Abstract
The benzimidazole anthelmintic fenbendazole (FBZ) is a common and effective treatment for pinworm infestation in laboratory animal colonies. Although many investigators have examined the potential for deleterious biologic effects of FBZ, more subtle aspects of the treatment remain untested. Accordingly, we evaluated differences in food intake when healthy male Sprague–Dawley rats were provided a standard nonmedicated laboratory rodent chow or the same chow supplemented with FBZ. We also tested for a preference for either food type when subjects were provided a choice of the 2 diets. Data from these experiments showed no differences in food intake or body weight when rats were maintained on either standard or FBZ-supplemented chow. When the rats were given access to both the standard and FBZ-supplemented diets, they showed a clear preference for the standard diet. The preference for the standard diet indicates that the rats can discriminate between the 2 foods and may avoid the FBZ-supplemented chow when possible. Investigators conducting experiments during treatment with FBZ in which differences in food preference are relevant should be aware of these data and plan their studies accordingly.
Abbreviation: FBZ, fenbendazole
Pinworm infestation is a common and troublesome situation that can arise in the maintenance and care of laboratory rat colonies.8,9 A popular and effective treatment strategy relies on a diet that has been supplemented with the benzimidazole anthelmintic fenbendazole (FBZ; methyl 5-[phenylthio]-2-bendzimidazole carbamate).4,6,7 According to a 1991 toxicology evaluation released by the World Health Organization, therapeutic levels of fenbendazole can be administered throughout the lifetime in rodents without significant toxic side effects.11 Moreover, previous experiments have failed to find carcinogenic effects of FBZ treatment at therapeutic doses (for review, see reference 20). However, various histological changes, such as hepatocellular hypertrophy and bile duct proliferation and hyperplasia, have been reported to occur in dosages exceeding 45 mg/kg.11,22 In addition, rats maintained on a diet containing FBZ had smaller litter sizes than those on an unmedicated diet.10 Nevertheless, deleterious effects resulting from therapeutic doses of FBZ appear to be minimal.
Therapeutic doses of FBZ have few, if any, behavioral effects. One study1 found that when FBZ was administered to female rats throughout pregnancy and gestation, the pups showed no deficits in digging maze performance and negative geotaxis, whereas the pregnant dams showed no difference in drinking or weight gain. However, the progeny of the FBZ-treated dams showed decreased performance in running-wheel and Morris water maze tests and demonstrated delayed righting reflex.1 Nevertheless, adult rats treated with FBZ failed to differ from controls in a variety of tests, including conditioning and timing tasks that are sensitive to neurotoxic drug effects.12
Although the potential for deleterious effects of FBZ has garnered much interest, more subtle issues remain untested. Diet and taste preference are important factors that may affect many types of experiments, especially those measuring food intake. To address the paucity of information related to intake of FBZ-supplemented food, we measured food intake and weight gain in healthy rats given standard or FBZ-supplemented chow. We also tested for a preference between standard and FBZ-supplemented diets. The data from these experiments indicate that rats consumed normal levels of the medicated diet when it was the only food available and did not alter their intake when switched from standard to FBZ-supplemented chow (or vice versa). Nevertheless, the rats showed a strong preference for the nonmedicated diet when given a choice.
Materials and Methods
Animals.
Adult male Sprague–Dawley rats (weight, 125 to 175 g) were obtained from Charles River Laboratories (Wilmington, MA). Animals were housed in a temperature (20 to 23 °C) and humidity (approximately 50%)-controlled colony room on a 12:12-h light:dark schedule. To facilitate measures of food intake, rats were housed conventionally in individual stainless steel hanging wire-mesh cages, with food and tap water provided ad libitum. On arrival, rats in both experiments were placed immediately into their respective experimental conditions and allowed access to a preweighed amount of food so that the first intake measures could be obtained the next day. All experimental protocols and housing arrangements were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo, which maintains full AAALAC accreditation. Health monitoring of the facility is conducted twice each year by using sentinels and includes testing for cilia-associated respiratory bacillus, Kilham rat virus, H1 virus, Mycoplasma pulmonis, rat parvovirus, pneumonia virus of mice, sialodacryoadenitis virus, Sendai virus, lymphocytic choriomeningitis virus, reovirus, fur mites, and pinworms. Testing during the time of the experiments described was negative for each of these agents.
Diets.
