Abstract
Gamma radiation is used to sterilize diets for specific pathogen-free (SPF) animals. Because a gamma-irradiated diet was linked to leukoencephalomyelopathy in SPF cats, we investigated the effects of ‘typical’ (28.9–34.3 kGy) and ‘high-end’ (38.4–48.7 kGy) doses of gamma irradiation and of pasteurization (at 107 °C for 15 min) on the amounts of fat; protein; carbohydrate (and taurine in cat diet); vitamins A, E, B1, B2, B6, and B12; and peroxide in commercially available dry cat, dog, and rodent diets. The only treatment-related changes occurred with vitamin A and peroxide. The typical and high-end doses of gamma irradiation reduced the vitamin A level of the cat diet to 42% and 30% of the untreated value, respectively—levels below recommended allowances for growth and reproduction. Only the higher irradiation dose reduced vitamin A in the rodent diet, and neither dose altered the canine diet. Pasteurization reduced the vitamin A content of the cat diet to 50% of its original level, which was within the recommended level for this species. Irradiation increased the peroxide content of all 3 animal diets: by approximately 11-fold with the typical dose and by 14- to 25-fold with the high-end dose. Therefore gamma irradiation can have profound, selective effects on the vitamin A and peroxide contents of dry diets, and caution is advised when feeding such diets long-term and exclusively to SPF animals, particularly cats. Furthermore, pasteurization (with its fewer deleterious effects) may represent an alternative method of decontaminating diets for rodents, dogs, and cats.
Abbreviation: AAFCO, Association of American Food Content Officials; LAC, Laboratory Animals Centre; NRC, National Research Council; SPF, specific pathogen-free
The increasing use of germ-free and specific pathogen-free (SPF) animals and genetically modified rodents (of known or uncertain immunocompetence) in research has led to a growing demand for sterilized animal diets.1,11,43 The methods used to sterilize diets include the application of heat by pasteurization20,43 or gamma irradiation.16,17,43 Food irradiation is used extensively to destroy microorganisms, a necessary process in the production of diets suitable for feeding to SPF animals.12,43 Pasteurization, which is the partial sterilization of foods at a temperature that destroys harmful microorganisms without marked changes in the chemistry of the food also can be used to ‘sterilize’ animal diets used in a barrier or SPF facility.12,43 Because these treatment processes are designed to kill microorganisms through destroying their proteins and other cellular components, it is not surprising that these methods also alter constituents of these diets.40,43 The physicochemical and biochemical changes in a food due to the application of gamma irradiation cause the formation of radiolytic products, whose risks are still the subject of ongoing research.22
The irradiation dose applied to a food product is measured in terms of kiloGrays (kGy). One kiloGray is equivalent to 1000 grays (Gy), 0.1 megarad (Mrad), or 100,000 rads. Exposure to gamma irradiation doses below 10 kGy is effective in enhancing food safety through the inactivation of pathogenic microorganisms such as Salmonella and Campylobacter and in extending the shelf-life of the diet by eliminating the microorganisms responsible for food spoilage.14,41 Irradiation doses of between 20 to 25 kGy12,43 and between 20 to 30 kGy11 are used most frequently to treat diets intended for SPF animals, whereas larger doses of 40 to 50 kGy are recommended for diets intended for gnotobiotic or germ-free animals, where absolute sterility is essential.11,43 During the irradiation process, the food is exposed to a controlled amount of gamma rays from a radioactive source, such as cobalt-60. The gamma rays evenly penetrate the food, rapidly killing food-poisoning bacteria, harmful parasites, and insects.36 The radiation dose and quantity of radiation energy absorbed by the food are the most critical factors in food irradiation.46 The ability of irradiation to kill a particular microbe is measured as the ‘D value,’ the amount of energy to kill 90% of the microbial population.48
Pasteurization of animal diets is achieved by exposure to a temperature of 107 °C for 15 to 20 min in an autoclave.12 Unlike sterilization, pasteurization does not kill all microorganisms in the food but instead achieves a ‘log reduction’ in the number of viable organisms.5
Animal diets vary in composition depending on the unique nutritional biochemistry of the species that it is intended for. The nutrient requirements of dogs and cats are described in the AAFCO Dog and Cat Food Nutrient Profiles4 from the Association of American Feed Content Officials (AAFCO). The values developed by the National Research Council (NRC) Committee on Animal Nutrition are the minimal nutrient requirements and recommended allowance for dogs and cats.29 The nutrient requirements of rodents have been described in the reports of the Laboratory Animals Centre (LAC) Diets Advisory Committee10 and those of the National Research Council.28 Because rodents are classified as omnivorous,28 the nutritional composition of rodent diets vary from that of the cat, a mammalian carnivore25 with obligatory requirements for dietary nutrients not essential for other mammals.27,39
