Abstract
Environmental enrichment of laboratory mice can improve the quality of research, but debate arises over the means of enrichment and its ability to be used in a sterile environment. One important form of enrichment is nesting material. Mice in the wild build dome-shaped, complex, multilayered nests, but this behavior is not seen in the laboratory, perhaps due to inappropriate nesting material rather than the nest-building ability of the mice. Here we focus on the use of naturalistic nesting materials to test whether they improve nest quality through the use of a ‘naturalistic nest score’ system; we also focus on materials that can be sterilized and easily used in existing housing systems. We first determined whether C57BL/6J mice build naturalistic nests when given shredded paper strips. We then compared these shredded paper strips with other commonly used nesting enrichments (facial tissues and compressed cotton squares). Nests were scored for 6 d. We found that the shredded paper strips allowed the mice to build higher quality nests than those built with any of the other materials. Nests built with tissues were of intermediate quality, and nests built with compressed cotton squares were of poor quality, similar to those built by the control group. These results suggest that C57BL/6J mice given appropriate nesting materials can build nests similar to those built by their wild counterparts.
Abbreviation: GLM, generalized linear model
Environmental enrichment of laboratory mice has the potential to improve mouse wellbeing, the public perception of mouse research, and the quality of scientific data.2,16,32 However considerable debate exists over how to provide enrichment for mice, as well as the benefits and costs of practical enrichments.4,16,24,27,31 This controversy stems from 2 related issues: most of the enrichments examined in the mouse literature are impractical in a real housing facility; and most commercial ‘enrichments’ have simply been assumed to be beneficial, without testing their effects on the animal. The concept of ‘biological relevance’ provides a way to address these issues as well as a framework for developing practical, effective enrichments.16,27,32 Here we illustrate this approach, using the development of a nest-building enrichment as an example.
The term environmental enrichment, at its broadest, is used for any change in husbandry or caging intended to benefit the animal's wellbeing.5 Changes in husbandry intended to benefit the animal may not always do so for 2 main reasons.32 First, the animal may not respond to the enrichment in the way intended. An animal may simply fail to perceive an enrichment as meaningful, it may find it aversive (as is the case for defensive burying of objects placed in the cage14), or it may try to defend the enrichment from its cage mates. For example, providing mice with shelves and nest boxes (resources that are thought to be beneficial but can be monopolized) can lead to increased aggression, immunosupression, and prolonged infections.1,23,27 Second, an enrichment may improve one measure of wellbeing but compromise another. For example, individually ventilated cages (IVC systems) reduce ammonia levels, airborne disease transmission, and the amount of human handling, but mice find the increased ventilations rates aversive,3 show elevated levels of fear or anxiety,21 and demonstrate immune suppression.25
Several authors have argued that only biologically relevant enrichments—those that allow animals to control stressors in their environment—will actually benefit wellbeing.16,27,32 For example, when provided with nesting material in ventilated cages, mice no longer find ventilation aversive.3 Furthermore, by giving animals homeostatic control over these stressors, such enrichments should reduce variability and benefit scientific outcomes16,32 because the environment in which the animal lives considerably affects all aspects of physiology. For example, unenriched or socially isolated animals typically show altered brain development and physiology,28 and the behaviors associated with unenriched environments may indicate altered brain function.16 Various lines of evidence suggest enrichment renders brains more ‘normal,’ not merely ‘different,’ in comparison to standard-housed animals. For instance, marsh tits (Parus palustris), which are naturally food-storing birds, have a greater hippocampus size than do nonfood storing Parus species. In captivity, however, only marsh tits with food-storing experience show greater hippocampal volume and neuron number, as well as fewer apoptotic cells, compared with those maintained with standard housing.11
A subset of biologically relevant enrichments incurs unintended costs. For example, shelters are biologically relevant, but they can induce aggression in group-housed animals.27,32 These and other conditionally beneficial enrichments may benefit certain types of animals under certain conditions—for example, shelters may provide excellent enrichment for singly housed mice.32 Although some forms of enrichment may be detrimental to some animals, providing nesting material to mice does not appear to incur these kinds of disadvantages 27,32 (but also see reference 20). Mice show strong preferences for cages that provide nesting material (reviewed in references 19 and 27), and nest building plays a central role in the natural history of wild mice, suggesting that nesting material may be a highly beneficial enrichment.
