Abstract
Escherichia coli mRNA interferases, such as MazF and ChpBK, are sequence-specific endoribonucleases encoded by toxin-antitoxin (TA) systems present in its genome. A MazF homologue in Staphylococcus aureus (MazFSa) has been shown to inhibit cell growth when induced in E. coli. Here, we determined the cleavage site for MazFSa with the use of phage MS2 RNA as a substrate and CspA, an RNA chaperone, which prevents the formation of secondary structures in the RNA substrate. MazFSa specifically cleaves the RNA at a pentad sequence, U↓ACAU. Bioinformatics analysis revealed that this pentad sequence is significantly abundant in several genes, including the sraP gene in the S. aureus N315 strain. This gene encodes a serine-rich protein, which is known to play an important role in adhesion of the pathogen to human tissues and thus in endovascular infection. We demonstrated that the sraP mRNA became extremely unstable in comparison with the ompA mRNA only when MazFSa was induced in E. coli. Further bioinformatics analysis indicated that the pentad sequence is also significantly abundant in the mRNAs for all the pathogenic factors in S. aureus. This observation suggests a possible regulatory relationship between the MazEFSa TA module and the pathogenicity in S. aureus.
The toxin-antitoxin (TA) systems were originally discovered on low-copy-number plasmids and were found to stably maintain the plasmid by selectively killing daughter cells which have lost the plasmid. This phenomenon is called postsegregational killing (15). However, later it was found that most bacteria also contain the TA systems on their genomes (26, 31). In the TA systems, the toxin genes are coexpressed with their cognate antitoxin genes present in the same operons and toxins and their cognate antitoxins form stable TA complexes in the cells under normal growth conditions. The Escherichia coli chromosome contains at least 16 TA operons (4, 13, 18-24, 26, 27, 31-33, 35). Since antitoxins are labile proteins which are easily degraded by stress-induced proteases such as Clp and Lon, the balance between toxin and antitoxin is altered under stress conditions, leading to the release of the toxin from the TA complexes. This results in growth arrest and eventual death (5, 9, 14).
Of these TA systems, the MazE (antitoxin)/MazF (toxin) system is one of the most extensively characterized (16). Structural studies have shown that two MazF dimers and one MazE dimer form a hexameric MazF2-MazE2-MazF2 complex (19). MazF has been shown to be a sequence-specific endoribonuclease that cleaves at ACA sequences present in mRNAs both in vitro and in vivo (30, 34). A number of MazF homologues have been identified in other bacteria. Interestingly, Myxococcus xanthus, a developmental soil-dwelling bacterium, was found to contain a solitary mazF gene, which is regulated by a serine/threonine protein kinase cascade and is essential for programmed cell death involved in obligatory cell lysis during differentiation to form fruiting bodies (25). On the other hand, Mycobacterium tuberculosis was found to contain at least seven MazF homologues (MazFMt1 to -Mt7) (37). MazFMt1 and MazFMt6 specifically cleave mRNA at UAC- and U-rich regions, respectively (37). Further characterization using a novel MS2 RNA-CspA primer extension method revealed that MazFMt3 recognizes unique pentad target sequences (UUCCU or CUCCU) and that MazFMt7 cleaves mRNA at UCGCU (36). In addition, bioinformatics analysis of the M. tuberculosis genome revealed that these pentad sequences were significantly underrepresented in genes which belong to the PE and PPE families, suggesting that the mRNA interferases may be involved in the pathogenesis of M. tuberculosis (36).
In this paper, using the same MS2 RNA-CspA primer extension method applied for MazFMt3 and -Mt7, we identified a unique pentad target sequence, UACAU, for the MazF homologue from Staphylococcus aureus, MazFSa. Further bioinformatics analysis revealed that this pentad sequence is highly abundant in the genes for pathogenic factors and particularly in the gene for the pathogenic adhesive factor SraP. We propose that MazFSa in S. aureus plays an important role in the pathogenicity of S. aureus. It should be noted that S. aureus is the one of the most common cause of infectious diseases. Particularly, methicillin-resistant S. aureus (MRSA), which is resistant to virtually all β-lactams, such as methicillin, oxacillin, penicillin, and amoxicillin, is most frequently found in Staphylococcus infections occurring in hospitals and health care facilities. Intriguingly, the gene for MazFSa is cotranscribed with the sigB operon, which is involved in the regulation of the expression of virulence factors under stress conditions (8, 28), suggesting that MazFSa may associate with the pathogenicity of this pathogen. Our findings may provide some insights into developing a novel therapeutic approach for S. aureus.
