Abstract
Fenbendazole (FBZ) is an anthelmintic drug widely used to treat and prevent pinworm outbreaks in laboratory rodents. Although data in nonrodent species indicate possible effects of fenbendazole on the bone marrow and lymphocyte proliferation and function, little has been reported regarding possible effects on the rodent immune system. The purpose of the current study was to determine the effects of a therapeutic regimen of FBZ on immune parameters in BALB/c mice. Both 9-wk on–off and 5-wk continuous medicated feed protocols were assessed. No significant differences between normal and FBZ diet treated mice were observed in the following parameters: complete blood count, blood chemistry, quantitation of major T and B cell markers in spleen, quantitation of T cell markers in the thymus, spleen cell proliferation to T and B cell mitogens, bone marrow colony-forming cell assays, skin graft rejection, and primary and secondary humoral immune responses. These data indicate that FBZ treatment does not affect many standard broad measures of immune function.
Abbreviation: Con A, concanavalin A; FBZ, fenbendazole; LPS, lipopolysaccharide
Fenbendazole (FBZ) is a broad-spectrum benzimidazole anthelmintic drug that has gained widespread use for the treatment and prophylaxis of pinworm infection.12,20,25 FBZ typically is administered through the use of medicated feed and is often used instead of ivermectin, which can have toxic effects in young animals.24 Although no toxic effects have been reported from the use of FBZ at therapeutic levels, the physiologic or biologic actions of the drug potentially might alter or interfere with ongoing research experiments.22,25 To this point, treatment with FBZ apparently decreases fecundity but not reproduction in rats.14,27 Furthermore, FBZ had no effect in rat behavioral studies.3,15 Recently, FBZ reportedly enhanced lipopolysacchide-induced inflammation in rats.13
FBZ treatment alters numerous immune parameters in nonrodent species including inducing myelosuppression and altering T and B cell responses.5,10,11,19,23,26 In contrast, FBZ treatment (150 ppm) for 23 wk did not alter the onset or incidence of diabetes in NOD mice.9 Furthermore, assays of T helper function and cytotoxicity remained unaffected in BALB/c mice treated for 2 wk with 100 ppm FBZ.21 In contrast, FBZ at 10mg/kg for 5 d in C57BL/6 mice resulted in increased spleen cell proliferation to mitogens.8 The goal of the current study was to examine BALB/c mice for changes in routine parameters and assessors of immune function during and after treatment with a commercially available FBZ-medicated (150 ppm) diet. Many treatment protocols using FBZ-medicated diet have been proposed.12,20 This study was conducted by using both a 9-wk on–off schedule as well as 5-wk continuous application.
Materials and Methods
Animals and housing.
All animals were maintained in accordance with the temperature and humidity recommendations of the Guide for the Care and Use of Laboratory Animals18 at the AAALAC-accredited facilities of the University of Miami. All experimental procedures were approved by the university's Animal Care and Use Committee. Sentinel mice maintained on dirty bedding were screened quarterly for: mouse hepatitis virus, Sendai virus, Mycoplasma pulmonis, pneumonia virus of mice, minute virus of mice, Thieler murine encephalomyelitis virus, mouse parvovirus, mouse rotavirus, lymphocytic choriomeningitis virus, and parasitic infections. Once a year, the panel was extended to include mouse norovirus, Ectromelia virus, K virus, Encephalitozoon cuniculi, polyoma virus, mouse adenovirus, reovirus, murine cytomegalovirus, hantavirus, mouse thymic virus, Clostridium piliforme, and cilia-associated respiratory bacillus. Helicobacter testing by PCR was conducted also. All results were negative during the course of this study.
BALB/c female mice purchased from Charles River Laboratories (Wilmington, MA) were used throughout the studies and were 8 wk of age on arrival. C57BL/6 mice used as skin-graft donors were obtained from an inhouse breeding colony. Mice were conventionally housed in groups of 5 animals per cage by using nonautoclaved shredded aspen bedding (Harlan Teklad, Madison, WI) and microisolation filter tops. They were given ad libitum access to rodent chow and municipal water by bottle. Regular chow was purchased from PMI International (Lab Diet 5001; Richmond, IN). Fenbendazole medicated diet was purchased from Harlan Teklad (Irradiated Global Diet 2018 with 150 ppm FBZ). The concentration of FBZ was verified through analytical testing at a reference laboratory.