In the wild, pregnant females tend to build the most complex nests, but nest-building ability extends to males and nonpregnant females.7,10,22 Mice build nests to provide shelter from the elements, predators, and competitors, but also as a way to compensate for changes in external temperatures.22 Therefore, nests provide external insulation and create a less thermally stressful habitat.12 The nests typically are built in secluded areas, and their quality at or right after birth is critical for the survival of offspring.10 If the nests remain undisturbed from outsiders, they generally take on a bowl shape or a dome with an exit hole on 1 side; this shape is most conducive to the survival of offspring.10 However, nests also can resemble something as simple as a shelf, but this configuration is less advantageous to the success of litters.6,7,10 Dense colonies of wild house mice can contain a communal nest consisting of several mothers with litters,7 and some females with communal nests share them not only with their own litters but also eventually with the litters of their daughters.13
Therefore, mice in the wild are expert and flexible nest builders, and this behavior is central to their survival, particularly in terms of dealing with challenging environments. However, in the laboratory, the use of nesting enrichments and nest-building behavior can vary, particularly between strains.8,9,30 In particular, C57BL/6J mice often build rather poor, flat nests when provided with commercial nest-building enrichments.15 We hypothesized that this variability does not reflect a lack of importance of nest building to C57BL/6J mice but rather that the enrichments provided are not biologically relevant (that is, the mice did not perceive such enrichments as suitable nest-building material). Accordingly, we predicted that if provided with more naturalistic nest-building material, C57BL/6J mice would build nests equivalent to those of their wild counterparts.
We first performed a pilot study to determine whether C57BL/6J mice would build naturalistic nests when given shredded paper strips. Having identified a material that allowed mice to build naturalistic nests, we then compared these shredded paper strips with other nesting enrichments to test our hypothesis that poor nest quality reflects the material provided, not the nest-building ability of the mice. Throughout the study, we focused on materials that could be sterilized and easily used in existing housing systems. If these materials are found to be suitable for nest building, then their use is more likely to be implemented across laboratories.
Materials and Methods
Animal housing.
All mice (C57BL/6J) originally were obtained from Jackson Laboratories (Bar Harbor, ME). In accordance with the ‘3Rs,’ we reuse animals whenever possible: in this case, these animals had been used previously in behavioral experiments but had never been exposed to nesting material. Mice were housed in 7.25 × 11.5 × 5 in. standard, nonventilated, clear plastic cages (RC71UPC, Unicage, Altdesign, Siloam Springs, AR). Cages were lined with aspen bedding (Harlan Teklad, Madison, WI), and food (2019, Harlan Teklad) and water were available ad libitum. The average temperature in the room was 20.6 to 21.1 °C, and average humidity was 50%. The room was on a 14:10-h light:dark cycle (lights on, 0600 to 2000). Housing and procedures were approved by our Institutional Animal Care and Use Committee.
Nest scoring.
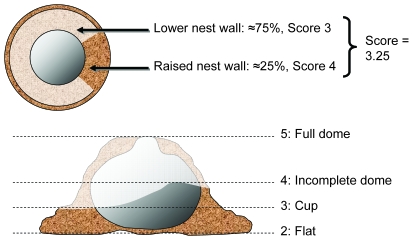
We initially intended to use a previously described nest scoring system,15 but the mice regularly built far better nests than the maximal score allowed on this scale. Another available scale9 was too qualitative (thus having very low resolution) and subjective (and therefore more likely to be inaccurate) for our purposes. We therefore derived a ‘naturalistic nest score’ (Table 1). In particular, mice were counted as having built a nest only if the nesting material contained a central hollow (Figures 1 and 2); a pile of material with mice underneath was not considered an actual nest. Nests then were further scored according to the height and closure of the walls surrounding the nest cavity. The score indicated the quality of the nest: higher scores indicated higher quality nests, whereas those with lower scores were of poor quality. In pilot work, the mean interrater reliability of our scale was 0.97.