Irradiation reduces the vitamin content of food, the effect of which may be indirect in that inadequate amounts of antioxidant vitamins (such as C, E, and β-carotene) may be available to counteract the effects of free radicals generated by normal cell metabolism.38 Problems may arise in situations where animals are exclusively fed irradiated diets, as in SPF facilities.47 Furthermore, the irradiation of diets containing fats with high levels of polyunsaturated fatty acids increases the onset of oxidative rancidity due to peroxidation of the contained unsaturated bonds.11,19, 37,43 The irradiation of a variety of commercial dry cat diets at 2.5 Mrad (25 kGy) resulted in considerable reductions in vitamin A levels, with up to 93% reduction observed in a diet with a relatively high-fat content.19 That study19 and previous work11 revealed a dose–response relationship for this phenomenon, with greater destruction of vitamins A and E with increasing irradiation dose. The most severe nutrient loss from autoclaving is to the heat-labile vitamins A and B1.44
Although the practice of feeding irradiated diet to laboratory rodents is well established,34,38 relatively little information is available on the effects of the long-term feeding of gamma-irradiated diets to dogs, and few toxicologic studies have been carried out in animals with foodstuffs irradiated with doses greater than 10 kGy.38 In 2001 the FDA recommended that gamma irradiation of animal diets of up to 50 kGy is acceptable for microbial control.18 We are aware of only 1 report on the effects of the long-term feeding of gamma-irradiated diets to cats, in which multiple cases of leukoencephalomyelopathy were described.9 No extensive controlled studies have examined the effects of gamma irradiation or pasteurization on the nutritive composition of commercially available diets recommended for the long-term feeding to rodents, cats and dogs. Therefore, the objective of this study was to investigate whether gamma irradiation or pasteurization of commercially available dry cat and dog food and of a rodent diet altered their nutritive composition.
Methods
Diets.
Dry cat diet (batch 06:24916:10B; Chicken, Liver, and Duck Flavour; Gilpa Umami Complete Diet for Adult Cats) and dry dog diet (batch 06:186 07:43B; Chicken and Rice; Gilpa Trinkets) were purchased in 15-kg bags from Gilbertson and Page (Hertfordshire, UK). Dry rodent diet (nonautoclavable, 12.5-kg bags, batch N266, T2018 Global Rodent Breeding diet) was purchased from Harlan Teklad (Oxfordshire, UK). To eliminate batch variability as a factor in the study, sufficient quantity of each batch of diet was purchased to conduct all of the analysis. The nutrients analyzed and the manufacturers’ stated contents for the 3 diets appear in Table 1.
Table 1.
Selected nutritional components in the control cat, dog, and rodent diets as provided on product specification sheets
| Vitamins |
Other nutritional components |
||||||||||
| Diet | A (retinol; IU/kg) | E (tocopherol; mg/kg) | B1 (thiamine; mg/kg) | B2 (riboflavin; mg/kg) | B6 (pyridoxine; mg/kg) | B12 (cobalamin; mg/kg) | Fat (g/kg) | Protein (g/kg) | Carbo-hydrate (g/kg) | Taurine (mg/kg) | Peroxide (mEq/ kg fat) |
| Cat | 12,000 | 150 | 12.0 | 8.0 | 6.0 | 0.05 | 140 | 310 | 505 | 1800 | <5.0 |
| Dog | 10,000 | 100 | 2.0 | 10.0 | 4.0 | 0.05 | 120 | 240 | 415 | – | <5.0 |
| Rodent | 15,400 | 101 | 16.5 | 14.9 | 18.5 | 0.08 | 60 | 189 | 573 | – | – |
–, not reported
Gamma irradiation.
Cat, dog, and rodent diets were prepared for irradiation by emptying 4 bags of purchased feed into a drum (height, 83 cm; radius, 29 cm). Each drum was cranked and taped to seal it prior to shipment for irradiation. The radiation facility consisted of a biological shield containing the gamma-radiation source (cobalt-60), a storage pool, and a mechanical system conveying the product around the source. One drum of each diet type was gamma-irradiated at a ‘typical’ radiation dose of 28.9 to 34.4 kGy, and a second drum of each diet type was gamma-irradiated at a ‘high-end’ radiation dose of 38.4 to 48.7 kGy. The delivered dose ranges were confirmed by certification from the radiation facility.
Pasteurization.
Two bags of each of the same batch of cat, dog, and rodent diets were pasteurized in an autoclave (GE71411–AR2, Getinge, Nottinghamshire, UK) at 107 °C at atmospheric pressure for 15 min. The diet was packed in polypropylene bags (Louis Blockx, Dottignies, Belgium) and in shallow layers (approximately 16 cm in depth) to allow the steam penetrate the diet.
Diet analysis.
