alveolar walls, filling of alveoli with viable and degenerate neutrophils, fibrin, edema and hemorrhage, and pleuritis consisting of mats of fibrinocelluar exudates along the pleura. Unique findings in the group treated with clindamycin are limited to the character of the interstitial component of the disease – the extent of lung involvement was similar between the groups (Figure 6B). A combination of necrosis and hyperplastic airway epithelium similar to that seen in other groups is present. However, fibrinonecrosis and acute coagulative necrosis are not the predominant findings. When present, these are limited to small, localized areas and entire lobes are never affected.
Table 1.
Histopathologic characterization of pneumonia.
| Virus only | Virus and Bacteria Mock Treatment |
Virus and Bacteria Ampicillin |
Virus and Bacteria Clindamycin |
|
|---|---|---|---|---|
| Severity of Airway | 1.3 ± 0.6a | 2.3 ± 0.6 | 3.0 ± 0.0 | 2.3 ± 0.6 |
| Disease | ||||
| Severity of Interstitial | 2.3 ± 0.6 | 3.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 |
| Disease | ||||
| Fibrino-purulent | 0.0 ± 0.0 | 2.7 ± 0.6 | 3.3 ± 0.6 | 1.3 ± 1.5 |
| Inflammation | ||||
| Interstitial Acute | 0.0 ± 0.0 | 2.7 ± 0.6 | 3.3 ± 0.6 | 1.7 ± 1.2 |
| Coagulative Necrosis |
Values are mean ± standard deviation of semi-qualitative scores on a scale of 0–4 as determined by a veterinary pathologist (K.L.B.) blinded to the study purpose and design and to the composition of the groups.
Figure 6.
Histopathology of mice treated for secondary bacterial pneumonia. Representative hematoxylin and eosin stained sections (40X) from of terminal airways and interstitium of lungs of mice infected with influenza virus PR8, then challenged 7 days later with S. pneumoniae and treated with either A) clindamycin or B) ampicillin. Lungs were taken 24 hours after start of treatment. in A) mononuclear inflammation (*) can be seen in the interstitium with a few admixed neutrophils. In B) fibrinonecrosis of the insterstitium is evident with effacement of the alveoli by lacy eosinophilic fibrin strands (arrows), copious infiltrates of both viable and degenerate neutrophils (*), and necrotic alveolar walls (arrowheads). AW = airway.
Discussion
Significant mortality accrues from severe lung infections despite the availability of effective antibiotics for the most common causes of pneumonia. Even with appropriate therapy, pneumococcal pneumonia causes mortality in 4–5% of uncomplicated cases, and 13% of cases with bacteremia [24–27]. These rates are essentially unchanged since the introduction of penicillin [21]. Based on limited case series and accumulated clinical experience, bacterial pneumonia following influenza is generally considered more difficult to treat and to have a high case fatality rate [3;4;12]. We have suggested that this may stem from the robust inflammatory response that occurs in response to combined infections [16;18]. Use of beta-lactam agents such as ampicillin may exacerbate the problem, since these agents act by lysing the bacteria, releasing pro-inflammatory substances like cell wall components, cytotoxins, and bacterial DNA which are recognized by the innate immune system and trigger the inflammatory response [28;29]. In support of this model, mice with severe pneumococcal pneumonia and bacteremia following influenza did not survive when treated with ampicillin, despite effective killing and clearance of bacteria [4]. In this study, we have extended this finding by using a less virulent strain of S. pneumoniae, which remains confined to the lung [16]. Ampicillin therapy only resulted in 56–62% survival in mice super-infected with this strain following influenza (Figure 1 and data not shown). Treatment in these mice was associated with increases in pro-inflammatory cytokines and chemokines, increased influx of inflammatory effector cells into the lungs, and worse histopathologic findings, despite rapid and complete clearance of bacteria.
Based on this model of the pathogenesis of these severe lung infections, we have suggested that treatment with non-lytic antibiotics may result in improved outcomes [30]. Clindamycin is a lincosamide antibiotic which targets the 50S ribosomal subunit, inhibiting protein synthesis, and is bacteriostatic at typical treatment doses. In our model, low dose therapy with clindamycin was poorly effective, clearing bacteria from the lungs in only 10/16 mice, but all mice which responded to the antibiotic survived. Increasing the dose of clindamycin improved clearance but also resulted in toxicity. Nonetheless, overall survival was improved compared to the cell-wall active agent. The improved outcomes appear to be due to a decreased inflammatory response, manifest by lower levels of pro-inflammatory cytokines and chemokines, fewer inflammatory cells in the lungs, and improved histopathology, despite slower clearance. Clindamycin treatment prior to therapy with ampicillin was equally efficacious – we hypothesize that protein synthesis shutdown prior to lysis by ampicillin decreased the release of pro-inflammatory toxins. Because of the incomplete response but favorable outcomes with clindamycin alone, we also studied a second antibiotic with a similar mechanism of action, azithromycin. Azithromycin is a 15-member ring macrolide which also targets the 50S ribosome, but which exhibits anti-inflammatory activities independent of its antimicrobial properties [31]. This antibiotic resulted in clinical cure in 11/12 mice (92%), although it is unclear whether the improved outcomes are solely due to the mechanism of action or due to this factor in addition to the anti-inflammatory properties of the drug.
