Abstract
A preferred method to genotype genetically engineered mice is through collection of distal tail tissue (tail biopsy) followed by DNA isolation. Currently, general or local anesthesia (or both) is recommended for biopsy after 3 wk of age, the time after which tail vertebrae are considered to be ossified. Our objective was to rigorously evaluate vertebral development, DNA content, and acute behavioral responses at different ages by harvesting tail biopsies of different lengths. We evaluated laboratory mice from 5 inbred strains and 1 outbred stock at each of 12 ages (3 to 42 d of age). Biopsies of 5-, 10-, and 15-mm lengths were obtained. Vertebrae were graded according to level of ossification by using complementary modalities of high-resolution microradiography, microcomputed tomography, and histology. Vertebral development progressed at different rates among the strains, with mature tail vertebrae containing endplates detectable in the tail of some strains by 10 d of age. Within the distal 2 mm of tail, end plates were not identified before 21 d of age. DNA yield (DNA weight/tissue weight) was greatest from the 5-mm biopsy harvest. Acute behavioral responses to biopsy varied by age and strain, and these differences were associated with vertebral maturation. Vertebral development progressed most rapidly in C57BL/6 mice, which also demonstrated the highest response rate to biopsy, whereas BALB/c mice had slower vertebral development and were less responsive. These findings support the collection of minimal lengths of tail tissue from mice at ages younger than 17 d, unless anesthesia or analgesia is provided.
Abbreviation: microCT, microcomputed tomography; microRad, microradiography; IMV, immature vertebrae; MV, mature vertebrae
Transgenic and gene-targeted mice provide the opportunity to study the function of genes throughout mammalian development and to model human disease. To determine whether mice are of the appropriate genotype for research studies, DNA must be isolated from viable tissue and analyzed, potentially by Southern blotting, PCR, or by the use of single-nucleotide polymorphism analysis.35 Although DNA can be isolated from tissues including blood,6,9,18,20 saliva,23 hair,40 digits,30 stool,4 ear pinnae7,16,37 and buccal32 and rectal25 mucosa, the preferred choice for rapid screening of large numbers of rodent genotypes is through collection (biopsy) of viable tissue from the distal aspect of the tail.5,10,11,16,19,28 According to the National Institutes of Health, “Obtaining tissue via tail biopsy is a safe, effective and humane procedure that causes minimal or transient pain and distress when performed properly.”33 Once genotyping has been performed, animal colony populations can be reduced to those numbers necessary for experimental efficiency.
Explicit guidelines have not been established for the minimal length of tail tissue to harvest and ideal age of animal to sample in order to maximize DNA quantification and minimize adverse physiologic impact. The biopsy procedure itself is momentary, yet involves the transection of multiple tissue types, including skin, nervous tissue, muscle, tendons, vasculature, cartilage and bone.43 Depending on the length of tail tissue removed, the animal's age at the time of biopsy and its genetic background, this procedure may carry a potential for acute and chronic pain.48 Guidance from the National Institutes of Health regarding the ‘ideal’ timing for tail biopsies states that mice should receive localized anesthesia and be 10 to 21 d old, or within an age range when “the tail tissue is soft (vertebra are not yet calcified).”33 An international working group advocates that the most humane age at which to perform tail biopsy is between 21 and 28 d of age, with provision of appropriate analgesia and anesthesia.38 The assignation of a time point after which general anesthesia is required is related to the time at which it is presumed that murine caudal (tail) vertebrae have undergone complete ossification and maturation with periosteal and endocortical innervation29,36, enabling the mice to sense and respond to painful stimuli. Due to the conflicting recommendations about the age at which to perform biopsies and the likelihood that vertebrae are indeed calcified at young ages, we hypothesized that current guidelines may be based more on custom than on scientific investigation of vertebral development and potential deleterious effects of biopsy lengths harvested at different ages.
Our study was undertaken to rigorously evaluate institutional guidelines promoted to research investigators and to develop science-based welfare standards. We wished to determine whether mice of different genetic backgrounds vary in the maturation and ossification of tail vertebra and to assess behavioral responses immediately following biopsy. We wanted to further determine whether optimal isolation of DNA for genotyping is dependent on the age of mice and length of biopsy sampled. Herein we demonstrate pronounced differences in caudal vertebral development between mice of different genetic backgrounds, differences in effects on quantity of DNA harvested linked to varying age and biopsy lengths sampled, and strain- and age-dependent behavioral responses to tail biopsy.
Materials and Methods
Animals.
All tested animals were cared for in compliance with the Guide for the Care and Use of Laboratory Animals.22 The University Committee on the Use and Care of Animals approved the described tail biopsy procedures in conscious animals. Facilities housing the animals were AAALAC-accredited. Mice (Mus musculus) of 5 commonly used inbred strains (C57BL/6NCrl [B6], 129S2/SvPasCrl [129], BALB/cAnNCrl [BALB/c], C3H/HeNCrl [C3H], and FVB/NCrl [FVB]) were procured from an approved vendor (Charles River Laboratories, Wilmington, MA), and 1 outbred stock (Swiss Webster [SW]) was transferred naïve from approved breeding protocols. Age-matched litters were combined to analyze ossification in groups of 6 mice at 12 distinct time points and grouped into 3 cohorts; a total of 432 mice were assessed. Gender was not taken into account in the present study.
Cohort 1.
Pups were evaluated between 3 and 14 d of age. These animals were analyzed for the ability to obtain quantifiable DNA for genotyping at very young ages, prior to expected vertebral maturation. Six pups each at the ages of 3, 7, 10, and 14 d were imaged with microradiography (microRad; Faxitron X-ray LLC, Wheeling, IL). The animals were euthanized by CO2 narcosis prior to imaging because of concerns of rejection or cannibalization after removing pups then replacing them with the dam at preweanling ages. Postmortem tail biopsies of 5, 10, and 15 mm were harvested for DNA quantification from each of 3 animals at each age. The entire tails of the remaining animals (n = 3) at each age were harvested for microcomputed tomography (microCT; eXploreLocus, GE Healthcare, Chalfont St Giles, UK) and histological analysis. No acute behavioral assessments were performed on this cohort.