Duplicate 1-kg bags of each treated diet (either gamma-irradiated at one of the 2 dose levels or pasteurized) and duplicate 1-kg bags of each untreated diet were transported by courier to a laboratory accredited by the United Kingdom Accreditation Service for analysis. The time interval between sterilization and analysis was between 3 and 7 d. To ensure verification of the reliability of the laboratory analyses, duplicate blinded diet samples from the same batch were analyzed and showed almost identical values. Back-up samples of all diets were retained for possible further analysis if required. Samples (500 g) of each of these diets were analyzed for fat; protein; carbohydrate; peroxide; and vitamin A, E, B1, B2, B6, and B12 content by using established analytical methodologies.2,3 The cat diet was also analyzed for taurine concentrations, because the cat requires a dietary supply of taurine to prevent central retinal degeneration.25
Results
The content of each analyte in treated dog, cat, and rodent diets are shown in Tables 2 through 4; the values in parentheses are the percentage of preirradiation diet figures. The typical recommended daily intake of each diet by its respective species is 105 g for adult cats, 200 to 350 g for adult medium-sized dogs, 5 g for mice, and 20 g for rats, as recommended by the manufacturers of the diets.
Table 2.
Vitamin and nutritional components in dry cat food before and after gamma irradiation or pasteurization
| Vitamins |
Other nutritional components |
||||||||||
| A (retinol; IU/kg) | E (tocopherol; mg/kg) | B1 (thiamine; mg/kg) | B2 (riboflavin; mg/kg) | B6 (pyridoxine; mg/kg) | B12 (cobalamin; mg/kg) | Fat (g/kg) | Protein (g/kg) | Carbohydrate (g/kg) | Taurine (mg/kg) | Peroxide (mEq/kg fat) | |
| Untreated | 20,700 | 207 | 8.6 | 8.8 | 5.6 | 0.2 | 133 | 355 | 357 | 2050 | 0.6 |
| Irradiated (28.9–34.4 kGy) | 8710 (42%) | 181 (87%) | 7.1 (83%) | 8.5 (97%) | 4.3 (77%) | 0.2 (100%) | 134 (101%) | 353 (100%) | 354 (99%) | 1890 (92%) | 6.6 (1100%) |
| Irradiated (38.4–48.7 kGy) | 6500 (31%) | 181 (87%) | 6.0 (70%) | 9.2 (105%) | 4.3 (77%) | 0.2 (100%) | 135 (102%) | 354 (100%) | 345 (97%) | 1820 (89%) | 12.6 (2100%) |
| Pasteurized | 10,300 (50%) | 173 (84%) | 5.8 (67%) | 8.5 (97%) | 5.4 (96%) | 0.2 (100%) | 136 (102%) | 348 (98%) | 343 (96%) | 1760 (86%) | 0.7 (117%) |
| AAFCO | 9000 | 30 | 5.0 | 4.0 | 4.0 | 0.02 | 90 | 300 | – | 1000 (E) 2000 (C) | – |
| NRC | 6666 | 31 | 6.3 | 4.0 | 2.5 | 0.02 | 90 | 300 | – | 1000 (E) 1700 (C) | – |
–, not reported; C, canned; E, extruded
Samples of dry cat food (500g) were analyzed for the content of the above nutrients after gamm a irradiation at 2 different doses (typical, 28.9–34.4 kGy; high end, 38.4–48.7 kGy) and pasteurization at 107 °C for 15 min. The values in parenthesis are the percentage change from preirradiation levels. Also included are the recommended minimal daily intake values of each nutrient for growth and reproduction for cats from the Association of American Feed Control Officials (AAFCO; 2007) and National Research Council (NRC; 2006), based on dry matter.
Table 3.
Vitamins and nutritional components in dry dog food before and after gamma irradiation or pasteurization
| Vitamins |
Other nutritional components |
|||||||||
| A (retinol; IU/kg) | E (tocopherol; mg/kg) | B1 (thiamine; mg/kg) | B2 (riboflavin; mg/kg) | B6 (pyridoxine; mg/kg) | B12 (cobalamin; mg/kg) | Fat (g/kg) | Protein (g/kg) | Carbo- hydrate (g/kg) | Peroxide (mEq/ kg fat) | |
| Untreated | 12,700 | 112 | 3.9 | 9.9 | 5.0 | 0.3 | 131 | 279 | 446 | 0.8 |
| Irradiated (28.9–34.4 kGy) | 12,200 (96%) | 114 (102%) | 3.5 (90%) | 9.5 (96%) | 4.2 (84%) | 0.2 (67%) | 134 (102%) | 277 (99%) | 441 (99%) | 8.9 (1113%) |
| Irradiated (38.4–48.7 kGy) | 11,900 (94%) | 114 (102%) | 3.1 (79%) | 9.5 (96%) | 4.2 (84%) | 0.2 (67%) | 134 (102%) | 277 (99%) | 443 (99%) | 11.3 (1413%) |
| Pasteurized | 12,100 (95%) | 112 (100%) | 2.7 (69%) | 9.7 (98%) | 4.8 (96%) | 0.2 (67%) | 135 (103%) | 266 (95%) | 435 (98%) | 0.7 (88%) |
| AAFCO | 5000 | 50 | 1.0 | 2.2 | 1.0 | 0.022 | 80 | 220 | – | – |
| NRC | 5050 | 30 | 2.25 | 5.3 | 1.5 | 0.035 | 85 | 200 | – | – |
Samples of dry dog food (500 g) were analyzed for the content of the above nutrients after gamm a irradiation at 2 different doses (typical, 28.9–34.4 kGy; high end, 38.4–48.7 kGy) and pasteurization at 107 °C for 15 min. The values in parenthesis are the percentage change from preirradiation levels. Also included are the recommended minimal daily intake values of each nutrient for growth and reproduction for cats from the Association of American Feed Control Officials (AAFCO; 2007) and National Research Council (NRC; 2006), based on dry matter. – = Not Reported.