Animals were bled by using the submandibular method, and blood was placed in a lithium-heparinized tube (Capijet, VWR, West Chester, PA) for complete blood count and chemistry analyses. Blood was placed in an anticoagulant-free tube (Capijet, VWR) for the antibody studies. Animals used for the in vitro assays were euthanized by cervical dislocation to minimize cellular changes in the lymphoid tissues. All other animals were euthanized by CO2 inhalation.
For the 9-wk on–off treatment schedule, time points of days 21, 35, and 63 were examined in 2 independent experiments. For the 5-wk continuous treatment schedule, time points of days 14, 21, and 35 were examined in 2 independent experiments. In the 9- wk group, mice received FBZ-containing feed for 1 wk followed by 1 wk of FBZ-free feed, yielding a 63-d treatment period. Chow intake did not appear to vary during this period, in that FBZ-treated mice mirrored the weight gain found in control (normal chow) mice. A minimum of 5 mice were evaluated per time point in each treatment group. Treatment groups included mice receiving the normal diet and the 5- and 9-wk FBZ treatment groups. Weekly throughout the treatments, the mice were weighed and examined for any changes in clinical appearance.
Complete blood count and blood chemistry analysis.
The complete blood count was performed by using an automated analyzer (Hemavet 950, Drew Scientific, Waterbury, CT). Parameters included: total WBC, total RBC, hemoglobin, hematocrit, RBC indices, WBC differential, and platelet count. After completion of the complete blood count, the lithium-heparin tube was centrifuged, and the plasma was analyzed by using an automated chemistry analyzer (Ortho Vitros 250, Ortho Diagnostics, Rochester, NY). The analyses included: glucose, BUN, creatinine, calcium, phosphorus, total protein, and alanine transaminase. Data were analyzed as the mean ± SE of a minimum of 5 mice per experimental group.
Preparation of cell suspensions.
Cell suspensions were prepared from the spleen and thymus by gentle homogenization in RPMI1640 media (without supplements, VWR). Bone marrow suspensions were prepared from bone marrow pulp extruded by injecting RPMI1640 media into the ends of tibia and femur bones. After centrifugation, cells were counted by using trypan blue exclusion. Tissue samples from 5 mice were pooled and examined per time point.
Flow cytometry.
Cells from spleen and thymus were labeled at 4 °C in the dark for 15 to 20 min with one or more of the following antibodies (BD Pharmingen, San Diego, CA): phycoerythrin-conjugated antiCD8 (clone 53-6.7); FITC-conjugated antiCD4 (clone GK1.5), FITC-conjugated antiCD3 (clone 145-2C11), FITC-conjugated antiIg. Cells were analyzed on a flow cytometer (FACScan, Becton Dickinson, San Jose, CA) with software provided by the manufacturer (CellQuest, Becton Dickinson) and using forward and side scatter gates previously identified to contain lymphoid cells.
Colony-forming cell assays.
Three colony-forming cell assays were conducted by using reagents purchased from StemCell Technologies (Vancouver, BC, Canada). Bone marrow was harvested from the femur and tibia bones, counted, and adjusted to a cell concentration as indicated in the protocols provided by the company. The bone marrow was plated in triplicate and incubated at 37 °C in 5% CO2 and 95% humidity in specific media (MethoCult, StemCell Technologies) to stimulate the production of erythrocyte, granulocyte, and preB cell progenitors. Colony-forming cells were counted after 3 to 12 d by using an inverted microscope. Data are presented as the mean ± SE of triplicate cultures.
In vitro proliferation of spleen cells.
Spleen cell proliferation assays were conducted by using sterile 96-well flat-bottom plates (Costar, Cambridge, MA). Cell suspensions from pooled tissues prepared for flow cytometry were adjusted appropriately for a final concentration of 1× 105 cells per well. Cells were incubated at 37 °C, 5% CO2, and 95% humidity with concanavalin A (ConA, 5 mg/ml, Sigma, St Louis, MO), antiCD3 monoclonal antibody (clone 145-2C11, 1:10 in tissue culture supernatant), and lipopolysaccharide (LPS, 50 μg/ml, Sigma) in RPMI1640 media supplemented with 10% FCS, L-glutamine, antibiotics, and nonessential amino acids (VWR). After 48 h, cultures were pulsed with 1 μCi 3H-thymidine (New England Nuclear, Boston, MA) for 18 h. The cultures were harvested, and uptake was measured by liquid scintillation. Data are presented as mean ± SE of triplicate cultures.