Table 1.
Naturalistic nest scoring system
| Description of nesting material | Score |
| Undisturbed: nesting material has not been moved; no sign of interaction or manipulation of the material. | 0 |
| Disturbed: interaction with the nesting material is evident (for example disturbed, chewed, spread around the cage) but has not been gathered to a nest site. If a nest site is present in the regular bedding material, either there is no concentration of the material in the nest site, or the material is merely piled on top of the nest cavity in the regular bedding. | 1 |
| Flat nest: nesting material has been gathered to a form a nest site in the cage, identified by a clear nest cavity in the middle of the material, or between the material and the cage wall. The nest is a flattened saucer shape with no, or incomplete, walls. | 2 |
| Flat nest with 1 side that is less than half of a sphere. | 2.25 |
| Flat nest with 2 sides that are less than half of a sphere. | 2.50 |
| Flat nest with 3 sides that are less than half of a sphere. | 2.75 |
| Cup: nesting material has been gathered to form a nest site in the cage. The nest has identifiable walls that form a ‘cup’ or ‘bowl’ (similar to a shallow soup bowl), such that the walls would not reach the widest point of an imaginary sphere that would fill the nest hollow (‘half of a sphere’). | 3 |
| Cup-shaped nest with 1 side that is half of a sphere | 3.25 |
| Cup-shaped nest with 2 sides that are half of a sphere | 3.50 |
| Cup-shaped nest with 3 sides that are half of a sphere | 3.75 |
| Incomplete dome: bedding material has been gathered to form a nest site in the cage. The walls reach (and may close back over) the widest point of an imaginary sphere that would fill the nest hollow (‘half of a sphere’). | 4 |
| Incomplete dome with 1 side that is more than half of a sphere. | 4.25 |
| Incomplete dome with 2 sides that are more than half of a sphere. | 4.50 |
| Incomplete dome with 3 sides that are more than half of a sphere. | 4.75 |
| Complete dome: nesting material has been gathered to form a nest site in the cage. The walls completely enclose the nest hollow. A small (mouse-sized) exit hole may be found on the side or the top of the dome | 5 |
Scores of 0 and 1 were possible only if the mice had not made a nest site by using the nesting material (or bedding if no nesting material was present). If the material was gathered or piled but no hollowed-out nest cavity was present within the material, a score of 1 was given. Scores of 2 through 5 were based on imagining the nest as a flattened sphere, which can be divided into 3 horizontal sections dividing the hollowed-out nest cavity in the center. Because mice usually reinforce the walls of the nest by building out the base, the best way to visualize the completeness of the nest was by the roughly spherical cavity in the middle: the nest was assigned a score by first identifying the lowest point on its edge (that is, assigning a score of 2 through 5) and then adding an additional 0.25 for each quarter of the nest that had a higher wall.
Nests of bedding material were scored as described for nesting material.
Figure 1.
The naturalistic nest score system. Scores are based on the shape of the nest as well as on how much the walls are built up around the nest cavity in order to form a dome. Both a top view and a side view are shown.
Figure 2.
Sample nests and their corresponding scores.
Experiment 1: pilot study.
Animals.
A total of 36 C57BL/6J mice (age: 3.5 mo) were housed in single-sex groups of 3, with a total of 6 groups of male mice and 6 groups of female mice. Mice were reused from previous experiments and had no prior experience of nest-building.
Overall design.