Table 4.
Vitamins and nutritional components in dry rodent food before and after gamma irradiation or pasteurization
| Vitamins |
Other nutritional components |
|||||||||
| A (retinol; IU/kg) | E (tocopherol; mg/kg) | B1 (thiamine; mg/kg) | B2 (riboflavin; mg/kg) | B6 (pyridoxine; mg/kg) | B12 (cobalamin; mg/kg) | Fat (g/kg) | Protein (g/kg) | Carbo- hydrate (g/kg) | Peroxide (mEq/ kg fat) | |
| Untreated | 9030 | 92 | 9.5 | 10.2 | 14.7 | 0.069 | 63 | 189 | 585 | 0.8 |
| Irradiated (28.9-34.4 kGy) | 8140 (90%) | 85 (92%) | 8.4 (88%) | 11.0 (108%) | 11.0 (75%) | 0.063 (91%) | 67 (106%) | 178 (94%) | 592 (101%) | 9.2 (1150%) |
| Irradiated (38.4-48.7 kGy) | 6030 (67%) | 85 (92%) | 8.9 (94%) | 10.0 (98%) | 9.5 (65%) | 0.069 (100%) | 63 (100%) | 189 (100%) | 588 (101%) | 19.9 (2488%) |
| Pasteurized | 7260 (80%) | 88 (96%) | 6.6 (69%) | 11.2 (110%) | 10.7 (73%) | 0.073 (106%) | 58 (92%) | 179 (95%) | 592 (101%) | 1.4 (175%) |
| LAC Diets Advisory | 7000 | 60 | 4.0 | 5.0 | 6.0 | 0.005 | 40 | – | – | – |
| NRC (mouse diet) | 2400 | 22 | 5.0 | 7.0 | 8.0 | 0.010 | 50 | 180 | – | – |
Samples of dry rodent diet (500 g) were analyzed for the content of the above nutrients after gamma irradiation at 2 different doses (typical, 28.9–34.4 kGy; high end, 38.4–48.7 kGy) and pasteurization at 107 °C for 15 min. The values in parenthesis are the percentage change from preirradiation values. In addition, the recommended level of each nutrient to be added per kilogram of diet for rodents is included (Laboratory Animals Centre [LAC] Diets Advisory Committee, 1977; National Research Council [NRC], 1995). – = Not Reported
Nutritive composition of dry cat diet (Table 2 and Figures 1 and 2).
Analysis of untreated cat diet showed that all measured constituents met or exceeded the NRC 2006 recommended allowance and the AAFCO 2007 minimal requirements for cats, where such levels have been set.4,29
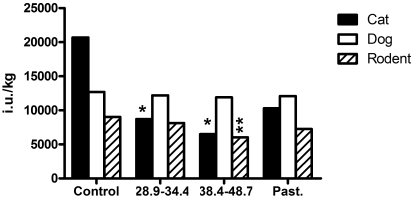
Figure 1.
Samples of dry food (500 g) were analyzed for vitamin A content after gamma irradiation at 2 doses (typical dose, 28.9–34.4 kGy; high-end dose, 38.4–48.7 kGy) and pasteurization at 107 °C for 15 min. *, value lower than the recommended minimal daily intake of 9000 IU/kg for growth and reproduction for cats as designated by the Association of American Feed Control Officials guidelines of 2007; **, value lower than the recommended minimal dietary intake of 7000 IU/kg as designated by the Laboratory Animals Centre Diets Advisory Guideline of 1977.
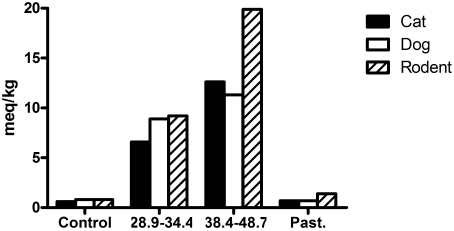
Figure 2.
Samples of dry food (500 g) were analyzed for peroxide content after gamma irradiation at 2 doses (typical dose, 28.9–34.4 kGy; high-end dose, 38.4–48.7 kGy) and pasteurization (Past.) at 107 °C for 15 min.