Skin grafting.
Allogeneic tail skin was grafted onto the tail skin of recipients according to the procedure of Melvold.7,16 Recipient mice were anesthetized by using a ketamine–xylazine mixture. C57BL/6 donor skin grafts (approximately 2 × 5 mm) were placed on open tissue beds of recipient tails, allowed to adhere, and covered with a polystyrene tube for 1 to 2 d. Mice were examined daily for pain and discomfort. This protocol has routinely been found to not require analgesia, and none was given as per our approved protocol. On day 3, grafts were examined and graded as satisfactory, unsatisfactory, or no graft. Only satisfactory grafts were examined the remainder of the experiment. Every 1 to 2 d thereafter, grafts were graded on a quantitative system for appearance, loss of hair, drying or scabbing of the graft, and color of the tissue. Complete rejection occurred with total loss of the grafted tissue. Recipients also received syngeneic grafts for internal experimental controls. Results are calculated as the mean rejection score ± SE for the 5 mice in each experimental group.
Assessment of primary and secondary antibody responses.
Mice were injected intraperitoneally with 0.05 ml of 1 mg/ml dinitrophenyl–human serum albumin, Sigma) emulsified v/v with Titermax (Sigma). For primary antibody responses, a single injection was given on each experimental time point during FBZ treatment. For secondary antibody response, an injection was given 2 wk prior to the start of the project while the mice were on a normal rodent diet. Follow-up injections were given on each experimental time point during FBZ treatment. For each time point, 5 mice were injected per experimental group. Fourteen days after the last injection, mice were bled by the submandibular method, and serum was tested by ELISA. For this procedure, ELISA plates (Maxisorp Immunomodule ELISA plates, Nunc, Rochester, NY) were coated with 1 μg/ml dinitrophenyl–human serum albumin and blocked with a 10% casein solution (Sigma). Serum samples were diluted 2-fold to determine the titer or endpoint of reactivity as tested and compared with serum from nonimmunized mice. Results are expressed as the mean titer ± SE of the 5 mice per experimental group.
Statistical analysis.
Mean and standard error were calculated for all tests. The Student t test was used to calculate P values. Differences associated with P values of less than or equal to 0.05 were considered significant. All statistical analyses were conducted by using Prism 4 software (GraphPad Software, La Jolla, CA).
Results
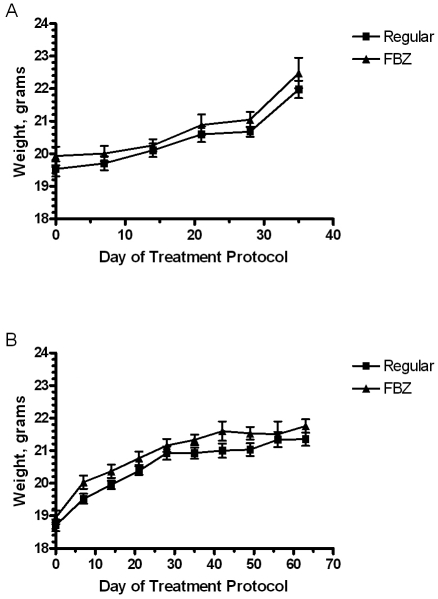
Throughout each treatment protocol, the clinical condition of the mice did not change grossly. Weights of the animals did not differ between those receiving normal and medicated diets, although all mice gained weight over the course of treatment regardless of diet (Figure 1). Hematology and chemistry analyses also revealed no significant differences (data not shown).
Figure 1.
Assessment of weight change in mice treated with fenbendazole- medicated diet continuously for 35 d and by an on–off protocol for 63 d. Data are mean ± SE of data from a pool of 12 mice collected at multiple time points during each treatment protocol. There are no significant differences between control and FBZ mice (P > 0.05).
Flow cytometric analysis of pooled thymocytes and splenocytes revealed no significant differences (Table 1). For purposes of statistical analyses, flow cytometric data were averaged over 3 experimental time points for each treatment schedule. When spleen cells were cultured, no significant differences in proliferation to the mitogens ConA or LPS or with the use of an anti-CD3 monoclonal antibody were observed (Table 2). In Table 2, proliferation data are represented by the last experimental time point, which was the termination of treatment for both schedules. Similar experiments were conducted at earlier time points during treatment, and no significant differences were found between treatment groups (data not shown).
Table 1.