Cohort 2.
Mice were evaluated between 17 and 28 d of age. These animals were expected to demonstrate the greatest maturation of vertebrae in the tail; specifically, the time points were grouped around age day 21, the typical age after which general anesthesia is recommended for tail biopsy. Three pups each at the ages of 17, 21, 24, and 28 d were briefly anesthetized with isoflurane delivered by a precision vaporizer (inhaled dose, 1% to 2%) and imaged by using microRad. After complete recovery from anesthesia, these animals underwent tail biopsy for 5-, 10-, and 15-mm samples, with scoring of acute behavioral responses (see Acute behavioral assessment). Additional animals (n = 3) at each age were euthanized by CO2 narcosis, and postmortem samples of 35 mm of tail tissue were harvested for microCT evaluation and histologic analysis.
Cohort 3.
Mice were evaluated between 31 and 42 d of age. These animals were expected to have completed tail vertebral ossification and primarily were evaluated for behavioral responses to biopsies performed without anesthetic intervention. Animals in this cohort, aged 31, 34, 38, and 42 d, were treated identically to cohort 2 animals.
All mice were housed on ventilated racks in microisolation caging containing corncob bedding (Bed-o'Cobs, The Andersons, Maumee, OH). Cages were provided with automatic water and ad libitum chow (LabDiet 5001, PMI International, Brentwood, MO). Mice were tested routinely to be free from pinworms by cecal exam and antibody-negative for tested pathogens including mouse hepatitis virus, mouse parvoviruses, rotavirus, Ectromelia, Sendai virus, pneumonia virus of mice, Theiler murine encephalomyelitis virus, reovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, mouse adenovirus, and polyomavirus.
Imaging.
Anesthetized mice from cohorts 2 and 3 and euthanized mice from cohort 1 were positioned in ventral recumbency for microRad. All animals recovered from anesthesia without incident while under close observation. MicroCT was performed on harvested 35-mm distal tail segments (the length limitation as determined by the field of view of the equipment) or shorter lengths of total tail from younger mice. MicroCT vertebral counts within the most distal 2, 5, 10, and 15 mm were compared with those identified from radiographs. Immature vertebrae (IMV) and mature vertebrae (MV) were enumerated for each animal by evaluators blinded to the genetic background and age of mice.
Histology.
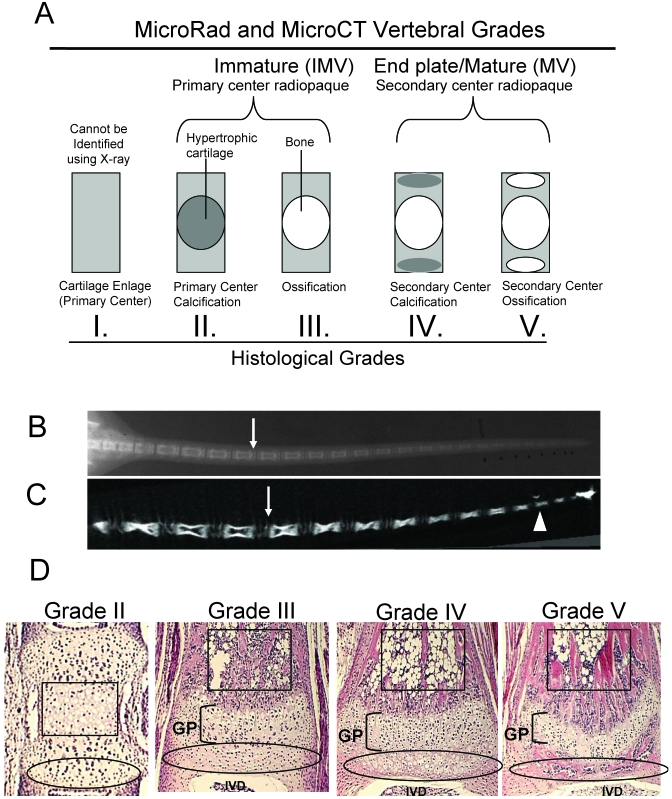
Tail segments up to 35 mm in length were decalcified and embedded longitudinally in paraffin. Planar sections (width, 7.0 µm) were stained with hematoxylin and eosin and graded (I through V) for maturation according to the following scale: I, IMV with nonmineralized cartilage enlage; II, IMV with mineralized, hypertrophic cartilage with minimal vascular invasion and no bone development in the primary center of ossification; III, IMV with primary center ossified; IV, MV with primary center ossified and mineralized hypertrophic cartilage in the secondary centers; and V, MV with both primary and secondary centers ossified, referred to as end plates (Figure 1).
Figure 1.
Methodology for vertebral grading of development. By using microRad (A, B) and microCT (A,C) vertebrae were classified as being either immature (IMV) or mature with end plates (MV). The white arrows indicate differing images of endplates, representing MV (B, C), whereas the white arrowhead depicts a representative IMV on microCT (C). Histologic samples were graded as II, III, IV, or V, depending on the presence or absence of hypertrophic chondrocytes or bone in primary and secondary ossification centers (D); black boxes outline primary centers of ossification and ovals outline secondary centers of ossification, growth plates (GP) are bracketed, and intervertebral disc spaces (IVD) are labeled.
Tail biopsy.
Animals were restrained manually and placed on a plastic block with permanent grooves denoting 5, 10, and 15 mm to provide consistency in sampling lengths. The tail was held in position next to the measured grooves and the tail wiped briefly with alcohol. A single-use scalpel blade was held perpendicular to the tail to make a transverse biopsy cut. Hemostasis was achieved after biopsy by manual pressure using a disposable gauze sponge. Biopsied animals were returned to their home cage for acute behavioral assessments. The sampled tail tissue was saved for subsequent DNA extraction and analysis.
DNA extraction and quantification.