Mice were split into 3 groups (2 cages of each sex) to assess 3 different nesting materials: 2 facial tissues (2.8 g; Kleenex, Kimberly-Clark, Neenah, WI), shredded paper strips (Enviro-dri Eco-bedding, Fibercore, Cleveland, OH), and the shredded paper strips (8 g) and 1 tissue. Mice were given the nesting material on day 1. Pictures were taken and nests were scored 24 h later (day 2) and again on day 3. Nesting materials were removed from the cage at the end of the study.
The shredded paper strips we used in this study were made of 1/8-in. strips of 100% recycled biodegradable paper fibers. The material is a sturdy opaque shredded and folded paper (Figure 3). As such, the synthetic bedding we provided resembles grass-like material that wild mice commonly use to build nests.6,22
Figure 3.
Example of a nest built with a combination of tissue and the shredded paper strips. Note how the tissue is intertwined with the shredded paper strips, creating a 2-layer nest.
Statistical methods.
Nest scores were compared by using a split-plot general linear model (GLM; version 14, Minitab Windows, Minitab, State College, PA) to accommodate repeated measures on cage. Therefore, cage was treated as a fixed, rather than random, blocking factor, because a cage of animals cannot be considered a random effect.18,26 Cage was nested within sex and treatment and crossed with day (of observation). For day, only the main effect was included. Model hierarchy required inclusion of the sex × treatment interaction in order to calculate the cage effect, but sex × treatment was not tested because we did not have any a priori hypotheses regarding this effect. Factors of interest were the sex and treatment main effects. Significant effects were examined post hoc with Tukey pairwise comparisons corrected to a family α level of 0.05. The assumptions of GLM (linearity, homogeneity of variance, and normality of error) were confirmed graphically post hoc,18 and the angular transformation was applied to meet these assumptions.
Experiment 2.
Animals.
A total of 32 C57BL/6J laboratory mice (age: 7.5 mo) bred from our existing colony were housed in pairs by gender, with a total of 8 pairs of male mice and 8 pairs of female mice. Housing conditions were equivalent to that in experiment 1. Mice had no prior experience of nest building before the experiment.
Overall design.
After determining in the pilot experiment that the shredded paper strips alone and with tissue provided suitable materials for nest building, we decided to confirm our data by comparing tissue, shredded paper strips, and compressed cotton squares (Nestlets, Ancare, Bellmore, NY), which are commonly provided to mice as nesting material. We also standardized the amounts of available nesting materials to control for a possible effect on nest-building ability.
Three different types of nesting materials were used: facial tissue, a commercially available nesting material comprising compressed cotton squares, and the shredded paper strips. Mice were split into 4 groups (2 cages of each gender), each of which received 70 g of aspen bedding and a different nesting material (8 g; controls received an additional 8 g of aspen bedding). Thus, all groups had a total of 78 g of bedding and nesting materials to manipulate for nest building. There were 16 cages of mice in the experiment in total.
Mice were grouped into 2 replicates, each containing 2 cages (1 male, 1 female) of each of the 4 treatments. Replicates were staggered by day so that day 1 of the first replicate occurred 1 d prior to day 1 of the second replicate. This timing was established to accommodate the limited number of video cameras available. Cages placed in the video stations were varied across days. Mice were placed in clean cages and given the assigned nesting material on day 1 at 1700. Material was placed at the end of the cage opposite the mouth of the water bottle, except for the control group for which the extra aspen bedding was spread uniformly across the cage. Pictures were taken, and nests were scored at 0900 (replicate 1) and 0930 (replicate 2) on days 2 through 4 (nests were unaltered during this time by the experimenter). On day 4, cages were changed, and new nesting material (same treatment groups) was given in the same manner at 1700. Pictures were taken again, and scoring was repeated as described. Video recording occurred every other day for each replicate in order to enable us to examine nest-building behavior and look for stereotypic behavior.
Mice were weighed at the start and end of the experiment (days 1 and 7) to check for any weight loss. Tail and flank wounds were counted at the start and the end of the experiment (to check for an increase in injurious aggression). Throughout the experiment, mice were monitored for any signs of injury.