Spleen and thymus cell flow cytometric data from FBZ-treated mice
| Continuous feed protocol (% of total population) |
On–off protocol (% of total population) |
|||
| Regular diet | FBZ diet | Regular diet | FBZ diet | |
| Spleen | ||||
| CD4+ | 23.8 ± 2.5 | 24.0 ± 1.3 | 21.6 ± 0.4 | 20.9 ± 2.6 |
| CD8+ | 12.9 ± 2.1 | 10.6 ± 1.4 | 10.2 ± 1.1 | 15.3 ± 1.0 |
| Ig+ | 40.2 ± 1.9 | 37.5 ± 3.6 | 25.0 ± 0.8 | 33.6 ± 8.7 |
| Thymus | ||||
| CD4−CD8− | 3.0 ± 0.3 | 3.6 ± 0.9 | 7.3 ± 4.5 | 4.7 ± 3.2 |
| CD4+CD8− | 9.7 ± 0.9 | 9.6 ± 1.2 | 6.4 ± 0.9 | 6.2 ± 0.2 |
| CD4−CD8+ | 3.5 ± 1.3 | 2.9 ± 0.6 | 3.2 ± 1.1 | 3.5 ± 0.6 |
| CD4+CD8− | 67.7 ± 7.7 | 62.1 ± 9.1 | 67.2 ± 11.3 | 83.7 ± 2.1 |
Mice were treated continuously for 35 d and by an on–off protocol for 63 d. Values shown reflect the mean ± SE of data collected from a pool of 5 mice collected at days 14, 21, and 35 for the continuous-feed protocol and days 21, 35, and 63 for the on–off protocol . There are no significant differences between control and FBZ mice (P > 0.05).
Table 2.
Spleen cell proliferation data from FBZ-treated mice
| Continuous feed protocol |
On–off protocol |
|||
| Stimulation | Regular diet | FBZ diet | Regular diet | FBZ diet |
| None (medium only) | 1212 ± 101 | 1154 ± 88 | 1874 ± 854 | 1922 ± 765 |
| ConA | 71370 ± 2241 | 73711 ± 9780 | 108389 ± 16562 | 83504 ± 13463 |
| LPS | 27371 ± 7399 | 15612 ± 988 | 57512 ± 7993 | 65245 ± 5380 |
| AntiCD3 | 55510 ± 7572 | 42030 ± 10174 | 30140 ± 2393 | 26647 ± 1576 |
Mice were treated continuously for 35 d and by an on–off protocol for 63 d. Values shown reflect mean ± SE of proliferation (counts per minute) obtained from triplicate cultures of a pool of 5 control or FBZ-treated mice on day 35 for the continuous-feed protocol and day 63 for the on–off protocol. There are no significant differences between control and FBZ mice (P > 0.05).
Bone marrow cultures yielded no significant differences in the number of colony-forming units of the erythrocyte, granulocyte, and preB cell lineages. The data presented in Table 3 are representative of data collected at earlier time points during treatment.
Table 3.
Bone marrow colony-forming assays of FBZ-treated mice
| Continuous feed protocol |
On–off protocol |
|||
| Progenitor assay | Regular diet | FBZ diet | Regular diet | FBZ diet |
| Erythroid | 67.0 ± 3.8 | 67.7 ± 6.3 | 174.0 ± 31.6 | 133.3 ± 29.8 |
| preB | 27.0 ± 3.8 | 28.3 ± 3.8 | 23.7 ± 4.8 | 18.3 ± 0.7 |
| Lymphoid | 31.7 ± 3.9 | 29.0 ± 3.5 | 36.7 ± 4.7 | 44.3 ± 8.7 |
Mice were treated continuously for 35 d and by an on–off protocol for 63 d. Values shown reflect mean ± SE of number of colonies obtained from triplicate cultures of a pool of 5 control or FBZ mice on day 35 for the continuous-feed protocol and day 63 for the on–off protocol. There are no significant differences between control and FBZ mice (P > 0.05).
