Abstract
Over the past 20 years, there has been a concerted effort to expand our understanding of the neural circuitry involved in the pathogenesis of psychiatric disorders. Distinct neuronal circuits and networks have been implicated in obsessive compulsive disorder (OCD) and major depressive disorder (MDD) involving feedback loops between the cortex, striatum, and thalamus. When neurosurgery is used as a therapeutic tool in severe OCD and MDD, the goal is to modulate specific targets or nodes within these networks in an effort to produce symptom relief.
Currently, four lesioning neurosurgical procedures are utilized for treatment refractory OCD and MDD: cingulotomy, capsulotomy, subcaudate tractotomy, and limbic leucotomy. Deep brain stimulation (DBS) is a novel neurosurgical approach that has some distinct advantages over lesioning procedures. With DBS, the desired clinical effect can be achieved by reversible, high frequency stimulation in a nucleus or at a node in the circuit without the need to produce an irreversible lesion. Recent trials of deep brain stimulation in both OCD and MDD at several neuroanatomical targets have reported promising early results in highly refractory patients and with a good safety profile. Future definitive trials in MDD and OCD are envisaged.
Keywords: obsessive compulsive disorder, major depression, neurosurgery, deep brain stimulation, neurocircuitry
Introduction
No topic in psychiatry is as controversial as treating psychiatric disorders with neurosurgery. Since the advent of scientific study of the human brain, biologically oriented psychiatrists have sought to unravel its mysteries by identifying underlying neuropathological mechanisms resulting in psychiatric disorders. It was hoped that such insights would lead to cures for the most debilitating forms of these disorders through targeting brain regions that were responsible for generating psychopathology. Unfortunately, the history of surgical interventions in psychiatry has been marred by misguided applications of erroneous or overly simplistic approaches to psychiatric disease, sometimes resulting in significant negative outcomes. The birth and subsequent demise of psychosurgery in the 20th century serves as an important caution in considering neurosurgical interventions today. However, despite the success of psychotherapeutic and psychopharmacological strategies to treat mental illness, there still remains a large proportion of patients who suffer with persistent and debilitating illness that fails to respond to standard treatments.
Until recently, many of our treatments in psychiatry were discovered serendipitously, including the initial discoveries of the effectiveness of antidepressants and electroconvulsive therapy. 1 Over the past several decades, there has been a concerted effort by means of neuroimaging to expand our understanding of the neural circuitry involved in the pathogenesis of psychiatric disorders. In this context, a novel neurosurgical treatment intervention, deep brain stimulation (DBS), has emerged that targets focused regions of the brain in an effort to alleviate psychiatric symptoms. A psychiatrist in general practice may well wonder what this new technology is and what it may offer therapeutically that is clinically distinct and relevant. We will review here the current literature pertaining to neurosurgical interventions in psychiatry, with a special emphasis on DBS and the underlying neurocircuitry involved in two major psychiatric disorders; obsessive compulsive disorder (OCD) and major depressive disorder (MDD).
History
Neurosurgical procedures to treat psychiatric disorders have been controversial, in part due to the significant morbidity associated with earlier approaches and, up to now, the irreversible nature of the procedure. In the past, there was also a lack of systematic follow-up to measure long-term functional outcomes following such interventions. Finally, the indiscriminate use of earlier crude neurosurgical procedures, such as prefrontal and transorbital lobotomy, resulted at times in irreversible personality change and cognitive decline. By the mid-1950s, over 30,000 frontal lobotomies were performed in the US alone.2,3 Although some patients benefited from this procedure, many suffered permanent adverse consequences. Thus, psychosurgery, a term originally coined by Egas Moniz, who was awarded the Nobel prize for his efforts in pioneering prefrontal leucotomy, ultimately fell into disrepute.
Given this troubled history, current approaches in neurosurgery to psychiatric disease are proceeding with due caution. In contemporary practice, patients must meet operationalized criteria for severity, chronicity, and disability and have a demonstrated inability to respond to standard available treatments, including psychopharmacology and psychotherapy. When surgical interventions are being considered, a multidisciplinary team consisting of psychiatrists, neurosurgeons, and other specialists involved in the care of the patient must review each case carefully to establish that conventional treatment has indeed failed and that a neurosurgical intervention is warranted. From an ethical standpoint, neurosurgery should be restricted to patients who have the requisite decision-making capacity, and the surgery must only be performed to restore function and relieve suffering. Needless to say, surgery should never be performed for political, law enforcement, or other nonmedical, sociological purposes.4
Finally, with the level of precision that is available with modern neurosurgical approaches, the emphasis has shifted away from ablative or lesioning procedures, however precisely placed they might be. Rather, the focus is one of stimulation or inhibition (modulation) of nodes or targets within neural circuits by means of precisely placed electrodes with the object of restoring normal activity and function within the identified circuit. A major virtue of this approach is the move away from irreversible or ablative lesions to one of reversible neuromodulation or what is termed functional neurosurgery.