MATERIALS AND METHODS
Strains and plasmids.
The E. coli BL21(DE3) strain was used for recombinant protein expression. Plasmids pET-28a-MazFSa and pBAD-MazESa were constructed from pET-28a (Novagen) and pBAD to express His6-tagged MazFSa and MazESa, respectively.
Purification of His6-tagged MazFSa in E. coli.
MazFSa tagged with His6 at the N-terminal end were purified from strain BL21(DE3) carrying pET-28a-MazFSa by using Ni-nitrilotriacetic acid resin (Qiagen) as described previously (32).
Purification of the CspA protein from E. coli.
CspA was purified as described previously (6).
Primer extension analysis in vitro.
For primer extension analysis of mRNA cleavage sites in vitro, the full-length MS2 mRNAs were partially digested with or without purified toxin protein MazFSa and with or without purified CspA protein at 37°C for 15 min. The digestion reaction mixture (10 μl) consisted of 0.8 μg of MS2 RNA substrate, 0.0625 μg of His6-tagged MazFSa, 32 μg CspA, and 0.5 μl of RNase inhibitor (Roche) in 10 mM Tris-HCl (pH 7.8). Primer extension was carried out at 47°C for 1 h with 20 μl of the reaction mixture as described previously (33). The reactions were stopped by adding 12 μl of sequence loading buffer (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, and 0.05% xylene cyanol EF). The samples were incubated at 90°C for 5 min prior to electrophoresis on a 6% polyacrylamide and 36% urea gel. The primers were 5′ labeled with [γ-32P]ATP, using T4 polynucleotide kinase.
Reverse transcriptase PCR (RT-PCR).
Reverse transcription of the isolated RNA was performed using the protocol for the Transcriptor first-strand cDNA synthesis kit (Roche Diagnostic GmbH, Mannheim, Germany) in accordance with the manufacturer's instructions. cDNA was synthesized from 1 μg of total RNA by using gene-specific primers (sraP-RTr3 [5′-CGT TGA ATC ACT AAA CGA CG-3′] for sraP and ompA-RTr1 [5′-TCA ACA ACA GAC TGA GCA CG-3′] for ompA). Reverse transcription at 55°C was performed for 30 min after 10 min of incubation at 50°C with the primers. The enzyme was heat inactivated for 5 min at 85°C, and the PCR steps included heating for 1 min at 94°C, cooling for 1 min at 50°C, and extension for 1 min at 72°C for 29 cycles (primers sraUP10 [5′-CAA CGA GTA TAT CAG GG-3′] and sraP-RTr3 for sraP fragment region 2) or 28 cycles (primers sraUP14 [5′-GTA GTT CGG TAC AAA CAT C-3′] and sraP-RTr5 [5′-CTC CAC CAG CTA CAT TA-3′] for sraP fragment region 1 and ompA-RTf2 [5′-CCG AAA GAT AAC ACC TGG TA-3′] and ompA-RTr1 for ompA). As a control to ensure that there was no residual DNA or DNA contamination, a cDNA synthesis procedure and PCRs were performed on the all mRNA templates without the inclusion of reverse transcriptase with the ompA primer set.
Bioinformatics analysis of the frequencies of MazFSa motifs in Staphylococcus aureus CDS.
We retrieved the genomic sequence of S. aureus N315 from NCBI RefSeq (accession no. NC_002745) and extracted all coding sequences (CDS) from the record. We first calculated the nucleotide composition of each CDS. The probability (p) of the appearance of the cleavage motif UACAU anywhere in the CDS is (percentage of U residues)2 (percentage of A residues)2 (percentage of C residues). Let L be the length of the CDS. Then, the expected number (E) of the motifs in the CDS is p(L − 4). Let K be the actual number of the motifs in the CDS. Then, the probability (P) of having K or fewer motifs in the CDS is
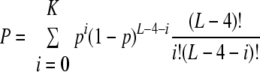 |
A very small P value suggests that the CDS may have evolved to eliminate the motif from its sequence. The probability (P) of having K or more target sites in the gene is
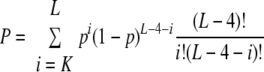 |
A very small P value suggests that the CDS is a prime target of MazFSa.
RESULTS
MazFSa is toxic in E. coli and is neutralized by MazESa.
The genes for MazFSa and MazESa were cloned into the pET-28a and pBAD33 plasmids, respectively. These two plasmids were cotransfected into the E. coli C43 strain. As shown in Fig. 1, the induction of the MazFSa caused cell growth arrest at 0.1 mM and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). However, the coinduction of MazESa with arabinose rescued cell growth, neutralizing the toxicity of MazFSa.