Tail biopsy samples of 5, 10, and 15 mm lengths were digested using a standard DNeasy tissue purification kit (Qiagen, Valencia, CA) and accompanying kit protocol, and DNA was eluted into 200 µl of diluent (Buffer AE: 10 mM Tris-Cl, 0.5 mM EDTA; pH, 9.0). DNA content was measured spectophotometrically at 260 and 280 nm. Mice were genotyped using PCR for the vitamin D receptor, a standard assay in our laboratory. cDNA was stained with ethidium bromide and visualized on 2% agarose gels by using UV lighting.
Acute behavioral assessment.
Visual observations by 2 evaluators blinded to the length sampled began at the time of biopsy. After biopsy, mice from cohorts 2 and 3 were returned to standard housing cages and were observed for 10 min, with a second 10-min observation phase at 1 h after the time of biopsy. Assessments were based on published pain-scoring systems39 and included notation of at least 1 of the following categories of behavior: body flinch at the time of biopsy; tail flick away from the original site of tail placement for biopsy; any audible expression from the animal; licking or attention to the tail tip after biopsy; and subdued activity relative to conspecifics also undergoing biopsy. Animals that demonstrated at least 1 behavioral response were categorized as responders; if no behavioral responses were noted, the animals were nonresponders. The percentage of responders was evaluated for the effects of age, biopsy length, and strain.
Statistical analysis.
The experiment was designed as a 6 × 3 × 12 (6 strains, 3 biopsy lengths, 12 time points) factorial experimental that limited discrete sample number (6 mice per time point) but allowed sample grouping through 3-way (species × biopsy length × age) ANOVA if warranted. Data were analyzed graphically for potential interaction effects and ANOVA performed. Main effects were evaluated by using Tukey posthoc analysis. Statistical analyses were performed by using Prism software (Graphpad Software, La Jolla, CA), and results were considered throughout all experiments to be statistically significant when the P value was less than 0.05.
Results
Vertebral development and maturation.
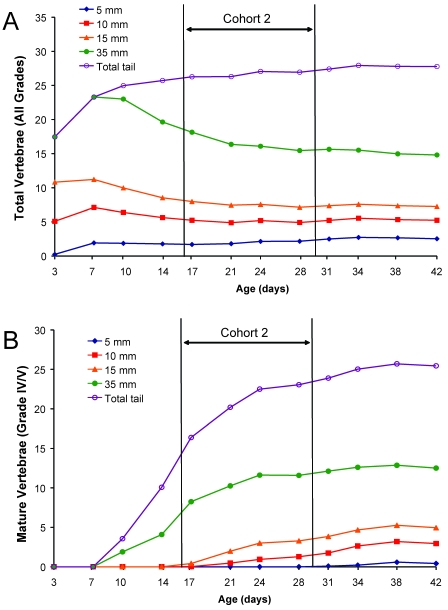
MicroRad was used to assess the absolute number of vertebrae per tail and those vertebrae with end plates (MV, grades IV and V; Figure 1 A, B) in discrete lengths of tail. Total IMV and MV were counted from radiographic films. Total coccygeal vertebral counts ranged in number from 27 to 29 by day 42 (Table 1). Vertebrae developed in a proximal to distal direction as mice aged (Figure 1 B, C). The total vertebral counts in each biopsy sample increased with increasing length harvested, with a sharp increase in total tail growth within the first week after birth (Figure 2 A). Although the total number of vertebrae in the tail increased with animal age, the number of vertebrae in each biopsy segment declined or plateaued due to vertebral maturation and elongation (Figure 2 A). Averaged across strains, MV were first discernable by microRad at day 10 in the total tail (Figure 2 B); however, no MV were observed on radiographs in any 5-mm distal tail segment until after day 31.
Table 1.
Differences in total coccygeal vertebrae based on genetic background
| Age (d) |
|||
| Strain | 3 | 21 | 42 |
| 129 | 16 | 26 | 27 |
| BALB/c | 21 | 27 | 28 |
| B6 | 15 | 26 | 27 |
| C3H | 18 | 25 | 28 |
| FVB | 16 | 27 | 28 |
| SW | 18 | 27 | 29 |
Values represent the mean vertebral number from 6 mice at each age using microRad.
Figure 2.
Timing of postnatal tail development by using microradiography, an inferior detection method for caudal vertebrae relative to imaging by microcomputed tomography (Figure 3). Vertebrae in microradiographs (n = 36 mice for each age group [strain factor collapsed]) were identified in discretely measured segments of tail (5, 10, 15, or 35 mm from tip) or in the total (entire) tail. (A) The total number of vertebrae (IMV and MV combined) in each biopsy segment length decreased over time, whereas vertebral counts in the total tail increased. (B) The number of mature vertebrae (grades IV and V) increased as a function of age.
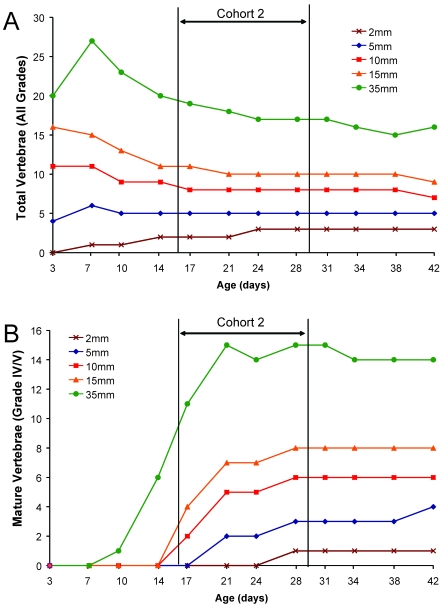
Comparison between the 2 imaging modalities of microRad and microCT revealed an approximately 2-wk difference in point of detection of MV across all genetic backgrounds, with microCT as the more sensitive imaging technique (Table 2). Within 5-mm distal tail biopsy segments, B6 and C3H mouse strains had MV detectable by day 21. MicroCT verified that, although calcified vertebrae were present in the distal 2mm of tail, none of these contained endplates prior to 21 d of age. Graphs documenting averaged microCT counts of vertebral maturation (Figure 3) across all strains are shifted to the left, demonstrating earlier detection of MV in measured biopsy lengths, relative to vertebral counts obtained by microRad (Figure 2). Graphs of both imaging modalities showed that those animals in cohort 2 (age, 17 to 28 d) underwent the most dramatic developmental changes, with maturation of vertebrae and the appearance of endplates. In 2-, 5-, 10-, and 15-mm segments of tail there were no vertebrae with end plates before day 14 (Figure 3 B), but between days 17 and 28, the number of vertebrae with end plates increased across all measurement lengths. Counts of MV tended to plateau across all genetic backgrounds in cohort 3 animals.