Brain Regions of Interest in OCD and Depression
Our understanding of the complex neurological underpinnings of mood and anxiety syndromes has improved over the past several decades, but much remains unknown. One reason for this is the lack of sophisticated animal models for these disorders, in part, because they are uniquely situated in our conscious subjectivity as humans. Analogous to neurological disorders, our understanding of the neurobiology of psychiatric disorders began with lesioning studies. Based on the seminal work of neurologists studying aphasias and movement disorders, researchers attempted to localize psychiatric syndromes to specific neuroanatomic regions. Once so defined, in theory the “abnormal” brain area could be surgically modulated for clinical benefit.
However, it has become clear that psychiatric syndromes cannot be localized in a single, so-called “abnormal” brain region. OCD and MDD are not located in a specific region of the brain. Rather, mood and anxiety disorders involve immensely complex interconnected systems or networks of organization within the brain. The current approach in treating psychiatric disorders neurosurgically is to modulate specific targets or nodes within these networks in an effort to produce symptom relief.2
OCD
OCD is an often-debilitating psychiatric disorder that is characterized by anxiety-provoking intrusive thoughts (obsessions) leading to compulsive behaviors or mental rituals to temporarily decrease the anxiety provoked by the obsessions. The 12-month prevalence of OCD in the US is approximately one percent.5 Symptoms generally begin in childhood and adolescence and often result in severe impairments in social and occupational functioning. Currently, behavioral psychotherapy and high-dose serotonergic antidepressant medications are considered to be the standard of care (APA Practice Guidelines, 2007). Follow-up studies, however, suggest suboptimal outcomes with current treatments with complete sustained remission of symptoms occurring in only 12 to 20 percent of patients receiving standard care.6 With more aggressive or optimized pharmacotherapy and behavioral therapies, an estimated 10 percent of OCD patients still manifest severe, intractable illness.7
Etiology
The etiology of OCD is currently hypothesized to relate to a combination of genetic and environmental factors. Epidemiological studies, including family and twin studies, strongly support a genetic component for OCD.8 Recent evidence highlights an abrupt onset of OCD symptoms in some cases in the context of group A beta-hemolytic streptococcal (GABHS) infection, implying that environmental factors may also contribute to the etiology of this disorder.9
Many authors have commented on obsessions and compulsions as being similar to involuntary motor behaviors. Freud hypothesized this connection in his psychoanalytic case report of the “Rat Man” stating, “a thought-process is obsessive or compulsive when, in consequence of an inhibition (due to a conflict of opposing impulses) at the motor end of the psychical system, it is undertaken with an expenditure of energy which is normally reserved for actions alone.”10 Other observers have also commented on the relationship of OCD to movement disorders, including Sydenham’s chorea, Tourette’s syndrome, Huntington’s disease, and involuntary tics.11
Recent studies have highlighted genetic links between Tourette’s syndrome and OCD, suggesting a similar neurological substrate for these disorders, specifically in the basal ganglia.8 Metabolic and volumetric neuroimaging studies of patients with OCD reveal abnormalities in several areas of the brain, including the caudate nucleus of the basal ganglia, as well as the orbitofrontal cortex.7 The cingulate gyrus also has demonstrated hypermetabolism in patients with OCD.12
Circuitry
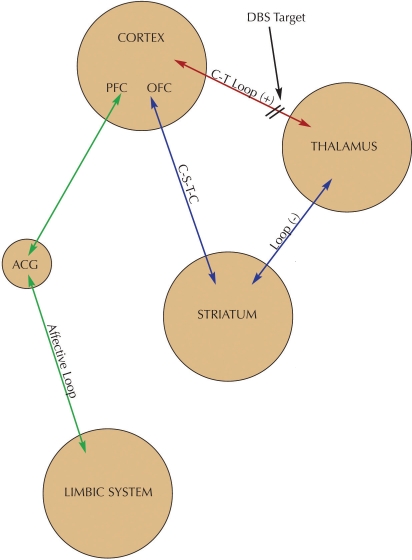
Based on these findings, distinct neuronal circuits have been implicated in symptoms of OCD involving feedback loops between the cortex, striatum, and thalamus. A multicircuit hypothesis of OCD states that the primary pathogenic mechanism is a dysregulation of the basal ganglia and limbic striatal circuitry working in concert with portions of the orbitofrontal and anterior cingulate cortex. Simplified, one can postulate the following three components to this model (Figure 1):2
FIGURE 1.