FIG. 1.
Induction of MazFSa and MazESa in E. coli. The sectors of the plates show different concentrations of IPTG (0, 0.1, and 1 mM), which induces the expression of His6-tagged MazFSa, and the rows of the plates show different concentrations of arabinose (0 and 0.2%), which induces the expression of MazESa. The upper half of each plate contains three different clones, which contain both vectors (pET-28a and pBAD) as a control. The lower half of each plate contains three different clones, which contain both pET-28a-MazFSa and pBAD-MazESa.
MazFSa specifically cleaves RNA at UACAU.
Primer extension experiments were carried out to determine the recognition sites for MazFSa with the use of MS2 RNA in the presence of 0.43 mM CspA, which is essential for melting the extensive secondary structures in the phage MS2 RNA (17). As shown in Fig. 2A to I, the addition of CspA significantly enhanced RNA cleavage in most cases. Notably, the RNA cleavage was detectable only in the presence of CspA for the cleavage sites shown in Fig. 2C, G, and H. Through these experiments, nine cleavage sites were identified, as listed in Table 1. The consensus sequence from these cleavage sites is UACAU, where MazFSa cleaves between the U residue in the first position and the A residue in the second position. There are a total of six UACAU sequences in the MS2 RNA (RefSeq accession no. NC_001417; NCBI website), all of which were cleaved by MazFSa. Three other cleavage sites were also identified (Fig. 2G, H, and I), each of which has a base substitution either at the second base or the fourth base in the consensus sequence (Table 1).
FIG. 2.
MazFSa cleaves specifically at UACAU sites. (A to I) In vitro cleavage of the MS2 RNA with His6-tagged MazFSa. Lane 1 represents a control reaction in which no proteins were added; lane 2 represents a control reaction in which only CspA protein was added; lane 3 represents MS2 RNA only with His6-tagged MazFSa; lane 4 represents MS2 RNA incubated with His6-tagged MazFSa and CspA protein. Cleavage sites are indicated by arrows on the RNA sequence and were determined using the RNA ladder shown on the right.
TABLE 1.
Cleavage sites of MazFSa in MS2 RNA
| Cleavage site |
|---|
| With U↓ACAU |
| UGACU U↓ACAU CGAAG |
| GGUUU U↓ACAU AAACG |
| GCUCC U↓ACAU GUCAG |
| UUUCU U↓ACAU GACAA |
| CGUUU U↓ACAU CAAGA |
| GUCGC U↓ACAU AGCGU |
| With sequence differing from U↓ACAU by 1 basea |
| GCGCG U↓ACGU AAAGU |
| GUGGU U↓CCAU ACUGG |
| UUGCU U↓ACUU AAGGG |
Base substitutions at the second or fourth base in the consensus sequence are in bold.
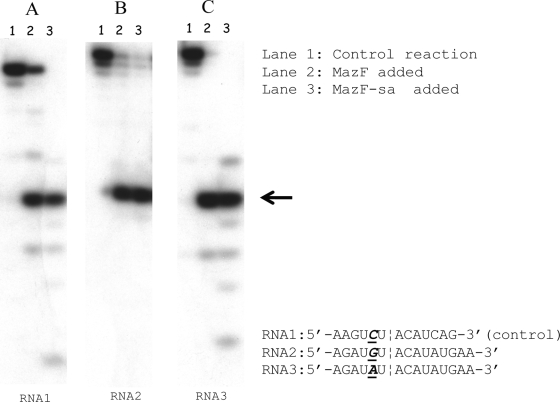
Since all six UACAU sequences in the MS2 RNA have only U or C at position −1, the result shown in Table 1 could not reveal the base requirement at this position. Therefore, three small RNAs were synthesized: RNA1 (5′-AAGUCUACAUCAG-3′ [control]), RNA2 (5′-AGAUGUACAUAUGAA-3′), and RNA3 (5′-AGAUAUACAUAUGAA-3′) (bases at position −1 are underlined; the consensus sequence is in italics). In RNA2 and RNA3, the base at position −1 was replaced with G and A, respectively, while the control RNA1 has a C residue at this position. The replacement of this C residue at the −1 position with G (Fig. 3B) or A (Fig. 3C) did not affect the cleavage activity of MazFSa in comparison with the control experiment (Fig. 3A). E. coli MazF was used in lane 2 for each experiment to demonstrate that the E. coli MazF is able to cleave all the RNAs between the sixth U and the seventh A residues as reported previously (33). These results indicate that MazFSa recognizes a pentad RNA sequence, most preferentially UACAU, with G/C/U residues C at position 2 and G/U at position 4 (Table 1).