Statistical methods.
Nest scores were compared by using a split-plot GLM identical to that used in experiment 1, except that there were 4, rather than 3, treatments, and the data did not require transformation.
For each cage we calculated the mean body weight of the mice before and after treatment. Body weights were analyzed by using a split-plot GLM. Cage was nested within sex and treatment, crossed with time point (before or after treatment). The treatment × time point interaction was evaluated to test whether body weights changed differentially according to treatment. No transformations were required.
No flank or tail wounds were detected in any of the mice; therefore these data were not analyzed.
Results
Experiment 1. Nest scores.
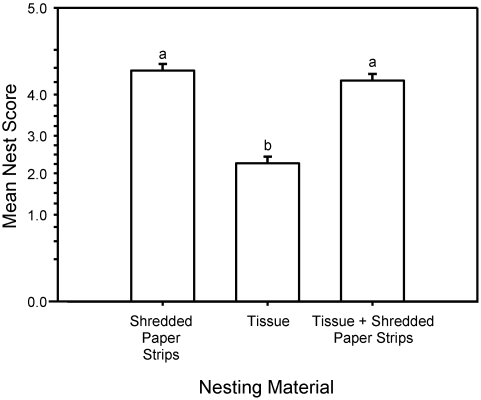
In this pilot study, nesting material affected nest quality (GLM, treatment main effect: F2,22 = 57.06; P < 0.001). Tukey post hoc comparisons revealed that the nests of mice provided with facial tissue alone scored lower than those of mice provided with the shredded paper strips only or in combination with tissue (that is, tissue < [shredded paper strips = tissue and shredded paper strips]; Figure 4). A cage's nest score was unaffected by the sex of the mice (GLM, sex main effect: F1,22 = 0.13; P = 0.721). All cages of mice given both facial tissue and the shredded paper strips built 2-layer nests, with the shredded paper strips forming the structural shell of the nest and the facial tissue shredded to line the nest (Figure 3).
Figure 4.
Mean nest quality scores in experiment 1. Tukey post hoc comparison shows that the mean nest score for tissue was significantly lower than that of the shredded paper strips or a combination of the two. Superscripts indicate means that do not differ.
Experiment 2: nest scores.
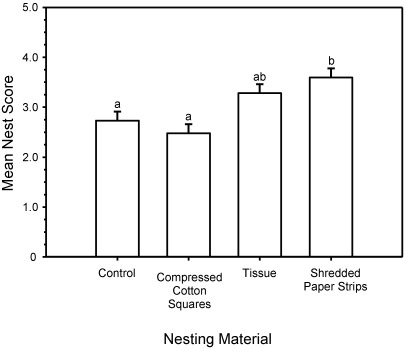
As in the pilot study, nesting material affected nest quality (GLM, treatment main effect: F3,75 = 7.78; P < 0.001). Tukey post hoc comparisons revealed that only the nests of mice provided the shredded paper strips scored higher than did those of control animals. The nests of mice with compressed cotton squares did not differ from those of controls, whereas mice given facial tissue only built nests of intermediate quality that were not significantly different from those of any other treatment (Figure 5). A cage's nest score was unaffected by the sex of the mice (GLM, sex main effect: F1,75 = 0.55; P = 0.460). Change in the mean body weight per cage was unaffected by treatment (GLM, treatment × time point: F3,12 = 0.24; P = 0.864).
Figure 5.
Mean nest quality scores for experiment 2. Tukey post hoc comparison shows that controls and the compressed cotton squares group (8 g) had significantly lower nest scores than did the group with shredded paper strips (8 g). Tissues (8 g) had nest scores that were not significantly different from any other group. Superscripts indicate means that do not differ.