Neural circuitry of OCD and DBS target. Hyperactivity in the CT loop or hypoactivity of the C-S-T-C loop may produce OCD symptoms. DBS targets the C-T loop and normalizes activity in the circuit.
KEY:
- PFC:
prefrontal cortex
- OFC:
orbitofrontal cortex
- C-T:
corticothalmic
- C-S-T-C:
cortico-striatal-thalamic-cortical
- ACG:
anterior cingulate gyrus
- OCD:
obsessive compulsive disorder
- DBS:
deep brain stimulation
A positive feedback loop from the orbital and prefrontal cortex to the thalamus via the anterior limb of the internal capsule. This cortico-thalamic pathway is excitatory and bidirectional.
A circuit linking the orbitofrontal cortex, caudate nucleus, globus pallidus, and the thalamus, known as the CSTC loop (cortex-striatum-thalamus-cortex). The overall output of this pathway is inhibitory and is thought to serve as a counterweight to the excitatory positive feedback loop as described in this article. This inhibitory pathway also receives serotonergic projections from the midbrain into the striatum.
A component linking portions of the limbic system, including the hippocampus, mammillary bodies and fornix, to the thalamus to the anterior cingulate cortex (ACC). These connections are hypothesized to contribute to the affective anxiety component of OCD symptoms.
Bringing together these three components of the circuit, OCD symptoms occur when there is an abnormal positive feedback in the orbito-fronto-thalamic circuit (# 1) that is, in turn, inadequately inhibited or modulated by the CSTC loop (# 2). One would then expect OCD symptoms to appear when the CSTC loop is abnormally decreased (too little inhibition), or when orbitofronto-thalamic activity is abnormally increased (too much excitation). From a therapeutic standpoint, increasing activity of the CSTC loop or decreasing activity of the orbitofronto-thalamic loop would be expected decreased symptoms of OCD. Lastly, decreasing activity in the limbic component of the circuit (# 3) would decrease the distressing negative affects associated with obsessions.
MDD
MDD is a complex and heterogeneous disorder that is highly prevalent, recurrent, and disabling. It is a major cause of disability and functional impairment worldwide. A large proportion of sufferers, in the range of 20 to 40 percent, fail to benefit adequately from currently available treatments (e.g., antidepressants, psychotherapies, and electroconvulsive therapy), due to either a lack of efficacy or lack of tolerability of our current treatment options.13
The etiology of depression is complex and still only partially understood. Genetics, early environmental factors, and neurohormonal factors have all been implicated in the pathophysiology of depression. A neurological basis for depression has been posited based on certain disorders having a higher association with depression, as seen with left hemisphere strokes, certain types of epilepsy, Huntington’s disease, and numerous other neurodegenerative disorders and injuries to discrete brain regions. Recent evidence suggests that specific neuronal pathways are implicated in depression. It is hypothesized that depressive symptoms occur when these neural systems do not exhibit appropriate, adaptive plasticity in response to external stimuli such as stressors. The dysfunction of specific pathways that help promote neuronal plasticity might also contribute to the depressive symptomatology.14
Systems model of depression
Depression is not the result of dysfunction in a single brain region or of a single neurotransmitter system. It can instead be conceptualized as a systems-level disorder affecting discrete but functionally integrated pathways. The symptoms of depression are not simply the result of one or more of these pathways not functioning appropriately, but also a failure of the other components of the system to maintain homeostasis in times of increased stress to the organism.15
Circuitry
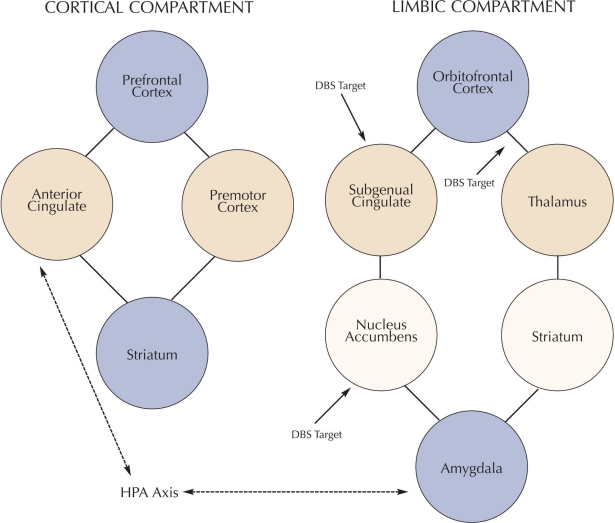
Neurobiological correlates of depressive illness can be grouped into the following three main components: cortical, subcortical, and limbic (Figure 2):
FIGURE 2.