FIG. 3.
MazFSa cleaves small RNA at UACAU sites. (A to C) In vitro cleavage of the three different RNAs (RNA1, RNA2, and RNA3) with His6-tagged MazFSa. Lane 1, a control reaction in which no proteins were added; lane 2, another control reaction, in which E. coli MazF protein was added; and lane 3, RNA incubated with His6-tagged MazFSa. Cleavage sites are indicated by arrows.
The target pentad sequence UACAU for MazFSa is highly abundant in the sraP gene.
As previously found with MazF homologues from M. tuberculosis, it is tempting to speculate that the highly specific cleavage site for MazFSa may be involved in regulation of a group of specific genes either by extremely low or high abundance in these genes. Note that a specific pentad sequence is expected to exist only once in every 1,000-base sequence, provided that the RNA has no bias in its base composition. Thus, the entire S. aureus N315 genome was examined to search for the open reading frames (ORFs) that contain the pentad sequence at a much lower frequency than expected or conversely at a much higher frequency than expected.
Interestingly, we found that there are certain genes which contain the MazFSa pentad sequence at a much higher frequency than expected. The gene which has the highest probability is the SA2447 gene in S. aureus N315, which has more than 99% identity to the sraP gene in the S. aureus COL strain. The ORF of this gene is 6,816 bases long, which, when the base composition is taken into consideration, is expected to contain 11 pentad sequences. Surprisingly, however, this ORF contains as many as 43 pentad sequences (Table 2), suggesting that the expression of this gene is likely to be highly sensitive to MazFSa.
TABLE 2.
Top 10 MazFSa-sensitive genes in the S. aureus genome
| CDS position | Length (bp) | Motif count
|
Probability | Locus | Gene | |
|---|---|---|---|---|---|---|
| Expected | Actual | |||||
| Complement (2755253-2762068) | 6,816 | 11.42 | 43 | 1 | SA2447 | |
| 1056358-1056567 | 210 | 0.14 | 3 | 0.99999 | SA0930 | |
| Complement (70209-70586) | 378 | 0.79 | 5 | 0.99984 | SA0062 | |
| 899902-901116 | 1,215 | 2.51 | 9 | 0.99972 | SA0794 | dltB |
| Complement (2528829-2529644) | 816 | 1.28 | 6 | 0.99964 | SA2252 | opp-1D |
| Complement (1675265-1676290) | 1,026 | 1.71 | 7 | 0.99961 | SA1466 | queA |
| 1395599-1395895 | 297 | 0.61 | 4 | 0.99959 | SA1222 | Truncated transposase |
| 940904-941293 | 390 | 0.65 | 4 | 0.99944 | SA0830 | |
| Complement (1859152-1860708) | 1,557 | 2.33 | 8 | 0.99931 | SA1626 | |
| 526376-526702 | 327 | 0.46 | 3 | 0.99870 | SA0456 | spoVG |
The stability of the sraP mRNA in the MazFSa-induced E. coli cells.
Next, we examined the sraP mRNA stability in E. coli in response to overexpression of MazFSa. For this purpose, the sraP gene from S. aureus, consisting of 6,546 bases (not including the first 270 bases for the signal peptide), was first cloned into the pBAD24 plasmid, which was then cotransformed into E. coli BL21(DE3) cells together with pET-28a MazFSa. At 90 min after SraP induction with arabinose, MazFSa expression was induced by adding 1 mM IPTG. The total RNA was extracted at each time point (0, 2, 5, 10, 20, 30, and 60 min) after IPTG induction. Since the mRNA for OmpA does not contain MazFSa cleavage sites, this mRNA was used as a control (Fig. 4E). In order to examine the stability of the sraP mRNA, we chose the 777-base region from base 392 to base 1168, containing five MazFSa target sequences (region 1), and the 641-base region from base 2693 to base 3333, containing nine MazFSa target sequences (region 2) (Fig. 4F) for semiquantitative detection of the mRNA by RT-PCR. As can be seen from Fig. 4, the sraP mRNA is stable in the absence of MazFSa (Fig. 4B and C) but became highly unstable when MazFSa was induced (Fig. 4A and C). At 5 min after the induction of MazFSa, the amount of the sraP mRNA was reduced 75% (region 1) or to 30% (region 2) of the amount observed at the 0-min time point, and at 30 min after MazFSa induction, it was reduced to 36% (region 1) or 14% (region 2) of the amount for this time point (Fig. 4A and C). Further degradation of the mRNA was not observed at 60 min. Region 2, containing nine cleavage sites, is more unstable than region 1, with five cleavage sites. Importantly, the ompA mRNA was very stable to the induced MazFSa, and its amount remained unchanged (Fig. 4E).