At Charles River Laboratories, the rats had been fed a commercial diet (5L79, PMI Nutrition International, St Louis, MO) since weaning. On arrival at our facility, all rats received commercial standard chow (18% protein; Global 2018, Harlan Teklad, Madison, WI) or medicated chow (Global 2018S with FBZ, Harlan Teklad). The medicated chow contains 150 ppm FBZ to provide a targeted minimum dose of 8 mg/kg body weight per day. Other than the addition of FBZ and increased vitamins, the diets used by the vendor and at our facility are similar in their composition (Table 1). Although the manufacturers report no change in the formulas of these diets since their inception, the data from their analyses differ over time for various reasons. An updated analysis for the medicated diet is unavailable currently, so all comparisons of the diets were based on analyses from similar time frames. Accordingly, we used analysis reports dated within 1 y of each other and provide the dates of the reports from which these numbers were taken (Table 1).
Table 1.
Nutritional information of the diets consumed before and after the present experiments
| At our facility |
||||
| Standard chow | FBZ chow | Diet at commercial animal facility | ||
| Digestible/metabolizable energy (MJ/kg) | 3.4/3.3 | 3.4/3.3 | 3.4/3.2 | |
| Crude protein | 18.9% | 18.8% | 18.0% | |
| Crude oil | 6.0% | 6.0% | 5.0% | |
| Carbohydrate | 57.33% | 57.26% | * | |
| Sugar | 4.93% | 4.91% | 2.9%* | |
| Starch | 41.24% | 41.19% | 33.9% | |
| Vitamin A | 15.4 IU/g | 30.7 IU/g | 44 IU/g | |
| Vitamin E | 101 mg/kg | 126 mg/kg | 80 IU/kg | |
| Vitamin K (menadione) | 51 mg/kg | 102 mg/kg | 3.4 mg/kg | |
| Vitamin B1 | 16.5 mg/kg | 117.60 mg/kg | 92 mg/kg | |
| Vitamin B2 | 14.9 mg/kg | 27.2 mg/kg | 8.0 mg/kg | |
| Niacin | 41.2 mg/kg | 87.3mg/kg | 60 mg/kg | |
| Vitamin B6 | 18.5 mg/kg | 26.8 mg/kg | 12 mg/kg | |
| Pantothenic acid | 33 mg/kg | 141.6 mg/kg | 24 mg/kg | |
| Biotin | 0.3 mg/kg | 0.82 mg/kg | 0.28 mg/kg | |
| Folate | 3.34 mg/kg | 8.41 mg/kg | 4.2 mg/kg | |
| Vitamin D3 | 1.5 IU/g | 2.05 IU/g | 1.5 IU/g | |
| Vitamin B12 | 0.08 mg/kg | 0.15 mg/kg | 0.019 mg/kg | |
| Date of report | June 2005 | December 2006 | April 2005 | |
Values that differ between the 2 diets used at our institution and selected similarities are shown. Asterisks indicate values that were calculated or omitted because comparable data were not available from the various vendors.
Intake and body weight measures.
Preweighed food was provided in standard stainless steel hoppers. After 24 h, rats were briefly removed from their cages and weighed, and the amount of food remaining, including any on the bottom of the cages or any that had spilled onto plastic sheets placed under each cage, was recorded. Intake was calculated as the weight (in grams) of food provided less that recovered.
Experiment 1.
Rats were tested over 12 d to assess whether the type of food provided, either standard or medicated, altered intake or weight gain. On arrival from the vendor, animals were transferred to cages that contained either standard chow (n = 12) or medicated chow supplemented with FBZ (n = 12). After 6 d, half of the animals from each group had their food switched to the type of chow to which they had not yet been exposed (standard to medicated or medicated to standard), yielding a total of 4 experimental groups, each comprising 6 animals. Accordingly, 2 of these groups received a continuous diet of either standard or medicated chow for the duration of the experiment; the remaining 2 groups began with either standard or medicated chow, which then was changed to medicated or standard chow, respectively.
Experiment 2.
On arrival from the vendor, rats were placed into cages containing 2 food hoppers. Beginning on the first day, half of the rats (n = 10) were given standard food in both hoppers, whereas the remaining rats (n = 10) received medicated food in both hoppers. After 6 d, the food in 1 hopper was substituted with either standard or medicated food such that all animals had access to both food types. The location of the hopper containing the medicated and standard foods was counterbalanced to control for any left–right preference effects. Specifically, once both diets were available, the medicated diet was given in the left hopper for half of the rats from each group and in the right hopper for the other half. Collection of spillage from under the cage was not included in the intake measures from this experiment because of the difficulty in determining the type of diet contained in the spillage.
Data analysis.
All data were analyzed by using SPSS software (version 14.0; SPSS, Chicago, IL). One-way ANOVA were used to test for differences in daily body weights for both experiments as well as differences in daily cumulative food intakes from Experiment 1. Mean daily food intake from Experiment 2, on days prior to animals having access to both food types, was analyzed by using 1-way ANOVA, and a repeated-measures design was used in analyzing data collected after that point. Statistical significance was defined as a P value of less than 0.05.