Neural circuitry of MDD and DBS targets. Circuitry is divided into cortical and limbic compartments with links between amygdala, ACG, and HPA axis forming a modulatory circuit between the cortical and limbic compartments. DBS interventions for MDD target nodes within the limbic compartment.
KEY:
- DBS:
deep brain stimulation
- MDD:
major depressive disorder
- HPA:
hypothalamic-pituitary axis
Cortical component. This component appears to give rise to the psychomotor and cognitive aspects of depressive symptoms and consists of the prefrontal cortex, the dorsal portion of the anterior cingulate gyrus, and areas of the premotor cortex. This cortical component has access to the striatum and then creates a feedback loop via the thalamus.
Subcortical component. This component involves the affective experiencing of depressive symptoms, including anhedonia and sadness. This aspect of the neural circuit includes, among others, the subgenual anterior cingulate (Brodman’s area 25), the orbitofrontal cortex, and limbic structures in the brain involved with negative emotions, including the nucleus accumbens and amygdala. This component also interacts with the striatum and subsequently the thalamus to create a loop. Brain imaging research and functional blood flow studies (positron emission tomography [PET] and functional magnetic resonance imaging [fMRI]) support depressive illness involving decreases in cortical regions with relative increases in limbic areas.11
Modulatory component. It is postulated that a modulatory component regulates the corticol and subcortical circuits and includes the critical neuroendocrine aspects of depressive symptoms. This “modulating pathway” involves the amygdala, pregenual anterior cingulate cortex, and the hypothalamic-pituitary-adrenal axis. It is hypothesized that it mediates the cortical and limbic circuits via inhibitory projections to these circuits.2
Neurosurgical Interventions in OCD and MDD
Frontal leucotomy, the first applied neurosurgical procedure for psychiatric symptoms, was a gross method of interrupting the white matter tracts associated with the frontal cortex. Unfortunately, such procedures were performed indiscriminately without careful patient selection and were associated with serious neuropsychiatric morbidity. Recent approaches to treating treatment-refractory patients with neurosurgery differ substantially from these early interventions. Surgery is reserved for only the most severe of cases, when psychopharmacological and or depression. On the basis of standard outcome measures, 36 to 50 percent of patients were considered to be treatment responders.26 A recent study by Cho, et al., of 16 patients with intractable major affective disorders who underwent a limbic leucotomy, followed patients for seven years post-surgery. In the seven-year follow-up, mean scores on the Hamilton Depression Rating Scale and the Beck Depression Inventory declined significantly from 42±5.76 to 20±11.98 (p<0.01) and from 32±9.13 to 19±14.29 (p<0.05), respectively. There was no surgical mortality, and only three patients experienced temporary minor complications.27
Neuromodulation
Deep brain stimulation has several advantages over the lesioning procedures as just described. Surgeons using DBS can potentially achieve a clinical effect without producing an irreversible lesion. In DBS procedures, stimulation electrodes are implanted into specific brain regions and continuous electrical high frequency stimulation is delivered from an implanted, externally programmable pulse generator, similar to a cardiac pacemaker. DBS is reversible in that the stimulator can be turned on or off, and the output of the device and stimulation can be controlled at the discretion of the clinician. This also allows researchers to create sham conditions that allow for blinded placebo controlled trials, a method that is unavailable for practical and ethical reasons in lesioning operations. However, DBS still requires neurosurgery and has the potential for serious medical and neurological side effects. Symptomatic hemorrhage, infection, and seizure may occur in 1 to 4 percent of cases, but death is exceedingly rare.28
Contemporary use of clinical brain stimulation began in 1987 with Benabid, et al., reporting on the first successful use of thalamic DBS as a treatment for Parkinson tremor.28 The subthalamic nucleus (STN) subsequently became a prime target in Parkinson’s disease, with the finding that STN lesions reversed motor signs of Parkinsonism in experimental animals. Currently, DBS is FDA approved to treat Parkinson’s disease and essential tremor. The mechanism of action of DBS is still unknown. Because clinical effects of DBS are similar to lesioning neurosurgery, it is hypothesized that DBS disrupts pathological neural activity, although there are several competing hypotheses regarding how this occurs.29
DBS in OCD
Recent trials of deep brain stimulation in OCD at several targets have indicated distinct benefit. One target is the anterior limb of the internal capsule, the site used in capsulotomy. Targeting of the anterior capsule is believed to disrupt activity in the loop fibers that connect the cortex with the thalamus, thus, in theory, disrupting that pathological circuit.