Table 2.
Time (d) of detection of IMV and MV vertebrae based on microRad and microCT
| Length of biopsy (mm) |
|||||||||
| 15 |
10 |
5 |
2 |
||||||
| IMV | MV | IMV | MV | IMV | MV | IMV | MV | ||
| 129 | microRad | 3 | 17 | 3 | 17 | 7 | 38 | NE | NE |
| microCT | 3 | 14 | 3 | 17 | 3 | 21 | 3 | >42 | |
| BALB/c | microRad | 3 | 24 | 3 | 24 | 3 | 38 | NE | NE |
| microCT | 3 | 21 | 3 | 21 | 3 | 21 | 17 | 31 | |
| B6 | microRad | 3 | 21 | 3 | 21 | 7 | 31 | NE | NE |
| microCT | 3 | 17 | 3 | 17 | 3 | 17 | 7 | 21 | |
| C3H | microRad | 3 | 17 | 3 | 21 | 7 | 34 | NE | NE |
| microCT | 3 | 14 | 3 | 17 | 3 | 17 | 7 | 21 | |
| FVB | microRad | 3 | 21 | 3 | 24 | 7 | 34 | NE | NE |
| microCT | 3 | 14 | 3 | 17 | 3 | 21 | 7 | 31 | |
| SW | microRad | 3 | 17 | 3 | 21 | 7 | 34 | NE | NE |
| microCT | 3 | 17 | 3 | 17 | 7 | 21 | 14 | 31 | |
NE, not evaluable; IMV, immature vertebrae (grade II or III); MV, mature vertebrae (grade IV or V)
For each strain, 6 mice per time point were evaluated by microRad and 3 mice per time point by microCT.
Figure 3.
Timing of postnatal tail vertebral development by using microcomputed tomography. A 35-mm segment of tail was scanned by using microCT and vertebrae (n = 18 mice for each age group [strain factor collapsed]) in measured segments of tail (2, 5, 10, or 15 mm) determined. (A) The total number of vertebrae in the 35-mm sections decreased over time, after a peak within the first week. Counts in the 10- to 15-mm segments decreased with increasing age. Counts in the shortest sections (2 and 5 mm) increased for the first 2 to 3 wk of age and then plateaued, with consistent counts through day 42. (B) The number of vertebrae that had end plates (MV; grades IV and V) increased as a function of age for each measured segment. On average, MV were not noted in the most distal 2 mm before day 21; however, MV are present in all sections greater than or equal to 5 mm before day 21.
Histologic evaluation.
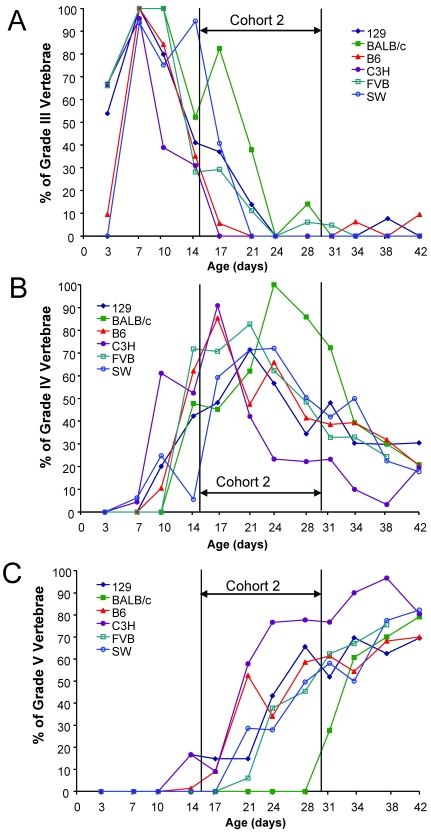
To differentiate mineralized cartilage as a precursor to ossification in end plates, tails were examined by histology. Similar to microRad and microCT, histology demonstrated a clear age-dependent progression in the maturation of vertebrae. Distinct, genotype-specific differences were noted in development of immature (grade III; Figure 4 A) to mature vertebrae (grades IV and V; Figures 4 B, C) in the 35-mm tail segment evaluated. As an example, the BALB/c mice had approximately the same number of IMV as other strains in cohort 1 (Figure 4 A), but the maturation of secondary centers of ossification is slowed relative to other strains. BALB/c mice did not show appearance of grade V vertebrae until day 31 (Figure 4 C), even though they had more grade IV vertebrae in older cohort 2 animals than did other strains and stock (Figure 4 B). Conversely, B6, 129, and C3H mice had grade V vertebrae as early as day 14, and more than 50% of vertebrae were grade V by day 28. A significant correlation (R2 = 0.88) existed between the percentage of mature grade IV or V vertebrae, determined by histology, and quantification of vertebrae with radiopaque end plates, determined by microCT. The correlation between grade V vertebrae by histology and end plates by microCT was significant also, but the R2 value was lower (R2 = 0.57). This result suggests that the presence of end plates as a maturation criterion may overestimate the extent of completed vertebral maturation when imaging with microCT alone.
Figure 4.
Histologic differences in postnatal vertebral development of the total tail varied across genetic backgrounds of mice. The percentage of vertebrae (n = 3 mice for each age group for each strain) was graded and quantified from the 35-mm sections of total tail for each strain as a function of age. (A) Grade III vertebrae, classified as immature yet ossified, were predominant in cohort 1 animals; (B) Grade IV vertebrae, classified as immature with secondary centers of calcification, were predominant in Cohort 2 animals; (C) Grade V vertebrae, those that are the most mature with ossified end plates, were predominant in Cohort 3 animals. Genetic differences in tail development for Grades IV and V were notable in Cohort 2 animals between C3H, B6, and BALB/c strains (B, C).