Results
Experiment 1.
Healthy male Sprague–Dawley rats were given either standard laboratory chow or chow that was supplemented with FBZ. After 6 d, the diet of half of the rats from each group was switched to the type of chow that had not been available previously, and measures were taken for an additional 6 d. Body weight did not differ across groups on any of the 12 d (F3,20≤ 1.79, P ≥ 0.18; Figure 1). Differences in cumulative intake between animals receiving standard and FBZ-supplemented diets did not reach statistical significance on any day (F3,20≤ 2.93, P ≥ 0.06; Figure 2). Moreover, switching to the alternative diet did not change intakes in either group.
Figure 1.
Daily body weight (mean ± SEM) of rats receiving standard and FBZ-supplemented diets. Diet had no effect on mean body weight on any day. The vertical line after day 6 represents the point at which the diet of 2 of the 4 groups was switched to the type of food that was not previously provided (FBZ to standard or standard to FBZ). The other 2 groups were maintained on the same diet, either FBZ-supplemented or standard, throughout the experiment.
Figure 2.
Cumulative intake (mean ± SEM) of standard and FBZ-supplemented diets. Neither the type of food provided nor a change in diet altered intake. The vertical line after day 6 represents the point at which the diet of 2 of the 4 groups was switched to the type of food that was not previously provided (FBZ to standard or standard to FBZ). The other 2 groups were maintained on the same diet, either FBZ-supplemented or standard, throughout the experiment.
Experiment 2.
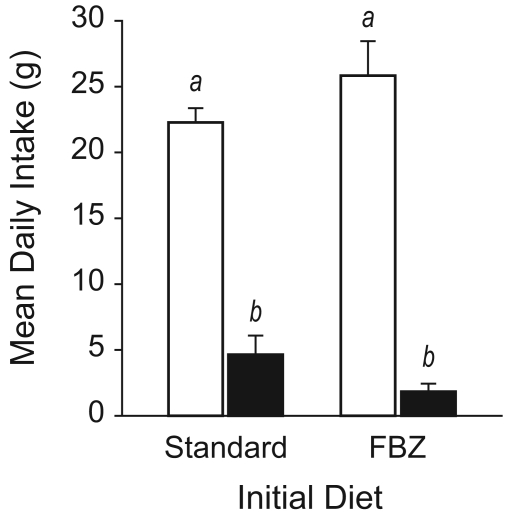
In a second experiment, rats were provided 2 food hoppers to test for a preference for either standard or FBZ-supplemented chow. All animals began the experiment with only 1 type of diet in both hoppers, with half of the rats receiving standard chow and the other half receiving medicated chow. After 6 d, animals were given access to both food types. As in Experiment 1, neither intake (F1,18=0.038, P = 0.85) nor body weight (F1,18≤0.53, P ≥ 0.48) differed as a function of the type of food provided. When both food types were offered, body weight did not differ as a function of the food provided (F1,18≤1.14, P ≥ 0.3; Figure 3), but animals reliably preferred the standard chow and almost completely avoided the medicated chow (F1,18=281.56, P < 0.001; Figure 4). This preference for standard chow did not depend on the food type provided initially (F1,18=0.225, P = 0.64; Figure 4).
Figure 3.
Body weight (mean ± SEM) of rats initially maintained on either standard chow (open bars) or FBZ-supplemented chow (filled bars). Body weight did not differ between the 2 groups either before (prechoice) or after (choice) both food types were made available.
Figure 4.
Daily food intake (mean ± SEM) by rats maintained initially on standard or FBZ-supplemented diets. Intake of standard chow (open bars) and FBZ-supplemented chow (filled bars) is shown for animals initially maintained on standard (left) or FBZ-supplemented (right) chow. Groups marked with different letters differed (P < 0.001) from each other.