Discussion
Because of its resemblance to nesting material used by wild mice, we predicted that the shredded paper strips would be most conducive to nest building compared with excess bedding, facial tissue, and compressed cotton squares. The results of this study suggest that providing more naturalistic nesting material allows mice to build more naturalistic nests. In experiment 1, mice built higher quality nests with the shredded paper strips than with facial tissues, perhaps because: 1) simpler nests are adequate for mice if the nests are built with tissues (for example, perhaps tissues are simply better insulators than other materials); 2) the weight of the material provided affects the quality of the nests; or 3) mice cannot build high-quality nests by using only facial tissues. We can exclude the first possibility by considering the nests built when tissues and the shredded paper strips were provided together: every cage of mice used tissues to line the nest and otherwise built similar nests to those built when the shredded paper strips were provided alone. In this case, the mice responded differently to materials with different properties, selecting one to line the nests and another to form the structure of the nest.
In experiment 2, more tissues were provided that equaled the weight of the shredded paper strips and compressed cotton squares allocated, but the nest scores for tissues still did not exceed those for the shredded paper strips. Scores for controls, compressed cotton squares, and tissues were not statistically different. Findings of both experiments 1 and 2 support both the second and third possibilities listed above: mice cannot build a high-quality nest with tissues alone, especially if the amount of tissue is limited. Although tissues did not support the same quality of nest building as the shredded paper strips (as shown, for example, by the differential use of these materials in experiment 1), they appear to have some features that make them somewhat attractive to the mice (for example, perhaps they are easy to shred and fluff up). In other studies, mice exerted considerable effort to gather and combine different materials to make composite nests.29,30 In this current study, instead of using the compressed cotton squares for nest building, the mice simply used them as a border around nests built of aspen bedding. This behavior further suggests that certain features of the material make it more or less preferred for nest building.
The current results contrast with previous findings,30 where facial tissue was preferred over the shredded paper strips. There are 2 possible explanations for this apparent discrepancy. First, unequal amounts of tissue and shredded paper strips were used in the previous study (as in our experiment 1). In addition, the previous study used less (5 g) of the shredded paper strips than we did and housed 6 mice in a single cage. Consequently, the design may have provided too little nesting material per mouse, too many mice to share a nest, or sufficient thermal advantage from huddling to devalue nesting material. Therefore, contrasting this previous study30 with our current results further reinforces the conclusion that quality and quantity of nesting material interact to determine nest quality and nesting material preferences. Second, the previous study evaluated preference for the materials, whereas we evaluated the quality of nests built with materials provided to the cages. Therefore, although the mice may have preferred facial tissue to the shredded paper strips, they would not necessarily have built superior nests with tissue. Mice might prefer a material that does not allow them to build naturalistic nests if their choice is dictated by additional features of the material (for example odor, color, or comfort). This interpretation is supported by another study:29 despite a lack of preferences between nest-building materials, mice selected different materials for different purposes within the structure of the nest.
The fact that nests constructed with the shredded paper strips were themselves more naturalistic than were those constructed by using compressed cotton squares underlines the importance of considering biological relevance when designing enrichments. The poor scores for the compressed cotton squares could conceivably reflect that compressed cotton squares do not provide sufficient material to build a more complex nest or that compressed cotton squares meet mouse needs in the absence of a complex nest. However, because mice that were provided multiple compressed cotton squares built no nest at all, these explanations are unlikely. Mice may use the shredded paper strips to control cold stress; our preliminary data indicate that mice alter the quality of nests in response to ambient temperature.17 This kind of control over a stressor in the mice's environment further illustrates the concept of biologically relevant enrichment.16,27,32 The fact that the quality of nest depends on temperature in other experiments means that the higher nest scores for the shredded paper strips cannot be merely a passive response to the material. Instead, nests built with the shredded paper strips appear to provide an adaptive response to the environment that is not possible with compressed cotton squares. We plan to test this hypothesis by investigating the thermal properties of nests in future experiments. In sum, these 2 experiments show that given appropriate material, even naive mice can build naturalistic nests in the laboratory.