Greenberg, et al., reported on results from data collected by four groups, which worked in collaboration over eight years, for a total of 26 patients.30 The percentage of patients meeting the full response criterion (≥35% YBOCS decrease) increased from 28 percent at one month to 61.5 percent at last follow-up. Conversely, the percentage with less than a 25-percent YBOCS decrease (no response) declined from 68 percent at one month to 27 percent at last follow-up. Overall, a total of 73 percent of patients had at least a 25-percent YOBCS improvement at last follow-up; a large majority of those improvements were a 35-percent or greater YBOCS reduction. Depression and anxiety also improved, as did self care, independent living, and work, school and social functioning. Surgical adverse effects included two asymptomatic, intracerebral hemorrhages, a single seizure, and a superficial wound infection. Device-related adverse events included a break in a stimulating lead and extension wire requiring a replacement in one patient each. Psychiatric adverse effects included transient hypomanic symptoms and worsened depression and OCD when DBS was interrupted by stimulator battery depletion.
Abelson, et al., examined the effects of DBS for treatment-refractory OCD in four patients with leads placed bilaterally in the anterior limbs of the internal capsule in a blinded, on-off design, with one patient experiencing substantial benefit in mood, anxiety, and OCD symptoms both during the blinded study and open, long-term follow-up. One patient committed suicide despite her OCD symptoms improving as per self report. One patient showed moderate benefit during open follow-up.31 Other small case series and a case report have reported benefit.32,33 Other brain regions have also received attention, including the nucleus accumbens with one case series reporting observed, but not quantified, clinical improvement in three out of four patients.34
DBS in depression
Based on neuroimaging findings that implicate hyperactivity of the subgenual cingulate region (BA 25) in treatment-resistant depression and association of decreases in cg25 activity with clinical improvement, Mayberg and colleagues targeted this area in a study of six patients implanted with bilateral leads in this area.35 At one month postoperatively, 2 of the 6 patients met the criteria for clinical response, which was defined as a decrease in the Hamilton Depression Rating Scale (HRDS-17, also known as HAM-D-17) of 50 percent or more from the pretreatment baseline. At the six-month study endpoint, antidepressant response was maintained in four subjects (66%). Three of these responders attained remission or near remission of illness. From a safety standpoint, two of the patients developed persistent wound infections and required explantation of the device.
Another site utilized in DBS for depression is the nucleus accumbens, a site implicated primarily in the reward systems of the brain. Schlaepfer, et al., have reported on three patients suffering psychotherapeutic alternatives have been utilized with no benefit, and a detailed informed consent process, which involves both patient and family, are central.
Stereotaxis
Advances in neurosurgery now allow precise lesioning techniques via stereotaxis. Stereotaxis is the method by which neurosurgeons visualize the brain as volume in a three-dimensional space. The brain is then referenced to a specific coordinate system that allows for precision in reaching subcortical brain structures with minimal disruption of the surrounding tissue. Neurosurgeons currently use stereotactic techniques with computer-based functional neuroimaging and physiologic recordings to allow for submillimeter accuracy.2 Currently, there are four ablative neurosurgical procedures utilized for treatment-refractory OCD and MDD: cingulotomy, capsulotomy, subcaudate tractotomy, and limbic leucotomy. Each of these procedures seeks to modulate the activity of the neurocircuitry described previously, including the interactions between various components of the frontal cortex and cingulate cortex and their interactions with the basal ganglia and the thalamus.