DNA yield.
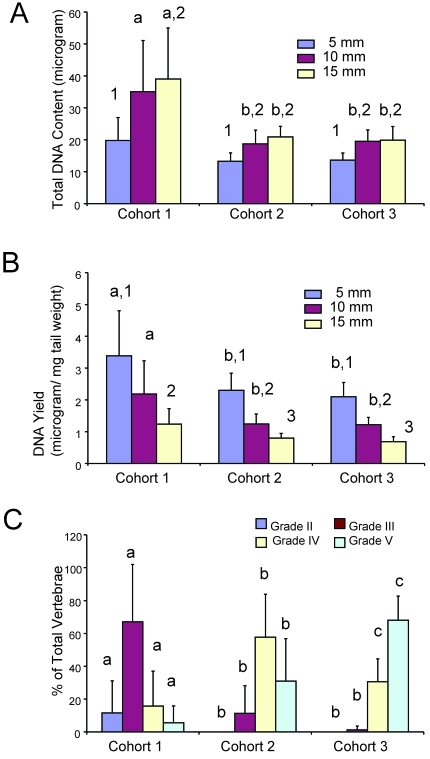
DNA was extracted from the tails and concentration of DNA determined spectrophotometerically by measuring absorbance at 260 and 280 nm. Because only a single sample was evaluated for each length–strain–age sample, multilevel data were analyzed graphically to determine whether data could be collapsed based on group. Strain background was not a primary determinant of DNA harvest (data not shown), therefore data were evaluated independent of strain. The longer the length of the tail sampled, the greater the weight of the collected tissue, as expected (data not shown). More DNA was obtained (Figure 5 A) from longer biopsy samples, with increased quantities harvested from the youngest animals (cohort 1). However, the relative yield (the amount of DNA relative to the weight of tissue) was highest in the shortest biopsy segment (5 mm; Figure 5 B) across all cohorts. As a function of age cohorts, cohort 1 mice (3 to 14 d) had significantly (P < 0.05) higher DNA content (Figure 5 A) and yield (Figure 5 B) than did cohorts 2 and 3 (age, 17 to 42 d combined). Cohort 1 animals had a higher percentage of IMV of grades II and III relative to cohorts 2 and 3 (Figure 5 C). Therefore the higher DNA content and yield from 5-mm biopsies in cohort 1 animals likely reflects increased cellularity and less mineralization in these distal tail tissues than are present in older animals.
Figure 5.
Total DNA content and DNA yield per tail biopsy differed by age and biopsy length. Each age cohort (n = 24 for each bar representing strain and age collapsed for each length within a cohort ) was graphed relative to tail biopsy length harvested. (A) Total DNA extracted was greatest in the youngest animals and in long biopsy lengths. (B) Relative yield of DNA extracted from biopsies shows a higher efficiency (by tissue weight) of DNA harvest from shorter biopsies and younger mice. Different letters (a, b) on the bar graphs indicate significant (P < 0.05) differences between age cohorts within the same length of biopsy. Different numbers (1 through 3) indicate lengths of biopsy are significant (P < 0.05) within a given age cohort. (C) Similarly vertebral development as a function of age was determined for each cohort. Different letters (a, b, c) on the bar graphs indicate significant (P < 0.05) differences between age cohorts within the same grade. Compared with other cohorts, cohort 1 mice have very low prevalence of grade IV and V vertebrae; therefore, tissues are more cellular and less mineralized in nature.
Acute behavioral responses.
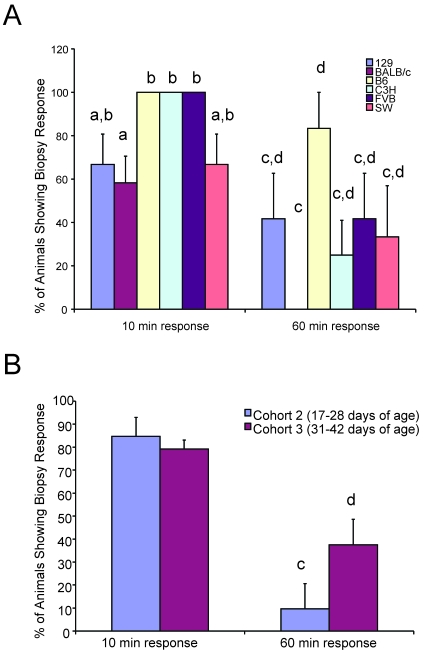
Acute behavioral responses as a function of genetic background, age, and biopsy length were evaluated, and animals collectively were classified by the absence or presence of response to the biopsy procedure; data were evaluated by 1-way ANOVA. The percentage of responders (that is, the percentage of mice with at least 1 response at the time of or shortly after biopsy) differed among the various genetic backgrounds and age cohorts. For example, fewer BALB/c mice responded within the 10- and 60-min observation periods, whereas a significantly (P < 0.05) greater number of B6, C3H, and FVB mice responded to the procedure (Figure 6 A). The number of animals with an observable response diminished over the course of the observation periods, but cohort 3 (animals as old as 42 d) had a significantly (P < 0.05) greater number of responders remaining at 60 min postbiopsy than did the younger cohort 2 animals (Figure 6 B). This result was expected given the presence of greater numbers of mature tail vertebrae in older animals.
Figure 6.
Strain- and age-associated changes in acute behavioral responses. (A) Variability between strains in the percentage of responders after tail biopsy is shown. Fewer BALB/c mice responded to the stimulus of biopsy, compared with other strains, at both 10- and 60-min observation periods after biopsy. Different letters above the columns indicate that those values are significant at P < 0.05 for either the 10-min (a, b) or 60-min (c, d) time point (n = 24 for each strain). (B) Cohort 2 mice show a significant reduction (marked with different letters [c, d]); P < 0.05) in behavioral responders over time compared with older cohort 3 animals (n = 72 animals for each cohort).