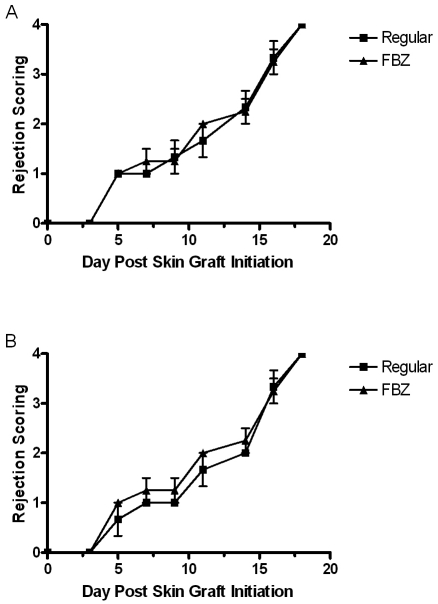
Two evaluations of in vivo immune function were undertaken. No significant differences in skin graft rejection were observed between mice with normal and FBZ-containing diets (Figure 2). Primary immunization yielded low titers of antigen-specific antibody in both treatment groups (Table 4). Secondary immunization reflected a large increase in titer. In studies of both treatment protocols, there were no significant differences in mice undergoing primary or secondary immunizations. By all in vivo assessments, no significant differences were found by using these methods at earlier time points during treatment (data not shown).
Figure 2.
Assessment of skin graft rejection in mice after treatment with fenbendazole-medicated diet continuously for 35 d and by an on–off protocol for 63 d. Data are mean ± SE of data from 5 mice collected at multiple time points after skin grafting. A score of 4 is considered a fully rejected graft. There are no significant differences between control and FBZ mice (P > 0.05). These results are representative of skin grafting performed at early time points during treatment.
Table 4.
Antibody titers of FBZ-treated mice
| Continuous feed protocol |
On–off protocol |
|||
| Immunization | Regular diet | FBZ diet | Regular diet | FBZ diet |
| Primary | 1843 ± 597 | 716 ± 215 | 7373 ± 819 | 8192 ± 0 |
| Secondary | 235900 ± 26210 | 157300 ± 44450 | 81920 ± 16384 | 106496 ± 24576 |
Mice were treated continuously for 35 d and by an on–off protocol for 63 d. Values shown reflect mean ± SE of titers obtained from 5 control or FBZ mice on day 35 for the continuous-feed protocol and day 63 for the on–off protocol. There are no significant differences between control and FBZ mice (P > 0.05).
Discussion
The aim of this study was to evaluate whether commonly used therapeutic regimens of the anthelmintic fenbendazole alters any routine parameters used to monitor immune function. Both in vitro and in vivo responses were studied in mice that were treated with FBZ-medicated diet in 5-wk continuous and 9-wk on–off schedules. The lymphoid tissues examined included bone marrow, thymus, and spleen. Both T and B cell function were evaluated by using in vivo methods.
Several previous reports have indicated that FBZ can result in severe myelosuppression in nonrodent species;10,19,23,26 these investigators postulated that the origin of the myelosuppression was idiosyncratic and dose-related. Prior to the current report, specific assessments had not been made in rodent species. The current study detected no significant differences in colony-forming cell assays for erythrocyte, granulocyte, and preB cell progenitors as a function of diet. Because the current studies were conducted by using commercially available diet containing 150 ppm FBZ, future studies might address possible dose- or mouse-strain-related differences to elucidate the origin of the myelosuppression in nonrodent species.
Flow cytometric evaluation failed to reveal any significant FBZ-associated differences in major thymocyte and splenocyte populations. FBZ-medicated diet previously was found to not affect splenic lymphocyte subpopulations as evaluated by flow cytometry of diabetic and nondiabetic control mice.9 Furthermore, in the current study, when splenocytes were cultured with mitogens and antiCD3, no significant differences in stimulation as measured by cell-proliferation assays were present between normal and FBZ diet groups in both the 5- and 9-wk treatment schedules. This result is consistent with the findings of unaltered ConA-induced proliferation in T cells from NOD mice treated for 23 wk.9 However the current data contrast with other findings, which indicated that FBZ treatment for 5 d resulted in increased responses in mitogens and altered splenic T cell subpopulations.8 These differences may be related to dosages, treatment schedules, mouse strains, and analytical methods. For example, in the cited study, FBZ was administered at a dose of 10 mg/kg twice daily for 5 d, and spleen cell proliferation was assessed after 72 h of incubation.8 Other investigators treated mice by medicated diet for 23 wk and assessed proliferation at 72 h.9 The current study used routine 5- and 9-wk treatment protocols and a 48-h spleen cell proliferation period.