Discussion
Pinworms (Aspicularis tetraptera in mice and Syphacia muris in rats) are a relatively common problem in the maintenance and care of laboratory rat and mouse colonies.8,9 Pinworms cause numerous biologic alterations in rodents, including modifications of growth, immune response, and behavior.17 Although infected animals often appear asymptomatic, pinworms can affect data collected from infected animals. Animals may have decreased weight gain, reduced growth rates, increased caloric demands, and compromised nutritional status.10 Fenbendazole-medicated feed is a common and efficacious method used to eradicate infestations from laboratory animal colonies.10 Fenbendazole (methyl 5-[phenylthio]-2-bendzimidazole carbamate) is a thio-substituted benzimidazole derivative widely used in veterinary medicine as a broad-spectrum anthelmintic. The drug acts by inhibiting glucose uptake by the parasitic worm and by polymerization of tubulin.14 Typical administration includes continuous feeding of medicated feed for 7 d, followed by unmedicated feed for 7 d, and repeated for 3 to 5 cycles.7 Although treatment of pinworms with a FBZ-supplemented diet does not appear to cause any physiologic side effects or toxicity when administered in therapeutic doses,5,11,17 the treatment may be associated with more subtle effects. When conducting experiments with animals that are currently undergoing treatment with FBZ, knowing about possible behavioral modifications that result from the treatment is important. The current study measured food intake and mean body weight of healthy male Sprague–Dawley rats that had no signs of pinworms and were maintained on either a standard laboratory diet or a diet supplemented with FBZ. We also tested whether rats preferred 1 diet over the other by measuring food intake when animals were given a choice between the 2 feeds.
Rats maintained on diets of either standard or medicated chow showed no diet-associated difference in food intake or weight gain. This finding is consistent with previous findings involving rats4,12 and Mongolian gerbils.21 In addition, food intake did not change when the type of feed was switched in this experiment. This lack of effect could indicate that the rats could not detect differences in the food types or did not prefer 1 diet over the other. However, when rats had access to both standard and medicated chow, they demonstrated a clear preference for the standard chow. Previous diet exposure is important in interpreting these data, and prompted us to purchase rats from a vendor that used a diet different from those used in the study. This consideration is particularly important because previous research demonstrates that taste preferences may be acquired when animals are shipped from 1 facility to another.16 The novelty of both diets used in the experiment here strongly suggests that preference due to familiarity with the diet did not account for the observed differences in intake.
Differences other than the addition of FBZ in the diets also might influence intakes. The most notable is the difference in vitamin content (Table 1). Preference for dietary vitamins has been reported for rats, but these preferences generally occur only during deficient conditions and seem universally to show greater intake of the food with the higher vitamin content.2,3,18,19 In this respect, the preferred diet in the present experiment had the lower vitamin content. Therefore, the previous reports on vitamin preference would have predicted the opposite response had vitamin content and preference been a factor here. Given the direction of any preference predicted by the content of the diets and the fact that these diets are all designed to address the nutritional requirements of laboratory rats,15 these issues likely did not play a role in the observed differences. As such, these data strongly suggest a preference for the FBZ-free food and, moreover, indicate that rats can detect a difference between the 2 food types.
The animals used in this study were not infected with pinworms and were otherwise healthy. Indeed, animals were not manipulated experimentally except for the changes in diet and the food intake measures involved. As such, whether these findings would generalize to animals infected with pinworms or those subjected to various experimental manipulations is unknown. Although rats with pinworm infections or those otherwise affected by experimentation would be unlikely to show a different food preference or fail to show the preference we observed, the ingestive behavior of rats with pinworms or undergoing specific experimental manipulations remains an open question.
The basis of the observed preference cannot be determined from the present experiments and remains a question for future study. Although difference in taste is a likely and conservative explanation, the difference also could be the result of a conditioned avoidance.13 However, any effect of FBZ that would generate a conditioned avoidance likely would have produced an observable difference in intake from experiment 1. Specifically, when animals were given access to standard chow before FBZ-supplemented chow, intake did not differ between the 2 food types. As such, the observed preference in experiment 2 likely resulted from differences in the taste or smell of the foods provided.
Pinworm infestation is relatively common in laboratory animal facilities. Treatment with FBZ-supplemented food is an efficient and cost-effective means of treating pinworm outbreaks.4 The present experiments provide important information for investigators using animals receiving a FBZ-supplemented diet. Specifically, these data indicate that rats consume normal amounts of FBZ-containing food and gain weight similarly to animals given standard chow. The data further indicate that rats can detect differences in FBZ-containing and standard diets and prefer the standard chow. As such, investigators using laboratory rats should be aware of this preference, especially when conducting experiments that measure food intake.
Acknowledgments
The authors thank Elizabeth Mietlicki and Erica Nowak for their assistance with the studies described here. DD is supported by NIH award DK73800.