Lesioning procedures
Cingulotomy
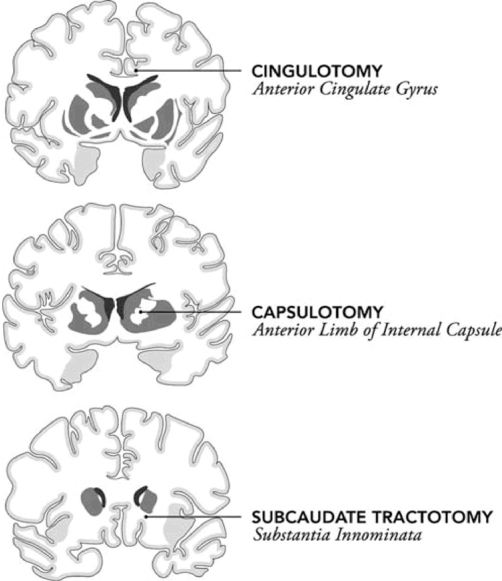
Neurosurgery of the cingulate gyrus has been reported since the 1940s, with Freeman and Watts reporting that severing fibers from the cingulate gyrus led to an improvement of anxiety symptoms and Whitty reporting a bilateral resection of the cingulate gyrus in 1952.2 Currently stereotactic, bilateral lesioning of the cingulate gyrus is the most common neurosurgical procedure for treatment refractory psychiatric syndromes, specifically OCD. The cingulate gyrus is critical in corticostriatal-thalamic pathways in mediating the transfer of information from the anterior cingulate cortex to the orbitofrontal cortex and to the limbic system.16 In a cingulotomy, the anterior portion of the cingulate gyrus is lesioned, interrupting tracts between the cingulate gyrus and the frontal lobes, eliminating the efferent projections of the anterior cingulate cortex.11 See Figure 3.
FIGURE 3.
Sites of lesioning neurosurgical procedures for OCD and MDD. Limbic leucotomy combines the cingulotomy and subcaudate tractotomy procedures. [Adapted from Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery 2007;61(1):1-11]
Results
One retrospective study demonstrated a mean 30-percent improvement in patients (n=33) who underwent cingulotomy for OCD.17 Eighteen of these patients were included in a follow-up study with a 30-percent success rate.18 Jung, et al., followed 17 patients with treatment refractory OCD who had received bilateral stereotactic cingulotomy for a period of 24 months and reported response in eight patients (47%) with a mean reduction of 48 percent in their baseline Yale-Brown Obsessive Compulsive Scale (YBOCS) scores.19
In a recent prospective study, Dougherty, et al.,18 reported on 44 patients undergoing cingulotomy for OCD, with 32 percent meeting criteria for response and 14 percent meeting criteria for partial response at 32 weeks post-surgery. Cosgrove, et al., recently reported on the safety of over 800 cingulotomies performed over a 40-year period at Massachusetts General Hospital, with no deaths and only two infections being reported.20 For intractable major depression, response rates have been high, with a 68-percent response rate (n=198) reported by Ballantine21 and a 60-percent response rate (n=34) by Greenberg, et al.22 A recent study by Steele, et al., reported on eight patients with treatment refractory MDD undergoing anteriorly placed cingulate cortex lesions with beneficial results.21
Capsulotomy
Developed by Lars Leksell and Jean Talairach in the 1940s, the anterior capsulotomy has been in use for treatment refractory OCD and depression for over five decades.2 This surgery targets the anterior limb of the internal capsule, which serves as a relay route between cortical structures and the thalamus. A prospective study of 15 patients who underwent bilateral anterior capsulotomy for OCD reported that 53 percent of the patients showed a 33-percent reduction in symptoms, 29 percent of the cases experienced a 50-percent reduction, and 17 percent were improved by as much as 66 percent. Complications were observed in three cases: one with transitory hallucinations, one with a single epileptic seizure, and one case who developed a progressive behavior disorder that became permanent.23 A more recent study by Liu, et al., reported on 35 patients with treatment refractory OCD who underwent stereotactic bilateral capsulotomy with a three-year follow-up. Results were robust: Twenty patients became OCD symptom-free (57%), 10 experienced significant improvement (29%), and five experienced no meaningful improvement (14%).24
Subcaudate tractotomy
Another neurosurgical procedure that is hypothesized to exert its effects by interrupting the relay between the cortex and thalamus via the striatum is subcaudate tractotomy. This procedure was developed by in London by Knight in 1965 and has primarily been used for treatment refractory depression. The target site is a region of white matter localized beneath the head of the caudate known as the substantia innominata.2 Poynton, et al., prospectively studied 23 patients undergoing a stereotactic subcaudate tractotomy for treatment refractory depression. There was no significant correlation except for the six-month assessment when lower Hamilton scores were found to be associated with better global outcome.25 Greenberg, et al., reviewed outcomes with subcaudate tractotomy that included 1,300 patients and reported 40 to 60 percent of patients benefiting.22
Limbic leucotomy
This procedure was developed by Kelly and Richardson in the 1970s. The surgery is essentially a combination of subcaudate tractotomy and cingulotomy. Montoya, et al., conducted preoperative evaluations and postoperative follow-up assessments of efficacy and complications for 21 patients who underwent limbic leucotomy for OCD from treatment-resistant depression who had not responded to trials of pharmacotherapy, psychotherapy, and electroconvulsive therapy, and were implanted with bilateral DBS electrodes in the nucleus accumbens.36 Stimulation parameters were modified in a double-blind manner, and clinical ratings were assessed at each modification. Clinical ratings improved in all three patients when the stimulator was on, and worsened in all three patients when the stimulator was turned off. Effects were observable immediately, and no side effects occurred in any of the patients.