Vitamins.
Although most parameters measured in the dry cat diet were unaffected by either the low or high gamma irradiation dose, vitamin A was reduced to 42% (typical dose) and 31% (high-end dose) of its untreated value. For the typical dose, these data corresponded to approximately 72% of the AAFCO minimal recommended level for growth and reproduction and 98% of the NRC recommended allowance. Pasteurization of the dry cat diet reduced vitamin A to 50% of its original value, but this value was still within the AAFCO minimal and NRC accepted levels for cats.
Peroxide.
Concentration of peroxide in the dry cat diet was increased to 11- and 21-fold after the typical and high-end irradiation treatments, respectively. Pasteurization modestly increased this parameter to 117% of its untreated value.
Other nutritional components.
Fat, protein, and carbohydrate levels were not affected by either gamma irradiation dose or by pasteurization. Taurine levels were only minimally affected by irradiation and by pasteurization, and the values remained well above the AAFCO and NRC recommended minimal levels for cats.
Nutritive composition of dry dog food (Table 3 and Figures 1 and 2).
Analysis of untreated dry dog food showed that all measured constituents exceeded the NRC 2006 recommended allowance and the AAFCO 2007 minimal requirements for dogs, where such levels have been set. With the exception of peroxide, all other parameters analyzed were unaffected by either irradiation dose or pasteurization, and all values exceeded the NRC 2006 and the AAFCO 2007 accepted levels.
Peroxide.
The concentration of peroxide in the dry dog diet was increased to 11- and 14-fold after the typical and high-end irradiation treatments, respectively. Pasteurization did not affect peroxide as compared with the value in untreated feed.
Other nutritional components.
Fat, protein, and carbohydrate levels were not affected by either gamma irradiation at either dose or by pasteurization.
Nutritive composition of rodent diet (Table 4 and Figures 1 and 2).
Analysis of untreated rodent diet showed that all measured constituents exceeded the LAC Diets Advisory Committee 1977 guidelines and the NRC 1995 requirements of rodents, where such levels have been set.
Vitamins.
Although most parameters were unaffected by either dose of gamma irradiation, vitamin A was reduced to 67% of its untreated value at the higher irradiation dose, corresponding to approximately 86% of the LAC Diets Advisory Committee recommendation and 251% of the NRC recommendation for this parameter for mouse diet. Vitamin B6 was reduced to 65% of its untreated value at the higher irradiation dose but remained above the LAC Diets Advisory Committee and the NRC accepted levels for this parameter.
Peroxide.
Concentration of peroxide in the rodent diet was increased to 11.5- and 25-fold after the typical and high-end irradiation treatments, respectively. Pasteurization modestly increased this parameter to 175% of its untreated value.
With the exception of peroxide, all parameters measured were unaffected by pasteurization and remained at or above the LAC Diets Advisory Committee and the NRC guidelines, where such levels have been set.
Other nutritional components.
Fat, protein, and carbohydrate levels were not affected by either gamma irradiation at either dose or by pasteurization.
Discussion
The results of this study confirm that gamma irradiation, at the doses used, has profound effects on the vitamin A (retinol) and peroxide content of the dry cat food analyzed. Interestingly, although the level of this vitamin was reduced after equivalent gamma irradiation of canine and rodent diets, the relative reduction was much smaller than in cat diet, despite the fact that peroxide levels were increased in all diets to a similar extent. Why the reduction in vitamin A was not similar across the 3 diets is unclear but may be due to diet manufacture, the fat source and content of the diet, the diet's water content, the pretreatment concentration of vitamin A, or the ratios of the various dietary components.