References
- 1.Barron S, Baseheart BJ, Segar TM, Deveraux T, Willford JA. 2000. The behavioral teratogenic potential of fenbendazole: a medication for pinworm infestation. Neurotoxicol Teratol 22:871–877 [DOI] [PubMed] [Google Scholar]
- 2.Bernard RA, Halpern BP. 1968. Taste changes in vitamin A deficiency. J Gen Physiol 52:444–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard RA, Halpern BP, Kare MR. 1961. Effect of vitamin A deficiency on taste. Proc Soc Exp Biol Med 108:784–786 [DOI] [PubMed] [Google Scholar]
- 4.Coghlan LG, Lee DR, Psencik B, Weiss D. 1993. Practical and effective eradication of pinworms (Syphacia muris) in rats by use of fenbendazole. Lab Anim Sci 43:481–487 [PubMed] [Google Scholar]
- 5.Franke DD, Shirwan H. 2006. Prophylactic fenbendazole therapy does not affect the incidence and onset of type 1 diabetes in nonobese diabetic mice. Int Immunol 18:453–458 [DOI] [PubMed] [Google Scholar]
- 6.Huerkamp MJ, Benjamin KA, Webb SK, Pullium JK. 2004. Long-term results of dietary fenbendazole to eradicate Syphacia muris from rat colonies. Contemp Top Lab Anim Sci 43:35–36 [PubMed] [Google Scholar]
- 7.Huerkamp MJ, Benjamin KA, Zitzow LA, Pullium JK, Lloyd JA, Thompson WD, Webb SK, Lehner ND. 2000. Fenbendazole treatment without environmental decontamination eradicates Syphacia muris from all rats in a large, complex research institution. Contemp Top Lab Anim Sci 39:9–12 [PubMed] [Google Scholar]
- 8.Jacoby RO, Lindsey JR. 1997. Health care for research animals is essential and affordable. FASEB J 11:609–614 [DOI] [PubMed] [Google Scholar]
- 9.Jacoby RO, Lindsey JR. 1998. Risks of infection among laboratory rats and mice at major biomedical research institutions. ILAR J 39:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston NA, Bieszczak JR, Verhulst S, Disney KE, Montgomery KE, Toth LA. 2006. Fenbendazole treatment and litter size in rats. J Am Assoc Lab Anim Sci 45:35–39 [PubMed] [Google Scholar]
- 11.Joint FAO–WHO Committee on Food Additives 1991. Evaluation of certain veterinary drug residues in food: 38th report of the joint FAO–WHO expert committee on food additives. Geneva: World Health Organization [Google Scholar]
- 12.Keen R, Macinnis M, Guilhardi P, Chamberland K, Church R. 2005. The lack of behavioral effects of fenbendazole: a medication for pinworm infection. Contemp Top Lab Anim Sci 44:17–23 [PubMed] [Google Scholar]
- 13.Rozin P, Kalat JW. 1971. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol Rev 78:459–486 [DOI] [PubMed] [Google Scholar]
- 14.Shoda T, Onodera H, Takeda M, Uneyama C, Imazawa T, Takegawa K, Yasuhara K, Watanabe T, Hirose M, Mitsumori K. 1999. Liver tumor-promoting effects of fenbendazole in rats. Toxicol Pathol 27:553–562 [DOI] [PubMed] [Google Scholar]
- 15.Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council 1995. Nutrient requirements of laboratory animals, 4th ed. Washington (DC): National Academy Press [Google Scholar]
- 16.Tordoff MG, Alarcon LK, Byerly EA, Doman SA. 2005. Mice acquire flavor preferences during shipping. Physiol Behav 86:480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth LA, Oberbeck C, Straign CM, Frazier S, Rehg JE. 2000. Toxicity evaluation of prophylactic treatments for mites and pinworms in mice. Contemp Top Lab Anim Sci 39:18–21 [PubMed] [Google Scholar]
- 18.Tribe DE, Gordon JG. 1953. Choice of diet by rats deficient in members of the vitamin B complex. Br J Nutr 7:197–201 [DOI] [PubMed] [Google Scholar]
- 19.Tribe DE, Gordon JG. 1955. Choice of diet by rats. Br J Nutr 9:200–202 [DOI] [PubMed] [Google Scholar]
- 20.Villar D, Cray C, Zaias J, Altman NH. 2007. Biologic effects of fenbendazole in rats and mice: a review. J Am Assoc Lab Anim Sci 46:8–15 [PubMed] [Google Scholar]
- 21.Wilkerson JD, Brooks DL, Derby M, Griffey SM. 2001. Comparison of practical treatment methods to eradicate pinworm (Dentostomella translucida) infections from Mongolian gerbils (Meroines unguiculatus). Contemp Top Lab Anim Sci 40:31–36 [PubMed] [Google Scholar]
- 22.Xu SX, Zheng D, Sun YM, Wang SH, Shao LQ, Huang QY, Xiang DL. 1992. Subchronic toxicity studies of fenbendazole in rats. Vet Hum Toxicol 34:411–413 [PubMed] [Google Scholar]