Lastly, in a third target area, in the largest study of DBS in MDD reported to date, 15 chronically depressed patients underwent bilateral DBS implantation in the ventral internal capsule/ventral striatum (VC/VS). Responses were seen in 7/15 (47%) at six months, 5/11 (45.5%) at 12 months, and 8/15 (53.3%) at last follow-up. This was accompanied by long-term improvements in depression severity, functioning, quality of life, and short-term memory. Safety outcomes were good with no hemorrhages, infections, or neurological deficits reported.37
Conclusions
From this overview of surgical procedures and DBS techniques for OCD and treatment-resistant depression, it is clear that although much remains unknown about the underlying neurobiology of these disorders, progress in helping the most severely afflicted is being made. Currently, large scale, multicenter, sham-controlled trials for DBS in major depression are being planned in the US. Thus, it is likely that further progress by means of functional neurosurgery approaches will be made before too long in this field. If this occurs, it will be of great benefit to the many patients suffering with OCD and MDD who have not responded to traditional treatments and remain extremely disabled by their illnesses.
References
- 1.Shorter E.A History of Psychiatry: From the Era of the Asylum to the Age of Prozac. 1997New York, NY: John Wiley and Sons, Inc.239–287 [Google Scholar]
- 2.Kopell BH, Greenberg B, Rezai AR.Deep brain stimulation for psychiatric disorders. J Clin Neurophysiol. 200421(1)51–67 [DOI] [PubMed] [Google Scholar]
- 3.Kramer M.The 1951 survey of the use of psychosurgery. Mettler FA, Overholster W.Proceedings of the Third Research Conference on Psychosurgery. 1954Washington, DC: United States Government Printing Office; 159–168(USPHS publication 221) [Google Scholar]
- 4.Nuttin B, Gybels J, Cosyns P, et al. Deep brain stimulation for psychiatric disorders. Neurosurg Clin N Am. 200314(2)xv–xvi [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 200562(6)617–627[see comment][erratum appears in Arch Gen Psychiatry. 2005;62(7):709 Note: Merikangas, Kathleen R [added]]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen JL, Goodman WK, Keller MB, et al. Patterns of remission and relapse in obsessive-compulsive disorder: a 2-year prospective study. J Clin Psychiatry. 199960(5)346–351 [DOI] [PubMed] [Google Scholar]
- 7.Saxena S, Rauch S.Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 20003(23)563–589 [DOI] [PubMed] [Google Scholar]
- 8.Grados MA, Riddle MA, Samuels JF, et al. The familial phenotype of obsessive-compulsive disorder in relation to tic disorders: the Hopkins OCD family study. Biologic Psychiatry. 200150(8)559–565 [DOI] [PubMed] [Google Scholar]
- 9.Murphy ML, Pichichero ME.Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med. 2002156(4)356–361 [DOI] [PubMed] [Google Scholar]
- 10.Freud S.Two case histories: “Little Hans” and the “Rat Man.” Standard Edition of the Complete Works of Sigmund Freud. 1909New York, NY: W.W. Norton & Company Ltd; pi–vi [Google Scholar]
- 11.Panksepp J. Textbook of Biological Psychiatry. Hoboken, NJ: Wiley-Liss, Inc.; 2004. [Google Scholar]
- 12.Saxena S, Brody AL, Schwartz JM, Baxter LR.Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Supplementum. 19983526–37 [PubMed] [Google Scholar]
- 13.Rush AJ.STAR*D: what have we learned? Am J Psychiatry. 2007164(2)201–204 [DOI] [PubMed] [Google Scholar]
- 14.Vaidya VA, Duman RS.Depresssion—emerging insights from neurobiology. Br Med Bull. 20015761–79 [DOI] [PubMed] [Google Scholar]
- 15.Mayberg HS.Positron emission tomography imaging in depression: a neural systems perspective. Neuroimag Clin North Am. 200313(4)805–815 [DOI] [PubMed] [Google Scholar]