Another interesting finding was that irradiation of rodent diet generated levels of peroxide that were roughly equivalent to those generated in the other diets, despite the fact that the rodent diet, with 6% fat, had less than half the fat content of the feline and canine diets (13% fat). The relatively greater levels of peroxide generated after irradiation of the rodent diet may be related to the larger component of polyunsaturated fatty acids (3.3%) in this diet as compared with the feline (2.91%) or canine (2.75%) rations19 and may suggest a direct relationship between the level of peroxide generated in the irradiated diet and the diet's preexisting polyunsaturated fatty acid content. The fatty acid composition of the fat in the diet and especially the degree of unsaturation of these acids is particularly important.45 Saturated and monounsaturated fatty acids such as oleic acid are resistant to the effects of radiation, linoleic acid is relatively resistant, whereas polyunsaturated fatty acids containing 3 or more double bonds are destroyed readily by irradiation.23 Although the rodent diet largely consisted of linoleic and linolenic acid, which should be less prone to oxidative attack, the cat diet contained arachidonic, eicosapentaenoic, and docosahexaenoic acid, which are fatty acids that are more susceptible to radiation damage. A previous study23 reported that doses of ionizing radiation within the 200 to 1000 krad (2 to 10 kGy) range recommended for food preservation are likely to cause extensive changes to the lipid component of the food, particularly if a large quantity of highly unsaturated omega 3, C22.5, and C22.6 fatty acids are present, because these are particularly sensitive to irradiation. The formation of peroxide in irradiated fat is dependent on factors such as the chemical composition of the fat, type of radiation used, total dose-rate of the radiation, dispersion of fat in the diet, nature of the medium used for dispersion, and the presence of water.45
In the present study, a gamma irradiation dose of 38.4 to 48.7 kGy reduced vitamin A levels to 98% of the NRC recommended allowance but to approximately 72% of the AAFCO minimal recommended level for cats. This result is similar to that of a previous study, 19 which found that irradiation at 2.5 Mrad (25 kGy) of several commercially available dry cat diets resulted in a loss of vitamin A content and an increase in the peroxide value, an estimate of the oxidative rancidity of the fat. Our study found that irradiation also produced modest reductions in vitamin A (retinol) content and large increases in peroxide content in the dry dog food and rodent diet analyzed, and the dose of irradiation was directly related to the reduction in the level of vitamin A. The increase in the peroxide content of all 3 dry diets analyzed is similar to that of previous studies.11,19,43 To date, the NRC and AAFCO have not set recommended limits for peroxide levels in animal diets.
Vitamin A deficiency is associated with a variety of disorders, including impaired growth, visual deficits, decreased reproductive fitness, decreased disease resistance, altered bone growth, and neurologic disease.26,32,42, Common neurologic signs associated with vitamin A deficiency include convulsions, seizures, incoordination, and ataxia.6,8,13,21 Vitamin A deficiency has previously been implicated in causing ataxic syndromes in both lions26,31 and cheetahs33 and is suspected to be involved in the development of leukoencephalomyelopathy in SPF cats.9 In that study, irradiation (36 to 47 kGy) reduced vitamin A content by 42% of its untreated value, which is comparable to findings in the present study.9 Vitamin A deficiency is thought to cause neuronal degeneration26,31 indirectly through compression of the CNS after thickening of the cranial bones31 or through elevating cerebrospinal fluid pressure.26 Elevation of cerebrospinal fluid pressure is the earliest detectable change associated with vitamin A deficiency,24 and has been attributed to an increased resistance to the bulk absorption of cerebrospinal fluid into the bloodstream.7,15,21 Although the irradiation doses we used reduced vitamin A to levels lower than the AAFCO minimal recommendations (9000 IU/kg) and the NRC recommended allowance (6666 IU/kg) for growth and reproduction for cats, the resultant vitamin concentrations are higher than the recommended adult maintenance levels of AAFCO (5000 IU/kg) and NRC (3333 IU/kg).
In contrast, pasteurization had a relatively modest effect on the vitamin A and peroxide contents of the dry cat, dog and rodent diet analyzed. Although the vitamin A content in all 3 dry diets was reduced after this treatment, the vitamin A concentration after pasteurization in all cases remained above the AAFCO and NRC minimal accepted level for dogs and cats and above the LAC and NRC guidelines for rodents.
Although analysis of the untreated laboratory diets showed differences from the composition listed on the product specification sheets, the listed constituent measurements are estimates and considerable variation between the stated and actual constituent values can occur.43
Our results raise questions regarding the suitability of gamma-irradiated diets for the long-term exclusive feeding of cats in particular, given that such feeding regimes have been associated with the development of leukoencephalomyelopathy in this species.9 Felids have a higher dietary requirement for vitamin A than other species because they do not convert β-carotene to vitamin A (retinol) and therefore rely on animal products for their source of retinol.35 Perhaps caution should also be applied to the long-term feeding of dogs exclusively on gamma-irradiated dry diets. However given that feeding diets irradiated 25 kGy to laboratory rodents in long-term toxicologic studies is well established with no apparent negative health effect, the findings of the present study may have less implications for the feeding of rodents.30,34,38
In conclusion, this study has shown that gamma irradiation, at the doses used, has profound and selective effects on the vitamin A and peroxide contents of dry animal diets, particularly on dry diets formulated for cats. As a consequence, irradiated diets should be used with caution during long-term exclusive feeding of cats. The findings of this study suggest that pasteurization may provide an alternative option in decontaminating animal diets given that it has less effect on important dietary constituents.