Similar approaches to therapy have previously been considered for severe bacterial infections. It is now well appreciated that the use of steroids in children with Haemophilus influenzae meningitis [32;33] or adults with pneumococcal meningitis [34] improves clinical outcomes. However, the effects of steroids on severe lung infections including acute respiratory distress syndrome (ARDS) have been disappointing, suggesting other options are needed. In a rabbit model of pneumococcal meningitis, Nau et al. demonstrated that treatment with a non-lytic antibiotic such as rifampin results in improved outcomes [35;35–37]. Limited data from these investigators suggests that the benefits of rifampin therapy in this model are preserved when used in combination with ceftriaxone [35]. In this study we also showed that combination therapy was successful when the protein synthesis inhibitor preceded the cell wall active agent by 24 hours; further work on the relative timing of administration may be helpful. In humans, two retrospective studies [38;39] and one prospective, multicenter trial [40] have concluded that the addition of a macrolide to a beta-lactam results in a significant reduction in mortality (compared with beta-lactam therapy alone) in adults with bacteremic pneumococcal pneumonia. The mechanism(s) responsible for this effect were not apparent, but were unrelated to beta-lactam resistance. The results of our study suggest that the improved outcomes are related to suppression of the inflammatory response.
Secondary bacterial infections are more common during pandemic years when highly virulent virus strains circulate. Accumulating evidence suggests this is in part due to the robust inflammatory response induced in the lung by these pandemic strains [18;41]. Over the last 10 years, highly pathogenic avian influenza viruses of the H5N1 subtype have emerged as a cause of significant mortality and a pandemic threat in Southeast Asia, parts of the Middle East, Europe, and Africa [42]. A hallmark of these infections is severe lung inflammation similar to that seen with ARDS [43–46]. The only criterion missing for one these viruses to become the next pandemic strain is ease of transmission [47]. It can be predicted based on history that when these viruses adapt to humans and begin to cause widespread disease, secondary bacterial infections will emerge as a leading cause of morbidity and mortality. Antivirals, if available, may help mitigate the severity of complications as suggested by our earlier work [4]. However, alternate strategies including non-lytic antibiotics and immunomodulation need to be considered [30;48].
Acknowledgements
This work was supported by USPHS grant AI-66349 and by the American Lebanese Syrian Associated Charities (ALSAC). We would like to thank Dr. Elaine Tuomanen for helpful discussions on the manuscript.
Footnotes
Statement on Conflict of Interest
The authors declare that no conflict of interest exists.
Prior Presentation
Part of this research has been presented previously at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) as abstract B-843.
References
- 1.Minino AM, Heron MP, Murphy SL, Kochanek KD. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55(19):1–119. [PubMed] [Google Scholar]
- 2.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 3.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19(3):571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis. 2004;190(3):519–526. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]
- 5.Hers JFP, Masurel N, Mulder J. Bacteriology and histopathology of the respiratory tract and lungs of fatal Asian influenza. Lancet. 1958;2:1164–1165. doi: 10.1016/s0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay MI, Jr., Herrmann EC, Jr., Morrow GW, Jr., Brownx AL., Jr. Hong Kong influenza: clinical, microbiologic, and pathologic features in 127 cases. JAMA. 1970;214(10):1825–1832. doi: 10.1001/jama.214.10.1825. [DOI] [PubMed] [Google Scholar]
- 7.Louria D, Blumenfeld H, Ellis J, Kilbourne ED, Rogers D. Studies on influenza in the pandemic of 1957–58. II Pulmonary complications of influenza. J Clin Invest. 1959;38:213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin CM, Kunin CM, Gottlieb LS, Barnes MW, Liu C, Finland M. Asian influenza A in Boston, 1957–1958. I. Observations in thirty-two influenza-associated fatal cases. AMA Arch Intern Med. 1959;103(4):515–531. doi: 10.1001/archinte.1959.00270040001001. [DOI] [PubMed] [Google Scholar]
- 9.Stone WJ, Swift GW. Influenza and influenzal pneumonia at Fort Riley, Kansas. JAMA. 1919;72(7):487–493. [Google Scholar]
- 10.Abrahams A, Hallows N, French H. A further investigation into influenza-pneumococcal and influenzo-streptococcal septicaemia. Lancet. 1919:1–11. [Google Scholar]
- 11.Scadding JG. Lung changes in influenza. Quart J Med. 1937;6:425–465. [Google Scholar]
- 12.Nicholson KG. In: Human influenza. Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza.London: Blackwell Science, Ltd.; 1998. pp. 222–223. [Google Scholar]
- 13.Finland M, Barnes MW, Samper BA. Influenza virus isolations and serological studies made in Boston during the winter of 1943–1944. J Clin Invest. 1945;24:192–208. doi: 10.1172/JCI101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186(3):341–350. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 15.Peltola VT, Boyd KL, McAuley JL, Rehg JE, McCullers JA. Bacterial sinusitis and otitis media following influenza virus infection in ferrets. Infect Immun. 2006;74(5):2562–2567. doi: 10.1128/IAI.74.5.2562-2567.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MW, Schmidt JE, Rehg JE, Orihuela C, McCullers JA. Induction of pro- and anti- inflammatory molecules in a mouse model of pneumococcal pneumonia following influenza. Comp Med. 2007;57(1):12–18. [PMC free article] [PubMed] [Google Scholar]