FIG. 4.
Analysis of stability of SraP and ompA mRNAs upon induction of MazFSa. (A) Total cellular RNA was extracted from E. coli BL21(DE3) cells containing pET-28a-MazF and pBAD-SraP at various time points (0, 2, 5, 10, 20, 30, and 60 min) after the addition of IPTG and subjected to RT-PCR. The amplified 641-bp region 2 fragment of the sraP mRNA contains nine cutting sites of MazFSa. (B) RT-PCR for the sraP region 2 fragment without IPTG induction of MazFSa. (C) RT-PCR for the 777-bp region 1 fragment of the sraP mRNA contains five cutting sites. (D) RT-PCR for the sraP region 1 fragment without IPTG induction of MazFSa. (E) RT-PCR for the ompA mRNA with IPTG induction of MazFSa. (F) Schematic representation of the sraP gene. The DNA sequences which correspond to the putative functional domains are delineated within each part of the DNA sequence. sp, signal peptide; srr1; serine-rich region 1; nrr, nonrepeated region; srr2, serine-rich region 2; cw, cell wall anchoring region. The positions of primers used for RT-PCR and the amplified DNA fragment are shown (sraUP14, sraP-RTr5, the 777-bp region 1 fragment, sraUP10, sraP-RTr3, and the 641-bp region 2 fragment). The cutting sites of MazFSa (UACAU) are shown with arrows. The stars above the arrows indicate the positions of the actual cutting sites in the amplified DNA fragment.
The pentad sequence is significantly abundant in the genes encoding pathogenic factors in S. aureus.
S. aureus (N315) has a total of 2,588 genes, and we analyzed all ORFs for the occurrences of the pentad sequence. The ORFs are retrieved from NCBI RefSeq (accession no. NC_002745). With a Perl script, we counted the number of pentad sequences in each gene, and these genes were sorted on the basis of these numbers of potential MazFSa cleavage sites in descending order. Since some genes have the same number of cleavage sites (K), we used the probability that the gene has K or more target sites to break the tie in sorting. The probability is calculated as follows. We first determined the nucleotide composition of each gene, that is, the gene-specific nucleotide composition was used in the calculation. The probability (p) of the appearance of the target pentad UACAU anywhere in the gene is (percentage of U residues)2 (percentage of A residues)2 (percentage of C residues). Let L be the length of the gene. The probability (P) of having K or more target sites in the gene is
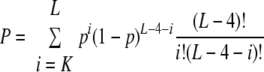 |
Among the 2,588 genes in the genome, 1,622 are characterized into 32 function types. For example, 109 genes are annotated as pathogenic factor. We then focused our study on the genes with function annotations. In the list of genes (sorted by number of target sites in descending order), four out of the top five genes belong to the pathogenic factor category. Therefore, we calculated the P value for the significance of the abundance of MazFSa cleavage sites in this category via hypergeometric distribution. Specifically, we calculated the probability that, among all possible ways to choose 5 out of 1,622 genes, 4 or more genes come from the pathogenic factor group (of 109 genes):
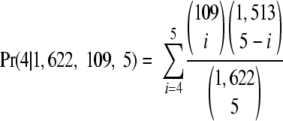 |
The P value was thus calculated to be 0.000092 (Table 3). This indicates that the target pentad sequence for MazFSa is significantly abundant in the pathogenic factor gene group in S. aureus. There are eight other gene groups, accounting for a total of 114 genes, which are not included in Table 3, as these gene groups have very low statistical significance (probability close to 1).
TABLE 3.