References
- 1.Adamiker D. 1975. A comparison of various methods for treating feedstuffs for laboratory animals. Food Irrad Info 5:19–42 [Google Scholar]
- 2.Analytical Methods Committee, Royal Society of Chemistry, London 1985. Determination of vitamin A in animal feedingstuffs by high-performance liquid chromatography. Analyst 110:1019–1126 [DOI] [PubMed] [Google Scholar]
- 3.Analytical Methods Committee, Royal Society of Chemistry, London 2000. Determination of thiamine and riboflavin in pet foods and animal feedingstuffs. Analyst 125:353–360 [Google Scholar]
- 4.Association of American Feed Control Officials (AAFCO) 2007. Dog and cat food nutrient profiles, p. 131–165. West Lafayette (IN): AAFCO [Google Scholar]
- 5.Blood DC, Studdert VP, Gay CD. 2006. Comprehenisve veterinary dictionary, 3rd edn. Philadelphia: Saunders Elsevier [Google Scholar]
- 6.Booth A, Reid M, Clark T. 1987. Hypovitaminosis A in feedlot cattle. J Am Vet Med Assoc 190:1305–1308 [PubMed] [Google Scholar]
- 7.Calhoun MC, Hurt HD, Eaton HD, Rousseau JE, Hall RC., Jr 1967. Rates of formation and absorption of cerebrospinal fluid in bovine hypovitaminosis A. J. Dairy Sci. 50:1489–1494 [DOI] [PubMed] [Google Scholar]
- 8.Carrigan MJ, Glastonbury JR, Evers JV. 1988. Hypovitaminosis A in pigs. Aust Vet J 65:158–160 [DOI] [PubMed] [Google Scholar]
- 9.Cassidy JP, Caulfield C, Jones BR, Worrall S, Conlon L, Palmer AC, Kelly J. 2007. Leukoencephalomyelopathy in specific pathogen-free cats. Vet Pathol 44:912–916 [DOI] [PubMed] [Google Scholar]
- 10.Clarke HE, Coates ME, Eva JK, Ford DJ, Milner CK, O'Donoghue PN, Scott PP, Ward RJ. 1977. Dietary standards for laboratory animals: report of the Laboratory Animals Centre Diets Advisory Committee. Lab Anim 11:1–28 [DOI] [PubMed] [Google Scholar]
- 11.Coates ME, Ford JE, Gregory ME, Thompson SY. 1969. Effects of gamma irradiation on the vitamin content of diets for laboratory animals. Lab Anim 3:39–49 [Google Scholar]
- 12.Coates ME. 1999. Nutrition and feeding, p. 45–60. In: Poole T. The UFAW handbook on the care and management of laboratory animals, 7th edn. Hoboken (NJ): Wiley [Google Scholar]
- 13.DeLahunta A. 1983. Cerebrospinal fluid and hydrocephalus. Vet Neuroanat Clin Neuorbiol 2: 30–52 [Google Scholar]
- 14.DeRouchey JM, Tokach MD, Nelssen JL, Goodband RD, Dritz SS, Woodworth JC, James BW, Real DE. 2003. Effect of irradiation of individual feed ingredients and the complete diet on nursery pig performance. J Anim Sci 81:1799–1805 [DOI] [PubMed] [Google Scholar]
- 15.Eaton HD. 1969. Chronic bovine hypo- and hypervitaminosis A and cerebrospinal fluid pressure. Am J Clin Nutr 22:1070–1080 [DOI] [PubMed] [Google Scholar]
- 16.Elias PS. 1980. The wholesomeness of irradiated food. Ecotoxicol Environ Saf 4:172–183 [DOI] [PubMed] [Google Scholar]
- 17.Elias PS, Cohen AJ. 1983. Recent advances in food irradiation, p. 203–233. Amsterdam: Elsevier Biomedical [Google Scholar]
- 18.Food and Drug Administration 2001. Irradiation in the production, processing, and handling of animal feed and pet food: irradiation. Fed Regist 66: 18539–18540 [Google Scholar]
- 19.Ford DJ. 1979. Observations on the influence of irradiation on fat and vitamin A in dry laboratory cat diets. Decontamination of animal feeds by irradation, p. 77–81. Vienna: International Atomic Energy Agency [Google Scholar]
- 20.Foster HL. 1964. A pasteurisation process for pelleted diets. Lab Anim Care 14:373–381 [PubMed] [Google Scholar]
- 21.Frye TM, Williams SN, Graham TW. 1991. Vitamin deficiencies in cattle. Vet Clin North Am Small Anim Pract 7:217–275 [DOI] [PubMed] [Google Scholar]
- 22.Grolichova M, Dvorak P, Musilova H. 2004. Employing ionizing radiation to enhance food safety: a review. Acta Vet (Brno) 73:143–149 [Google Scholar]
- 23.Hammer CT, Wills ED. 1979. The effect of ionizing radiation on the fatty acid composition of natural fats and on lipid peroxide formation. Int J Radiat Biol Relat Stud Phys Chem Med 35:323–332 [DOI] [PubMed] [Google Scholar]
- 24.Hayes KC, McCombs HL, Faherty TP. 1971. The fine structure of vitamin A deficiency II. Arachnoid granulations and CSF pressure. Brain 94:213–224 [DOI] [PubMed] [Google Scholar]