Compared with the current study, one study performed more specific analyses of T cell function in mice after a 2-wk treatment with 100 ppm FBZ-medicated diet.21 In vitro methods were used to assess the generation of allospecific and influenza-specific T cells responses, and no significant differences between normal and FBZ-treated BALB/c mice were found. Furthermore, the same study quantitated influenza-specific T cell proliferation and antibody production. In the current study, despite a trend toward decreased antibody production in the 5-wk continuous treatment protocol, no significant differences were observed in the titers generated in response to immunization. Overall, previously published results are consistent with the findings of the current study regarding evaluation of the in vivo immune responses of skin graft rejection and primary and secondary antibody production.
In contrast to the lack of changes in broad in vivo measures, other work from our group has demonstrated that FBZ treatment reduce some functions of proB cells in young (8 to 12 wk) and old (22 to 24 mo) BALB/c mice.17 Using specific measures of transcription factors, mRNA expression, and DNA binding, that study detected these effects on the fourth round of treatment in the on–off schedule. Most of these changes, including mild depression of the B cell proliferative response to LPS, returned to normal levels by 6 wk after treatment. These data indicate that FBZ treatment has the potential to affect experimental results from sensitive immunologic assays. Future studies should be considered to assess the effects of FBZ treatment on specific cell populations including T cell subsets, B cells, and antigen-presenting cells.
FBZ has been reported to block mitosis of human lymphocytes in vitro.11 In addition, FBZ treatment of sheep reduced peripheral blood lymphocyte responses to ConA and phytohemagglutinin;5 this study also indicated that FBZ decreased the in vivo response to a particulate antigen. These studies may reflect species differences in sensitivity to FBZ and are consistent with the earlier-cited reports of FBZ-induced myelosuppression in nonrodent species.
A recent survey indicated that parasitic infections in rat and mouse colonies are quite prevalent.6 These infections, particularly with pinworms, are known to affect the physical condition and health of laboratory rodents.2 With regard to the immune system, pinworm- infected mice have increased myelopoiesis and erythropoiesis, altered reactivity to cytokines, and altered immune tolerance, which can lead to the development of autoimmune diseases.1,4 These and other biologic changes support the importance of eradication of pinworms from research colonies. Commercially available diets and proven treatment regimens make eradication a feasible undertaking.12,20 However, researchers may understandably be concerned that treatment for pinworms may alter experimental results. In contrast to other treatments, FBZ-medicated diet appears to have minimal (if any) effect on frequently used immunocompetent rodent strains.25 The current study provides evidence that FBZ does not affect broad in vitro and in vivo measures of the immune system of 8-wk-old BALB/c mice.
References
- 1.Agersborg SS, Garza KM, Tung KS. 2001. Intestinal parasitism terminates self tolerance and enhances neonatal induction of autoimmune disease and memory. Eur J Immunol 31:851–859 [DOI] [PubMed] [Google Scholar]
- 2.Baker DG. 1998. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev 11:231–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barron S, Baseheart BJ, Segar TM, Deveraux T, Willford JA. 2000. The behavioral teratogenic potential of fenbendazole: a medication for pinworm infestation. Neurotoxicol Teratol 22:871–877 [DOI] [PubMed] [Google Scholar]
- 4.Bugarski D, Jovcic G, Katic-Radivojevic S, Petakov M, Krstic A, Stojanovic N, Milenkovic P. 2006. Hematopoietic changes and altered reactivity to IL17 in Syphacia obvelata-infected mice. Parasitol Int 55:91–97 [DOI] [PubMed] [Google Scholar]
- 5.Cabaj W, Stankiewicz M, Jonas WE, Moore LG. 1994. Fenbendazole and its effect on the immune system of the sheep. N Z Vet J 42:216–220 [DOI] [PubMed] [Google Scholar]