The target pentad sequence for MazFSa is significantly abundant in the pathogenic factor gene group in S. aureus
| Gene function | Significance | Gene no. |
|---|---|---|
| Transport binding | 0.167369 | 259 |
| Protein secretion | 0.091665 | 12 |
| Sensor | 0.019894 | 19 |
| Membrane bioenergetics | 0.167291 | 60 |
| Cell wall | 0.346614 | 65 |
| Cell division | 0.138731 | 16 |
| Coenzyme metabolism | 0.655797 | 70 |
| RNA modification | 0.344803 | 25 |
| Antibiotic production | 0.002466 | 1 |
| DNA repair or modification | 0.000223 | 28 |
| Transformation competence | 0.35868 | 11 |
| DNA replication | 0.022992 | 23 |
| DNA packaging | 0.048104 | 8 |
| Protein synthesis | 0.395914 | 99 |
| RNA synthesis | 0.310106 | 143 |
| Amino acid metabolism | 0.785883 | 149 |
| Carbohydrate metabolism | 0.752385 | 135 |
| Nucleic acid metabolism | 0.599606 | 75 |
| DNA recombination | 0.310621 | 21 |
| Protein modification | 0.396901 | 31 |
| Adaptation to atypical | 0.462806 | 47 |
| Pathogenic factor | 0.000092 | 109 |
| Phage related | 0.511815 | 52 |
| Lipid metabolism | 0.439539 | 51 |
DISCUSSION
S. aureus is a human pathogen causing a multitude of diseases, directly by infection (such as in the skin) or indirectly through toxins in cases of food poisoning and toxic shock syndrome. MRSA is a type of S. aureus which is often resistant to multiple antibiotics. The estimated number of people developing serious MRSA infection in 2005 predominantly through health care delivery systems was about 94,360, out of which approximately 18,650 people died. S. aureus is an opportunistic pathogen, having a complex network of global regulatory elements which enables it to rapidly sense its environmental changes and to respond appropriately to gain the capacity to survive under unfavorable conditions (7). These regulatory elements include the alternative sigma factor σB, which controls the expression of a variety of genes, including virulence determinants and global regulators (8, 28). The mazEFSa TA module is reported to be cotranscribed with the sigB operon, which includes regulator genes of sigB, such as rsbW, rsbV, and rsbU. Responding to environmental stresses, such as heat shock and salt stress, the sigB promoter sigBp1, which is located just upstream of mazEF genes, is rapidly activated, resulting in simultaneous activation of the mazFSa gene (28). Although it is known that sigB transcript levels do not necessarily reflect the σB content and also that the σB content is not a direct measure for σB activity, as σB activity depends on a posttranslational process involving several regulatory elements (28), the exact regulatory relationship of mazEF to the genes in the sigB operon remains to be elucidated.
Recently, a MazF homologue in S. aureus was reported to cleave at VUUV′ (V and V′ are A, C, or G and may or may not be identical), cleaving at the 3′ or 5′ end of the second U residue (11). In the present study, we unambiguously demonstrated that MazFSa is an mRNA interferase recognizing a pentad sequence. To identify a consensus pentad sequence, it is absolutely essential to use an RNA substrate which is longer than 3 kb, since statistically a unique pentad sequence can be found only once in every 1,024-base sequence, provided that the RNA contains equal numbers of bases and has random sequence. In addition to a long RNA substrate (the MS2 RNA used in our experiment is 3,569 bp in length), the use of an RNA chaperone, such as CspA, is also highly useful, since RNA substrates of such lengths usually contain large numbers of stable secondary structures, which have to be unwound for cleavage by mRNA interferases acting on single-stranded substrates (36).
Furthermore, the consensus sequence reported by Fu et al. (11, 12) was determined with the use of only the 5′-end portion (600 bases) of the 1.5-kb ctpA mRNA. This mRNA contains only one UACAU sequence in the 3′-end region which was not examined for MazFSa cleavage in their primer extension experiments. Clearly, the cleavage sites identified by them are the minor cleavage sites, which usually contain a 1-base difference from UACAU (Table 4), similar to those found in MS2 RNA (Table 1). Noticeably, MazFSa was used at a higher concentration in their experiments (15 pmol/reaction) than in our experiment (4.37 pmol/reaction), which might have also contributed toward the cleavage of RNA at nonspecific sequences.
TABLE 4.
Previously reported cleavage sites for MazFSa in the ctpA mRNAa
| Cleavage site with sequence differing from U↓ACAU by: |
|---|
| One base |
| GGCAA U↓UCAU AUCAA |
| GUUGU U↓GCAU AUAUU |
| AAACU U↓AAAU AAAAU |
| AAUCU U↓AAAU AGUGA |
| AUUAU U↓ACAA AAAAC |
| Two bases |
| AAGCA A↓ACUU AAAUA |
| UGGCA↓UUCGU CCUAA |
The cleavage sites were determined by Fu et al. (11). The VUUV′ sequences proposed in that study are underlined, and substitutions in the UACAU motif are in bold.