Discussion
Mouse strains show well-recognized and distinct differences in overall bone mass and formation3,41 and regeneration27. Our study supported these findings by elucidating distinct and functionally significant differences in tail vertebral development across mice of 6 different genetic backgrounds. Although our study evaluated coccygeal vertebral development through 42 d of age, evidence indicates that additional vertebrae develop beyond this time point. For example, we counted 27 vertebrae in B6 mice at 6 wk of age, whereas other investigators have documented 28 vertebrae at 10 wk2 and 29 to 31 vertebrae by 15 to 25 wk42 in the same strain. Given that ossification progresses generally in a proximal to distal manner,34 the extent of ossification at a given age depends on maturation of segments at the tail tip.42
Complementary imaging modalities were essential for obtaining accurate vertebral counts and assessing vertebral maturation to 42 d of age. Traditionally, morphologic skeletal assessments of rodent ossification have not used high-resolution imaging technologies, nor have they evaluated proximal and distal tail vertebrae.12,14,15,17,21,24,26,31,34,42-47 Although microradiography (microRad) is useful for whole-body imaging of rodents to 5× magnification, in our study, this modality did not provide the resolution needed to fully evaluate maturation or to enumerate distal tail vertebrae typically harvested during biopsy. Immature vertebrae show central radiopacity on radiographs, which represents either mineralized hypertrophic cartilage preceding osteogenesis or ossification. Radiopaque vertebrae with end plates represent those that are more mature with both diaphyseal radiopacity and epiphyseal radiopacity. To enhance the assessment of the most distal aspects of the tail, we imaged tail samples with microCT.
MicroCT has emerged as a central tool for the descriptive and quantitative analysis of skeletal anatomy. MicroCT morphometry uses a detailed 3-dimensional anatomic reconstruction of the entire structural component being investigated.2 This technology has been used to characterize murine skeletal long bones29 and phenotypes, including mutations resulting in tail abnormalities.13 The 35-μm voxel resolution used in the present study allowed quantification of mineralized vertebrae within the distal 2 mm of the tail across the 6 genetic backgrounds of mice. At 21 days of age, mature vertebrae with end plates were present in 2-mm distal tail lengths of 2 strains (C3H and B6). Within the 5 mm biopsy length, MV were visible in every analyzed strain and stock of mice at day 21.
The leading limitation of X-ray–based imaging, used in microRad and microCT, was an inability to differentiate whether endplates were composed of mineralized hypertrophic cartilage (grade IV) or mature bone (grade V). Therefore, histologic analysis of longitudinal tail tissue sections permitted a third level of morphologic evaluation. We were able to classify vertebrae into 5 developmental grades depending upon the presence or absence of hypertrophic chondrocytes and true bone formation. The primary drawback of histology was the technical challenge of planar sectioning of embedded tails, particularly in very young mice. In our study, multiple longitudinal sections from each animal at each age had to be evaluated in order to find those that contained full lengths of sectioned tails from base to tip. In a recent publication, histologic evaluation of transverse sections of the distal 2 mm of mouse tail at ages 12 to 32 wk confirmed that bony vertebrae were present;1 this result was expected given the older ages of the mice used in the study.
The tail biopsy procedure was performed according to institutional recommendations and was structured to emulate minimal (5 mm), moderate (10 mm), and maximal (15 mm) sampling techniques. Interestingly, the youngest cohort of animals (cohort 1) provided a higher yield of DNA and had increased DNA content in the distal tail compared with older cohorts. This greater DNA yield in younger animals likely is due to the presence of highly cellular cartilage, from which DNA can be extracted more easily than from bone. We verified that mice between 17 and 31 d of age undergo a phase of marked tail development, with maturation of vertebrae and the appearance of endplates. These findings support the harvest of the minimal biopsy lengths from preweaned animals at approximately 14 to 17 d of age to maximize DNA extraction from less-developed tail tissue.
All mice in our study were between 2.5 and 6 wk of age when evaluated for behavioral responses during the first hour after conscious tail biopsy. Both strain- and age-associated differences in behavioral responses were associated with vertebral maturation. Surprisingly, length of biopsy did not have a significant effect on behavioral responses. The correlation between ossification and pain responses, secondary to tail biopsy for genotyping, has recently been discussed.1,8 Inhalant anesthesia with methoxyflurane and ether were administered to telemeterized mice prior to tail biopsy, with the findings that mice between 12 to 32 wk of age did not have prolonged physiologic effects after tail biopsy and that the tested anesthetics were not advisable for the procedure.1 Tail biopsy, when compared with collection of other murine tissues (ear, hair, mucosal cells) for DNA isolation, had no measurable effects indicating distress or considerable pain; in fact, simple restraint compared to all tissue collection methods resulted in similar physiologic changes in tested animals.8
Our scoring system did not correlate to quantitative pain assessments, yet it did provide qualitative assessment of abnormal behaviors after the stimulus of biopsy. Responses differed among genetic backgrounds. A greater percentage of B6 mice responded to the biopsy procedure at 10 and 60 min as compared with BALB/c mice. These findings were consistent with the more rapid coccygeal vertebral maturation in B6 mice. BALB/c mice show the most delayed vertebral development, and this strain had the lowest percentage of mice responding behaviorally to the biopsy.
With increasing age, we verified that more animals responded to the stimulus of conscious tail biopsy. The mice in cohort 2, whose tail vertebrae were less mature, exhibited fewer prolonged (60 min postbiopsy) responses to the procedure than older cohort 3 mice. These results were not surprising given the expectation that mineralized mouse bone is innervated with both unmyelinated and myelinated sensory and sympathetic neurons capable of conducting sensory input from the periphery to the spinal cord, as described for C3H mice previously29 and as highlighted by evaluations of the distal 6-mm of tail tissue.1 If responsiveness to tail biopsy is related to vertebral maturation, as supported by our study, the current institutional standards of requiring anesthesia or analgesia (or both) only for mice biopsied after 21 d of age may require reevaluation, particularly if greater than 2-mm of distal tail is harvested. Furthermore, it may be advisable to consider the strain background prior to selection of biopsy age, as tail vertebral development does not proceed at uniform rates across genetic backgrounds.