- 25.MacDonald ML, Rogers QR, Morris JG. 1984. Nutrition of the domestic cat, a mammalian carnivore. Annu Rev Nutr 4:521–562 [DOI] [PubMed] [Google Scholar]
- 26.Maratea KA, Hooser SB, Ramos-Vara JA. 2006. Degenerative myelopathy and vitamin A deficiency in a young black-maned lion (Panthera leo). J Vet Diagn Invest 18:608–611 [DOI] [PubMed] [Google Scholar]
- 27.Morris JG. 2002. Idiosyncratic nutrient requirements of cats appear to be diet-induced evolutionary adaptations. Nutr Res Rev 15:153–168 [DOI] [PubMed] [Google Scholar]
- 28.National Research Council 1995. Nutrient requirements of laboratory animals, p. 1–153. Washington (DC): National Academy Press [Google Scholar]
- 29.National Research Council 2006. Nutrient requirements of dogs and cats, p. 1–370. Washington (DC): National Academy Press [Google Scholar]
- 30.Oller WL, Greenman DL, Suber R. 1985. Quality changes in animal feed resulting from extended storage. Lab Anim Sci 35:646–650 [PubMed] [Google Scholar]
- 31.O'Sullivan BM, Mayo FD, Hartley WJ. 1977. Neurological lesions in young captive lions associated with vitamin A deficiency. Aust Vet J 53:187–189 [DOI] [PubMed] [Google Scholar]
- 32.Palmer N. 1993. Diseases of bone, p. 87–88. In: Jubb KVF, Kennedy PC, Palmer N. Pathology of domestic animals. San Diego: Academic Press [Google Scholar]
- 33.Palmer AC, Callanan JJ, Guerin LA, Sheahan BJ, Stronach N, Franklin RJ. 2001. Progressive encephalomyelopathy and cerebellar degeneration in 10 captive-bred cheetahs. Vet Rec 149:49–54 [DOI] [PubMed] [Google Scholar]
- 34.Paterson JS. 1978. The production and use of pathogen-free animals, p. 410–429. In: Short DJ, Woodnott DP. The IAT manual of laboratory animal practice and techniques. Hertfordshire (UK): Granada Publishing [Google Scholar]
- 35.Puls R. 1994. Vitamin A, p. 14–34 In: Vitamin levels in animal health. Clearbrook (British Columbia): Sherpa International [Google Scholar]
- 36.Roberts T. 1998. Cold pasteurization of food by irradiation, p. 1–12. In: Food safety, publication 458-300 Blacksburg (VA): Virginia Polytechnic and State University [Google Scholar]
- 37.Schreiber M, Nassett ES. 1959. Digestion of irradiated fat in vivo. J Appl Physiol 14:639–642 [Google Scholar]
- 38.Scientific Committee on Food, European Commission [Internet] Revision of the opinion of the scientific committee on food on the irradiation of food. Available at: http://ec.europa.eu/food/fs/sc/scf/out193_en.pdf [Google Scholar]
- 39.Scott PP. 1965. Minerals and vitamins in feline nutrition, p. 75–88. In: Graham-Jones O. Canine and feline nutritional requirements. Oxford (UK): Pergamon Press [Google Scholar]
- 40.Short DJ. 1978. Sterilization and disinfection, p. 121-142. In: Short DJ, Woodnott DP. The IAT manual of laboratory animal practice and techniques. Hertfordshire (UK): Granada Publishing [Google Scholar]
- 41.Stewart EM. 2004. Food irradiation: more pros than cons? Biologist 51:91–96 [Google Scholar]
- 42.Summers BA, Cummings JF, DeLahunta A. 1995. Degenerative diseases of the central nervous system, p. 208–350. In: Veterinary neuropathology St Louis: Mosby Yearbook [Google Scholar]
- 43.Tobin G, Stevens KA, Russell RJ. 2007. Nutrition, p. 321-383 In: Fox JG, Barthold S, Davisson M, Newcomer CE, Quimby FW, Smith A. The mouse in biomedical research, 2nd edn. Philadelphia: Elsevier [Google Scholar]
- 44.Wescott RB, Gardner JA. 1962. Apparatus and methods for the steam sterilization of feed for germfree laboratory animals. Technical manuscript 7. Fort Detrick (MD): US Army Chemicals Corps Biological Laboratories [Google Scholar]
- 45.Wills ED. 1980. Studies of lipid peroxide formation in irradiated synthetic diets and the effects of storage after irradiation. Int J Radiat Biol Relat Stud Phys Chem Med 37:383–401 [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization (WHO) 1988. Food irradiation. A technique for preserving and improving the safety of food, p. 18–43. Geneva: WHO [Google Scholar]
- 47.World Health Organization (WHO) 1994. Safety and nutritional adequacy of irradiated food. Geneva: WHO [Google Scholar]
- 48.Zagory D. [Internet]. Produce irradiation: the not-so-silver bullet. The Packer, 3 Apr 2000. Available at: http://www.nsf.org/business/nsf_davis_fresh/articles_pro_irradiation.pdf.