- 17.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187(6):1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 18.McAuley JL, Hornung F, Boyd KL, et al. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe. 2007;2(4):240–249. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23(1 Suppl):S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 20.Peltola VT, Murti KG, McCullers JA. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J Infect Dis. 2005;192(2):249–257. doi: 10.1086/430954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60(5):759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- 22.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Childhood. Thorax. 2002;57 Suppl 1:i1–i24. doi: 10.1136/thorax.57.90001.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallares R, Linares J, Vadillo M, et al. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333(8):474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 25.Rahav G, McCullers JA, Engelhard D, et al. Invasive pneumococcal infections. A comparison between adults and children. Medicine (Baltimore) 1997;76(4):295–303. doi: 10.1097/00005792-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Metlay JP, Hofmann J, Cetron MS, et al. Impact of penicillin susceptibility on medical outcomes for adult patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2000;30(3):520–528. doi: 10.1086/313716. [DOI] [PubMed] [Google Scholar]
- 27.Feikin DR, Schuchat A, Kolczak M, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995–1997. Am J Public Health. 2000;90(2):223–229. doi: 10.2105/ajph.90.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullers JA, Tuomanen EI. Molecular pathogenesis of pneumococcal pneumonia. Front Biosci. 2001;6:D877–D889. doi: 10.2741/mccullers. [DOI] [PubMed] [Google Scholar]
- 29.Orman KL, English BK. Effects of antibiotic class on the macrophage inflammatory response to Streptococcus pneumoniae. J Infect Dis. 2000;182(5):1561–1565. doi: 10.1086/315861. [DOI] [PubMed] [Google Scholar]
- 30.McCullers JA, English BK. Improving therapeutic strategies for secondary bacterial pneumonia following influenza. Fut Virol. 2008;3(4) doi: 10.2217/17460913.3.4.397. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivetic TV, Bosnjak B, Hrvacic B, et al. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur J Pharmacol. 2006;539(1–2):131–138. doi: 10.1016/j.ejphar.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 32.Odio CM, Faingezicht I, Paris M, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991;324(22):1525–1531. doi: 10.1056/NEJM199105303242201. [DOI] [PubMed] [Google Scholar]
- 33.Lebel MH, Freij BJ, Syrogiannopoulos GA, et al. Dexamethasone therapy for bacterial meningitis. Results of two double- blind, placebo-controlled trials. N Engl J Med. 1988;319(15):964–971. doi: 10.1056/NEJM198810133191502. [DOI] [PubMed] [Google Scholar]
- 34.Quagliarello V, Scheld WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327(12):864–872. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 35.Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev. 2002;15(1):95–110. doi: 10.1128/CMR.15.1.95-110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt H, Zysk G, Reinert RR, et al. Rifabutin for experimental pneumococcal meningitis. Chemotherapy. 1997;43(4):264–271. doi: 10.1159/000239577. [DOI] [PubMed] [Google Scholar]
- 37.Nau R, Wellmer A, Soto A, et al. Rifampin reduces early mortality in experimental Streptococcus pneumoniae meningitis. J Infect Dis. 1999;179(6):1557–1560. doi: 10.1086/314760. [DOI] [PubMed] [Google Scholar]
- 38.Martinez JA, Horcajada JP, Almela M, et al. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2003;36(4):389–395. doi: 10.1086/367541. [DOI] [PubMed] [Google Scholar]
- 39.Garcia VE, Mensa J, Martinez JA, et al. Lower mortality among patients with community-acquired pneumonia treated with a macrolide plus a beta-lactam agent versus a beta-lactam agent alone. Eur J Clin Microbiol Infect Dis. 2005;24(3):190–195. doi: 10.1007/s10096-005-1295-9. [DOI] [PubMed] [Google Scholar]
- 40.Baddour LM, Yu VL, Klugman KP, et al. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170(4):440–444. doi: 10.1164/rccm.200311-1578OC. [DOI] [PubMed] [Google Scholar]
- 41.Tumpey TM, Garcia-Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79(23):14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302(5650):1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 43.Beigel JH, Farrar J, Han AM, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353(13):1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 44.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuen KY, Chan PK, Peiris M, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351(9101):467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 46.Cheung CY, Poon LL, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360(9348):1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 47.McCullers JA. Preparing for the next influenza pandemic. Pediatr Infect Dis J. 2008;27 doi: 10.1097/INF.0b013e3181684d41. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedson DS. Confronting an imminent pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis. 2008 doi: 10.1016/S1473-3099(08)70070-7. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]