Environmental enrichment in the laboratory is intended to benefit animal wellbeing5 and improve the quality of scientific data.16 The lack of aggression marks and injuries in all of the groups suggests that, at the very least, the nesting materials were not a detrimental form of enrichment (unlike the increase in aggression often seen with shelters). However, the broader effects of these nesting materials on brain function and behavior, thermoregulation, and consequent physiology (including immune function, growth, and reproduction) have yet to be determined. The effects of these materials should also be evaluated in other mouse strains. Another aspect to consider is the age at which the mice were tested. An interesting study would be the determination of whether the synthetic shredded paper strips are suitable nesting material for mice across age groups rather than adult mice only. Further, nest building did not differ significantly between genders in our study. In contrast, in the wild, pregnant female mice tend to build the highest quality nests.10 Although the female mice in our study were not pregnant, the effect (if any) of the estrus cycle on nest building could be evaluated. This assessment, along with a larger sample size, might have revealed a sex effect on nest building. Current and future work in our laboratory will focus on these aspects in order to understand the scope and limitations of nesting materials as beneficial environmental enrichment in the laboratory.
Acknowledgments
We would like to thank Brian Wood for contributing Enviro-dri Eco-bedding (Fibercore, Cleveland, OH). These experiments were supported in part by PULSe (Purdue University Life Sciences program) and Purdue School of Agriculture. This paper is not an implicit or explicit endorsement by the authors or by Purdue University for or against any particular commercial enrichment product.
References
- 1.Barnard CJ, Behnke JM, Sewell J. 1996. Environmental enrichment, immunocompetence, and resistance to Babesia microti in male mice. Physiol Behav 60:1223–1231 [DOI] [PubMed] [Google Scholar]
- 2.Baumans V. 2005. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR J 46:162–170 [DOI] [PubMed] [Google Scholar]
- 3.Baumans V, Schlingmann F, Vonck M, Van Lith HA. 2002. Individually ventilated cages: beneficial for mice and men? Contemp Top Lab Anim Sci 41:13–19 [PubMed] [Google Scholar]
- 4.Bayne K. 2005. Potential for unintended consequences of environmental enrichment for laboratory animals and research results. ILAR J 46:129–139 [DOI] [PubMed] [Google Scholar]
- 5.Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. 2003. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav 76:481–486 [DOI] [PubMed] [Google Scholar]
- 6.Berry RJ. 1970. The natural history of the house mouse. Field Studies 3:219–262 [Google Scholar]
- 7.Berry RJ, Bronson FH. 1992. Life-history and bioeconomy of the house mouse. Biol Rev Camb Philos Soc 67:519–550 [DOI] [PubMed] [Google Scholar]
- 8.Bond TL, Neumann PE, Mathieson WB, Brown RE. 2002. Nest building in nulligravid, primigravid and primiparous C57BL/6J and DBA/2J mice (Mus musculus). Physiol Behav 75:551–555 [DOI] [PubMed] [Google Scholar]
- 9.Broida J, Svare B. 1982. Strain-typical patterns of pregnancy-induced nest-building in mice: maternal and experiential influences. Physiol Behav 29:153–157 [DOI] [PubMed] [Google Scholar]
- 10.Brown RZ. 1953. Social behavior, reproduction, and population changes in the house mouse (Mus musculus L). Ecol Monogr 23:217–240 [Google Scholar]
- 11.Clayton NS, Krebs JR. 1994. Hippocampal growth and attrition in birds affected by experience. Proc Natl Acad Sci USA 91:7410–7414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clough G. 1982. Environmental effects on animals used in biomedical research. Biol Rev Camb Philos Soc 57:487–523 [DOI] [PubMed] [Google Scholar]