Our study shows that the mRNA for SraP is extremely sensitive to the endoribonuclease activity of the MazFSa since it has a significantly large number of MazFSa cleavage sites. Therefore, when MazFSa is induced under stress conditions, the synthesis of the SraP protein is likely to be severely inhibited. SraP is a high-molecular-mass protein, consisting of a total of 2,271 amino acid residues, which is homologous to GspB of Streptococcus gordonii. GspB is a large surface glycoprotein that is able to enhance the binding of the pathogen to human platelets (2). Consistent with this fact, SraP has been reported to be a virulence determinant in endovascular infection, and it is interesting to note that its C terminus contains a cell wall-anchoring motif (LPXTG) which is known to play important roles in virulence (29). Note that S. aureus is the main infective endocarditis pathogen and that the interaction of S. aureus with human platelets plays a crucial role in the pathogenesis of cardiovascular infections (10). As SraP mediates the direct binding of S. aureus to platelets, the present findings provide new insights into our understanding of how MazFSa is involved in the regulation of the pathogenicity of this pathogen. The gene of mazESa has was found to be transcribed at a constant level from early log phase to stationary phase in a microarray analysis, and mazESa and mazFSa were cotranscribed under regulation of the SigBP1 promoter (28). The microarray assay suggested that SraP is expressed from early exponential phase and that its expression level is higher in stationary phase than in log phase (3). Siboo et al. assessed the binding of S. aureus to human platelets and the effect of SraP expression on virulence in a rabbit endocarditis model using stationary-phase culture (29). They also reported that SraP from clinical isolates of S. aureus was expressed in mid-log phase (29). Analysis of this expression pattern indicated that MazEF is expressed at a constant level but that the expression of SraP is enhanced during late growth phase.
Recently, the global regulatory effect of the ClpP protease of S. aureus on genes involved in microbial virulence was reported. This study demonstrated that mazFSa is downregulated in a ClpP protease-defective mutant while sraP is upregulated in the mutant strain in comparison with the level for the wild-type strain (23). For E. coli, it is known that MazE is degraded by ClpPA (1), which leads to release of MazF (16). A similar regulatory mechanism is likely operating in S. aureus, affecting the expression of SraP. It is possible that MazFSa may be involved in regulation of expression of pathogenic factors, although additional work is needed to further elucidate the direct or indirect relationship between the mazEFSa module and pathogenic factors.
Our findings suggest that the mazEFSa system may be an excellent target for developing novel antibiotics against S. aureus. If the formation of the MazFSa-MazESa complex is blocked by a compound, MazFSa is activated to inhibit the synthesis of the SraP protein, resulting in the reduction of the platelet binding ability of this pathogen, with the concomitant reduction of its virulence. Importantly, as a result of the inhibition of the TA complex formation, the mazEFSa operon expression is likely to be depressed, which further stimulates the release of active MazFSa in the cell.
Previously, we found that mRNA interferases from M. tuberculosis recognize unique pentad sequences, which are underrepresented in the gene family involved in the pathogenicity of M. tuberculosis. The present paper demonstrates a second example of mRNA interferases being associated with the pathogenicity of a bacterium. It seems that there are at least three classes of mRNA interferases: class I mRNA interferases are those which cleave at specific 3-base sequences and are involved in cell growth regulation such, as E. coli MazF; class II mRNA interferases are those which have much higher specificity recognizing pentad sequences and are associated with bacterial pathogenicity; and class III mRNA interferases are those which have also higher specificity and are required for programmed cell death (Y. Yamaguchi and M. Inouye, unpublished). It remains to be elucidated if there is another class of mRNA interferases, recognizing sequences of 6 bases or longer. Such mRNA interferases, if found, must be involved in regulating only a very specific set of genes.
Acknowledgments
We thank Sangita Phadtare and Jason Schifano for critical reading of the manuscript.
This work was partially supported by a research fund from Takara-Bio., Inc., Japan.
Footnotes
Published ahead of print on 27 February 2009.