- 6.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276 [DOI] [PubMed] [Google Scholar]
- 7.Cray C, Mateo MO, Altman NH. 1993. In vitro and long-term in vivo immune dysfunction after infection of BALB/c mice with mouse hepatitis virus strain A59. Lab Anim Sci 43:169–174 [PubMed] [Google Scholar]
- 8.Dvoroznakova E, Boroskova Z, Dubinsky P, Velebny S, Tomasovicova O, Machnicka B. 1998. Changes in cellular immunity of mice treated for larval toxocarosis with fenbendazole. Helminthologia. 35:189–195 [Google Scholar]
- 9.Franke DD, Shirwan H. 2006. Prophylactic fenbendazole therapy does not affect the incidence and onset of type 1 diabetes in nonobese diabetic mice. Int Immunol 18:453–458 [DOI] [PubMed] [Google Scholar]
- 10.Gary AT, Keri ME, Wiedmeyer CE, Turnquist SE, Cohn LA. 2004. Bone marrow hypoplasia associated with fenbendazole adminstration in a dog. J Am Anim Hosp Assoc 40:224–229 [DOI] [PubMed] [Google Scholar]
- 11.Holden HE, Crider PA, Wahrenberg MG. 1980. Mitotic arrest by benzimidazole analogs in human lymphocyte cultures. Environ Mutagen 2:67–73 [DOI] [PubMed] [Google Scholar]
- 12.Huerkamp MJ, Benjamin KA, Zitzow LA, Pullium JK, Lloyd JA, Thompson WD, Webb SK, Lehner ND. 2000. Fenbendazole treatment without environmental decontamination eradicates Syphacia muris from all rats in a large, complex research institution. Contemp Top Lab Anim Sci 39:9–12 [PubMed] [Google Scholar]
- 13.Hunter RL, Choi DY, Kincer JF, Cass WA, Bing G, Gash DM. 2007. Fenbendazole treatment may influence lipopolysaccharide effects in rat brain. Comp Med 57:487–492 [PubMed] [Google Scholar]
- 14.Johnston NA, Bieszczak JR, Verhulst S, Disney KE, Montgomery KE, Toth LA. 2006. Fenbendazole treatment and litter size in rats. J Am Assoc Lab Anim Sci 45:35–39 [PubMed] [Google Scholar]
- 15.Keen R, Macinnis M, Guilhardi P, Chamberland K, Church R. 2005. The lack of behavioral effects of fenbendazole: a medication for pinworm infection. Contemp Top Lab Anim Sci 44:17–23 [PubMed] [Google Scholar]
- 16.Kohn HI, Melvold RW. 1974. Spontaneous histocompatibility mutations detected by dermal grafts: significant changes in rate over a 10-year period in the mouse H-system. Mutat Res 24:163–169 [DOI] [PubMed] [Google Scholar]
- 17.Landin AM, Frasca D, Zaias J, Van der Put E, Riley R, Altman NH, Blomberg B. 2008 Personal communication. [Google Scholar]
- 18.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 19.Neiffer DL, Lydick D, Burks K, Doherty D. 2005. Hematologic and plasma biochemical changes associated with fenbendazole administration in Hermann tortoises (Testudo hermanni). J Zoo Wildl Med 36:661–672 [DOI] [PubMed] [Google Scholar]
- 20.Pritchett KR, Johnston NA. 2002. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp Top Lab Anim Sci 41:36–46 [PubMed] [Google Scholar]
- 21.Reiss CS, Herrman JM, Hopkins RE., 2nd 1987. Effect of anthelminthic treatment on the immune response of mice. Lab Anim Sci 37:773–775 [PubMed] [Google Scholar]
- 22.Sajid MS, Iqbal Z, Muhammad G, Iqbal MU. 2006. Immunomodulatory effect of various antiparasitics: a review. Parasitology 132:301–313 [DOI] [PubMed] [Google Scholar]
- 23.Stokol T, Randolph JF, Nachbar S, Rodi C, Barr SC. 1997. Development of bone marrow toxicosis after albendazole administration in a dog and cat. J Am Vet Med Assoc 210:1753–1756 [PubMed] [Google Scholar]
- 24.Toth LA, Oberbeck C, Straign CM, Frazier S, Rehg JE. 2000. Toxicity evaluation of prophylactic treatments for mites and pinworms in mice. Contemp Top Lab Anim Sci 39:18–21 [PubMed] [Google Scholar]
- 25.Villar D, Cray C, Zaias J, Altman NH. 2007. Biologic effects of fenbendazole in rats and mice: a review. J Am Assoc Lab Anim Sci 46:8–15 [PubMed] [Google Scholar]
- 26.Weber MA, Terrell SP, Neiffer DL, Miller MA, Mangold BJ. 2002. Bone marrow hypoplasia and intestinal crypt cell necrosis associated with fenbendazole administration in five painted storks. J Am Vet Med Assoc 221: 369, 417–419 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization WHO food additive series, no. 29. Toxicological evaluation of certain veterinary drug residue. 1991. Available at http://www.inchem.org/documents/jecfa/jecmono/v29je01.htm [Google Scholar]