- 13.Crowcroft P, Rowe FP. 1963. Social organization and territorial behavior in the wild house mouse (Mus musculus L.). Proc Zool Soc London 140:517–531 [Google Scholar]
- 14.De Boer SF. 2003. Defensive burying in rodents: ethology, neurobiology, and psychopharmacology. Eur J Pharmacol 463:145–161 [DOI] [PubMed] [Google Scholar]
- 15.Deacon RMJ. 2006. Assessing nest building in mice. Nat Protocols 1:1117–1119 [DOI] [PubMed] [Google Scholar]
- 16.Garner JP. 2005. Stereotypies and other abnormal repetitive behaviors: potential impact on validity, reliability, and replicability of scientific outcomes. ILAR J 46:106–117 [DOI] [PubMed] [Google Scholar]
- 17.Gaskill BN, Rohr S, Pajor EA, Lucas JR, Garner JP. 2008. Comparison of mouse temperature preferences in laboratory housing with or without nesting material. (Ireland): University College Dublin. Conference Proceedings [Google Scholar]
- 18.Grafen A. 2002. A state-free optimization model for sequences of behaviour. Anim Behav 63:183–191 [Google Scholar]
- 19.Heizmann V, Jonas I, Hirschenauer K, Havelec L. 1998. Choice tests with groups of mice: nestbox, nesting material, and tubes as enrichment items for laboratory mice. J Exp Anim Sci 39:43–60 [Google Scholar]
- 20.Kaliste EK, Mering SM, Huuskonen HK. 2006. Environmental modification and agonistic behavior in NIH/S male mice: nesting material enhances fighting but shelters prevent it. Comp Med 56:202–208 [PubMed] [Google Scholar]
- 21.Kallnik M, Elvert R, Ehrhardt N, Kissling D, Mahabir E, Welzl G, Faus-Kessler T, de Angelis MH, Wurst W, Schmidt J, Holter SM. 2007. Impact of IVC housing on emotionality and fear-learning in male C3HeB/FeJ and C57BL/6J mice. Mamm Genome 18:173–186 [DOI] [PubMed] [Google Scholar]
- 22.Latham N, Mason G. 2004. From house mouse to mouse house: the behavioral biology of free-living Mus musculus and its implications in the laboratory. Appl Anim Behav Sci 86:261–289 [Google Scholar]
- 23.McGregor PK. 1990. Varied cages result in more aggression in male CFLP mice. Appl Anim Behav Sci 26:277–281 [Google Scholar]
- 24.Moons CP, Van Wiele P, Odberg FO. 2004. To enrich or not to enrich: providing shelter does not complicate handling of laboratory mice. Contemp Top Lab Anim Sci 43:18–21 [PubMed] [Google Scholar]
- 25.Neigh GN, Bowers SL, Korman B, Nelson RJ. 2005. Housing environment alters delayed-type hypersensitivity and corticosterone concentrations of individually housed male C57BL/6 mice. Anim Welf 14:249–257 [Google Scholar]
- 26.Newman JA, Bergelson J, Grafen A. 1997. Blocking factors and hypothesis tests in ecology: is your statistics text wrong? Ecology 78:1312–1320 [Google Scholar]
- 27.Olsson IA, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of ‘environmental enrichment.’ Lab Anim 36:243–270 [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig MR, Bennett EL. 1996. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res 78:57–65 [DOI] [PubMed] [Google Scholar]
- 29.Sherwin CM. 1997. Observations on the prevalence of nest-building in nonbreeding TO strain mice and their use of two nesting materials. Lab Anim 31:125–132 [DOI] [PubMed] [Google Scholar]
- 30.Van de Weerd HA. 1997. Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim 31:133–143 [DOI] [PubMed] [Google Scholar]
- 31.Wolfer DP, Litvin O, Morf S, Nitsch RM, Lipp HP, Wurbel H. 2004. Laboratory animal welfare: cage enrichment and mouse behaviour. Nature 432:821–822 [DOI] [PubMed] [Google Scholar]
- 32.Würbel H, Garner JP. 2007. Refinement of rodent research through environmental enrichment and systematic randomization. NC3Rs:1–9 [Google Scholar]