- 16.Lipsman N, Neimat JS, Lozano AM.Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery. 200761(1)1–11 [DOI] [PubMed] [Google Scholar]
- 17.Jenike MA, Baer L, Ballantine T, et al. Cingulotomy for refractory obsessive-compulsive disorder. A long-term follow-up of 33 patients. Arch Gen Psychiatry. [see comment].199148(6)548–555 [DOI] [PubMed] [Google Scholar]
- 18.Dougherty DD, Baer L, Cosgrove GR, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry 2002159(2)269–275 [DOI] [PubMed] [Google Scholar]
- 19.Jung HH, Kim CH, Chang JH, et al. Bilateral anterior cingulotomy for refractory obsessive-compulsive disorder: long-term follow-up results. Stereotact Function Neurosurg. 200684(4)184–189 [DOI] [PubMed] [Google Scholar]
- 20.Cosgrove GR, Rauch SL.Stereotactic cingulotomy. Neurosurg Clin North Am. 200314(2)225–235 [DOI] [PubMed] [Google Scholar]
- 21.Ballantine HT, Jr, Bouckoms AJ, Thomas EK, Giriunas IE.Treatment of psychiatric illness by stereotactic cingulotomy. Biologic Psychiatry. 198722(7)807–819 [DOI] [PubMed] [Google Scholar]
- 22.Greenberg BD, Price LH, Rauch SL, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin North Am. 200314(2)199–212 [DOI] [PubMed] [Google Scholar]
- 23.Oliver B, Gascón J, Aparicio A, et al. Bilateral anterior capsulotomy for refractory obsessive-compulsive disorders. Stereotac Function Neurosurg. 200381(1–4)90–95 [DOI] [PubMed] [Google Scholar]
- 24.Liu K, Zhang H, Liu C, et al. Stereotactic treatment of refractory obsessive compulsive disorder by bilateral capsulotomy with 3 years follow-up. J Clin Neurosci. 200815(6)622–629 [DOI] [PubMed] [Google Scholar]
- 25.Poynton A, Kartsounis L, Bridges P.A prospective clinical study of stereotactic subcaudate tractotomy. Psychol Med. 199525(4)763–770 [DOI] [PubMed] [Google Scholar]
- 26.Montoya A, Weiss AP, Price BH, et al. Magnetic resonance imaging-guided stereotactic limbic leukotomy for treatment of intractable psychiatric disease. Neurosurgery. 200250(5)1043–1049 [DOI] [PubMed] [Google Scholar]
- 27.Cho DY, Lee WY, Chen CC.Limbic leukotomy for intractable major affective disorders: a 7-year follow-up study using nine comprehensive psychiatric test evaluations. J Clin Neurosci. 200815(2)138–42 [DOI] [PubMed] [Google Scholar]
- 28.Hardesty DE, Sackeim HA.Deep brain stimulation in movement and psychiatric disorders. Biologic Psychiatry. 200761(7)831–835 [DOI] [PubMed] [Google Scholar]
- 29.Lozano AM, Eltahawy H.How does DBS work? Suppl Clin Neurophysiol. 200457733–736 [DOI] [PubMed] [Google Scholar]
- 30.Greenberg BD, Gabriels LA, Malone DA, Jr, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2008 May 20; doi: 10.1038/mp.2008.55. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abelson JL, Curtis GC, Sagher O, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biologic Psychiatry. 200557(5)510–516 [DOI] [PubMed] [Google Scholar]
- 32.Cosyns P, Gabriels L, Nuttin B.Deep brain stimulation in treatment refractory obsessive compulsive disorder. Verh K Acad Geneeskd Belg. 200365(6)385–399 [PubMed] [Google Scholar]
- 33.Anderson D, Ahmed A.Treatment of patients with intractable obsessive-compulsive disorder with anterior capsular stimulation. J Neurosurg. 200398(5)1104–1108[see comment] [DOI] [PubMed] [Google Scholar]
- 34.Sturm V, Lenartz D, Koulousakis A, et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive and anxiety disorders. J Chem Neuroanat. 200326(4)293–299 [DOI] [PubMed] [Google Scholar]
- 35.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 200545(5)651–660 [DOI] [PubMed] [Google Scholar]
- 36.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 200833(2)368–377 [DOI] [PubMed] [Google Scholar]
- 37.Rezai A. Deep brain stimulation (DBS) for highly treatment-resistant depression: long-term outcomes from a prospective multicenter trial.; Chicago IL: Apr 29, 2008. [Google Scholar]