Regardless of genetic background, all mouse tail vertebrae have calcification and ossification within the distal 5 mm of the tail at early ages, with mature endplates present prior to the typical age for weaning (21 d). Various strains we analyzed, including B6 and C3H, have mature vertebrae within 5 mm of distal tail by day 17 of age. These findings unequivocally refute the common statements made in contemporary institutional guidelines that imply calcification has not occurred in the tail until day 21 of age. Current standards suggest that a range of 2 to 15 mm of distal tail tissue is adequate for DNA isolation for genotyping. This range can now be modified and the suggestion made that tail biopsies less than 5 mm in length are sufficient for genotyping in mice no older than 17 d of age, unless anesthesia or topical analgesia is provided.
Acknowledgments
We would like to thank the following people for their assistance: K McKelvey in the University of Michigan (UM) Undergraduate Research Opportunity Program; S Woodhouse in the Michigan State University Merck–Merial Summer Program; J Knowlton, J Baker, R Taylor, and J Combs in the UM Orthopedic Research Laboratories; P Arrowsmith, E Wilkinson, and H Rush in the UM Unit for Laboratory Animal Medicine; and L Hakkinen in the Department of Animal Biology at the University of Pennsylvania. We are extremely grateful to the American College of Laboratory Animal Medicine (ACLAM) Foundation and the University of Michigan for funding these endeavors. Aspects of this work were presented at the National American Association of Laboratory Animal Science Meeting and the ACLAM Forum. Dr K Hankenson was supported by grants R01 AR49862 and K01 RR RR00161 from the National Institutes of Health.
References
- 1.Arras M, Rettich A, Seifert B, Kasermann HP, Rulicke T. 2007. Should laboratory mice be anaesthetized for tail biopsy? Lab Anim 41:30–45 [DOI] [PubMed] [Google Scholar]
- 2.Bab I, Hajbi-Yonissi C, Gabet Y, Muller R. 2007. Microtomographic atlas of the mouse skeleton. New York: Springer–Verlag [Google Scholar]
- 3.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. 1996. Genetic variability in adult bone density among inbred strains of mice. Bone 18:397–403 [DOI] [PubMed] [Google Scholar]
- 4.Broome RL, Feng L, Zhou Q, Smith A, Hahn N, Matsui SM, Omary MB. 1999. Noninvasive transgenic mouse genotyping using stool analysis. FEBS Lett 462:159–160 [DOI] [PubMed] [Google Scholar]
- 5.Burkhart CA, Norris MD, Haber M. 2002. A simple method for the isolation of genomic DNA from mouse tail free of real-time PCR inhibitors. J Biochem Biophys Methods 52:145–149 [DOI] [PubMed] [Google Scholar]
- 6.Campbell DB, Hess EJ. 1997. Rapid genotyping of mutant mice using dried blood spots for polymerase chain reaction (PCR) analysis. Brain Res Brain Res Protoc 1:117–123 [DOI] [PubMed] [Google Scholar]
- 7.Chen SZ, Evans GA. 1990. A simple screening method for trangenic mice using the polymerase chain reaction. Biotechniques 8:32–33 [PubMed] [Google Scholar]
- 8.Cinelli P, Rettich A, Seifert B, Burki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184 [DOI] [PubMed] [Google Scholar]
- 9.Couse JF, Davis VL, Tally WC, Korach KS. 1994. An improved method of genomic DNA extraction for screening transgenic mice. Biotechniques 17:1030–1032 [PubMed] [Google Scholar]
- 10.Drews R, Drohan WN, Lubon H. 1994. Transgene detection in mouse tail digests. Biotechniques 17:866–867 [PubMed] [Google Scholar]
- 11.Elder B. 2002. DNA isolation methods for genotyping rodents. Lab Anim (NY) 31:49–53 [DOI] [PubMed] [Google Scholar]
- 12.Feik SA, Storey E. 1983. Remodelling of bone and bones: growth of normal and transplanted caudal vertebrae. J Anat 136:1–14 [PMC free article] [PubMed] [Google Scholar]
- 13.Ford-Hutchinson AF, Cooper DM, Hallgrimsson B, Jirik FR. 2003. Imaging skeletal pathology in mutant mice by microcomputed tomography. J Rheumatol 30:2659–2665 [PubMed] [Google Scholar]
- 14.Fukuda S, Tomita S, Matsuoka O. 1977. [Comparative studies on bone growth in experimental animals. 1. Bone growth and ossification in mice (author's transl)]Jikken Dobutsu 26:103–113 [DOI] [PubMed] [Google Scholar]
- 15.Garrard G, Harrison GA, Weiner JS. 1974. Genotypic differences in the ossification of 12-day-old mice at 23 degrees C and 32 degrees C. J Anat 117:531–539 [PMC free article] [PubMed] [Google Scholar]
- 16.Gaw A, Mancini FP, Ishibashi S. 1995. Rapid genotyping of low-density lipoprotein receptor knockout mice using a polymerase chain reaction technique. Lab Anim 29:447–449 [DOI] [PubMed] [Google Scholar]
- 17.Green EL. 1951. The genetics of a difference in skeletal type between two inbred strains of mice (BALBc and C57BL). Genetics 36:391–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross M, Rotzer E. 1998. Rapid DNA extraction method for genetic screening. Eur J Med Res 3:173–175 [PubMed] [Google Scholar]
- 19.Henneberger C, Grantyn R, Rothe T. 2000. Rapid genotyping of newborn gene mutant mice. J Neurosci Methods 100:123–126 [DOI] [PubMed] [Google Scholar]
- 20.Hofstetter JR, Zhang A, Mayeda AR, Guscar T, Nurnberger JI, Jr, Lahiri DK. 1997. Genomic DNA from mice: a comparison of recovery methods and tissue sources. Biochem Mol Med 62:197–202 [DOI] [PubMed] [Google Scholar]
- 21.Hughes PC, Tanner JM. 1970. A longitudinal study of the growth of the black-hooded rat: methods of measurement and rates of growth for skull, limbs, pelvis, nose-rump and tail lengths. J Anat 106:349–370 [PMC free article] [PubMed] [Google Scholar]
- 22.Institute of Laboratory Animal Resources, National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C [Google Scholar]
- 23.Irwin MH, Moffatt RJ, Pinkert CA. 1996. Identification of transgenic mice by PCR analysis of saliva. Nat Biotechnol 14:1146–1148 [DOI] [PubMed] [Google Scholar]
- 24.Johnson ML. 1933. The time and order of appearance of ossification centers in the albino mouse. Am J Anat 52:241–271 [Google Scholar]
- 25.Lahm H, Hoeflich A, Rieger N, Wanke R, Wolf E. 1998. Identification of transgenic mice by direct PCR analysis of lysates of epithelial cells obtained from the inner surface of the rectum. Transgenic Res 7:131–134 [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Leichter J. 1983. Skeletal development in fetuses of rats consuming alcohol during gestation. Growth 47:254–262 [PubMed] [Google Scholar]
- 27.Li X, Gu W, Masinde G, Hamilton-Ulland M, Rundle CH, Mohan S, Baylink DJ. 2001. Genetic variation in bone-regenerative capacity among inbred strains of mice. Bone 29:134–140 [DOI] [PubMed] [Google Scholar]
- 28.Lin CS, Magnuson T, Samols D. 1989. A rapid procedure to identify newborn transgenic mice. DNA 8:297–299 [DOI] [PubMed] [Google Scholar]
- 29.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O'Leary P, Mantyh PW. 2002. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113:155–166 [DOI] [PubMed] [Google Scholar]
- 30.Malumbres M, Mangues R, Ferrer N, Lu S, Pellicer A. 1997. Isolation of high-molecular-weight DNA for reliable genotyping of transgenic mice. Biotechniques 22:1114–1119 [DOI] [PubMed] [Google Scholar]
- 31.McLaren A, Michie D. 1955. Factors affecting vertebral variation in mice. II. Further evidence of intrastrain variation. J Embryol Exp Morphol 3:366–375 [PubMed] [Google Scholar]
- 32.Meldgaard M, Bollen PJ, Finsen B. 2004. Noninvasive method for sampling and extraction of mouse DNA for PCR. Lab Anim 38:413–417 [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health (NIH) 2002 NIH guidelines for genotyping of rodents (June 12, 2002), http://oacu.od.nih.gov/ARAC/FinalGenotyping0602.pdf.
- 34.Patton JT, Kaufman MH. 1995. The timing of ossification of the limb bones, and growth rates of various long bones of the fore and hind limbs of the prenatal and early postnatal laboratory mouse. J Anat 186:175–185 [PMC free article] [PubMed] [Google Scholar]
- 35.Petkov PM, Cassell MA, Sargent EE, Donnelly CJ, Robinson P, Crew V, Asquith S, Haar RV, Wiles MV. 2004. Development of a SNP genotyping panel for genetic monitoring of the laboratory mouse. Genomics 83:902–911 [DOI] [PubMed] [Google Scholar]
- 36.Pinkert CA. 2003. Transgenic animal technology: alternatives in genotyping and phenotyping. Comp Med 53:126–139 [PubMed] [Google Scholar]
- 37.Ren S, Li M, Cai H, Hudgins S, Furth PA. 2001. A simplified method to prepare PCR template DNA for screening of transgenic and knockout mice. Contemp Top Lab Anim Sci 40:27–30 [PubMed] [Google Scholar]
- 38.Robinson V, Morton DB, Anderson D, Carver JFA, Francis RJ, Hubrecht R, Jenkins E, Mathers KE, Raymond R, Rosewell I, Wallace J, Wells DJ. 2003. Joint working group on refinement: refinement and reduction in production of genetically modified mice. Lab Anim 37Suppl 1:27–33 [Google Scholar]
- 39.Roughan JV, Flecknell PA. 2003. Evaluation of a short-duration behaviour-based postoperative pain scoring system in rats. Eur J Pain 7:397–406 [DOI] [PubMed] [Google Scholar]
- 40.Schmitteckert EM, Prokop CM, Hedrich HJ. 1999. DNA detection in hair of transgenic mice—a simple technique minimizing the distress on the animals. Lab Anim 33:385–389 [DOI] [PubMed] [Google Scholar]
- 41.Sheng MH, Baylink DJ, Beamer WG, Donahue LR, Rosen CJ, Lau KH, Wergedal JE. 1999. Histomorphometric studies show that bone formation and bone mineral apposition rates are greater in C3H/HeJ (high-density) than C57BL/6J (low-density) mice during growth. Bone 25:421–429 [DOI] [PubMed] [Google Scholar]
- 42.Shinohara H. 1999. The mouse vertebrae: changes in the morphology of mouse vertebrae exhibit specific patterns over limited numbers of vertebral levels. Okajimas Folia Anat Jpn 76:17–31 [DOI] [PubMed] [Google Scholar]
- 43.Shinohara H. 1999. The musculature of the mouse tail is characterized by metameric arrangements of bicipital muscles. Okajimas Folia Anat Jpn 76:157–169 [DOI] [PubMed] [Google Scholar]
- 44.Smetana K, Jr, Holub M. 1990. Ossification in nude mice. 1. Macroscopical study. APMIS 98:729–734 [DOI] [PubMed] [Google Scholar]
- 45.Smetana K, Jr, Holub M, Funda D. 1991. Ossification in nude mice. 2. A histological, histochemical, and immunohistochemical study. APMIS 99:1024–1030 [PubMed] [Google Scholar]
- 46.Young AD, Phipps DE, Astroff AB. 2000. Large-scale double-staining of rat fetal skeletons using alizarin red S and alcian blue. Teratology 61:273–276 [DOI] [PubMed] [Google Scholar]
- 47.Yukawa M, Hayashi N, Takagi K, Mochizuki K. 1999. The normal development of Mongolian gerbil foetuses and, in particular, the timing and sequence of the appearance of ossification centres. Anat Histol Embryol 28:319–324 [DOI] [PubMed] [Google Scholar]
- 48.Zhuo M. 1998. NMDA receptor-dependent long term hyperalgesia after tail amputation in mice. Eur J Pharmacol 349:211–220 [DOI] [PubMed] [Google Scholar]