REFERENCES
- 1.Aizenman, E., H. Engelberg-Kulka, and G. Glaser. 1996. An Escherichia coli chromosomal “addiction module” regulated by 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc. Natl. Acad. Sci. USA 936059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 441081-1094. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff, M., P. Dunman, J. Kormanec, D. Macapagal, E. Murphy, W. Mounts, B. Berger-Bächi, and S. Projan. 2004. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 1864085-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, J. M., and K. J. Shaw. 2003. A novel family of Escherichia coli toxin-antitoxin gene pairs. J. Bacteriol. 1856600-6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buts, L., J. Lah, M. H. Dao-Thi, L. Wyns, and R. Loris. 2005. Toxin-antitoxin modules as bacterial metabolic stress managers. Trends Biochem. Sci. 30672-679. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, S., W. Jiang, S. D. Emerson, and M. Inouye. 1993. The backbone structure of the major cold-shock protein CS7.4 of Escherichia coli in solution includes extensive beta-sheet structure. J. Biochem. (Tokyo) 114663-669. [DOI] [PubMed] [Google Scholar]
- 7.Cheung, A. L., A. S. Bayer, G. Zhang, H. Gresham, and Y. Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 401-9. [DOI] [PubMed] [Google Scholar]
- 8.Donegan, N. P., and A. L. Cheung. 30 January 2009, posting date. Regulation of the mazEF toxin-antitoxin module in Staphylococcus aureus and its impact on sigB expression. J. Bacteriol. doi: 10.1128/JB.01713-08. [DOI] [PMC free article] [PubMed]
- 9.Engelberg-Kulka, H., B. Sat, M. Reches, S. Amitai, and R. Hazan. 2004. Bacterial programmed cell death systems as targets for antibiotics. Trends Microbiol. 1266-71. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald, J. R., T. J. Foster, and D. Cox. 2006. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 4445-457. [DOI] [PubMed] [Google Scholar]
- 11.Fu, Z., N. P. Donegan, G. Memmi, and A. L. Cheung. 2007. Characterization of MazFSa, an endoribonuclease from Staphylococcus aureus. J. Bacteriol. 1898871-8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu, Z., S. Tamber, G. Memmi, N. P. Donegan, and A. L. Cheung. 23 January 2009, posting date. Overexpression of MazFSa in Staphylococcus aureus induces bacteriostasis by selectively targeting mRNAs for cleavage. J. Bacteriol. doi: 10.1128/JB.00907-08. [DOI] [PMC free article] [PubMed]
- 13.Garcia-Contreras, R., X. S. Zhang, Y. Kim, and T. K. Wood. 2008. Protein translation and cell death: the role of rare tRNAs in biofilm formation and in activating dormant phage killer genes. PLoS ONE 3e2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdes, K., S. K. Christensen, and A. Lobner-Olesen. 2005. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 3371-382. [DOI] [PubMed] [Google Scholar]
- 15.Hayes, F. 2003. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 3011496-1499. [DOI] [PubMed] [Google Scholar]
- 16.Inouye, M. 2006. The discovery of mRNA interferases: Implication in bacterial physiology and application to biotechnology. J. Cell. Physiol. 209670-676. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, W., Y. Hou, and M. Inouye. 1997. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 272196-202. [DOI] [PubMed] [Google Scholar]
- 18.Kamada, K., and F. Hanaoka. 2005. Conformational change in the catalytic site of the ribonuclease YoeB toxin by YefM antitoxin. Mol. Cell 19497-509. [DOI] [PubMed] [Google Scholar]
- 19.Kamada, K., F. Hanaoka, and S. K. Burley. 2003. Crystal structure of the MazE/MazF complex: molecular bases of antidote-toxin recognition. Mol. Cell 11875-884. [DOI] [PubMed] [Google Scholar]
- 20.Keren, I., D. Shah, A. Spoering, N. Kaldalu, and K. Lewis. 2004. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 1868172-8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korch, S. B., T. A. Henderson, and T. M. Hill. 2003. Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p) ppGpp synthesis. Mol. Microbiol. 501199-1213. [DOI] [PubMed] [Google Scholar]
- 22.Makarova, K. S., N. V. Grishin, and E. V. Koonin. 2006. The HicAB cassette, a putative novel, RNA-targeting toxin-antitoxin system in archaea and bacteria. Bioinformatics 222581-2584. [DOI] [PubMed] [Google Scholar]
- 23.Michel, A., F. Agerer, C. R. Hauck, M. Herrmann, J. Ullrich, J. Hacker, and K. Ohlsen. 2006. Global regulatory impact of ClpP protease of Staphylococcus aureus on regulons involved in virulence, oxdative stress response, autolysis, and DNA repair. J. Bacteriol. 1885783-8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motiejunaite, R., J. Armalyte, A. Markuckas, and E. Suziedeliene. 2007. Escherichia coli dinJ-yafQ genes act as a toxin-antitoxin module. FEMS Microbiol. Lett. 268112-119. [DOI] [PubMed] [Google Scholar]
- 25.Nariya, H., and M. Inouye. 2008. MazF, an mRNA interferase, mediates programmed cell death during multicellular Myxococcus development. Cell 13255-66. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen, K., A. V. Zavialov, M. Y. Pavlov, J. Elf, K. Gerdes, and M. Ehrenberg. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112131-140. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt, O., V. J. Schuenemann, N. J. Hand, T. J. Silhavy, J. Martin, A. N. Lupas, and S. Djuranovic. 2007. prlF and yhaV encode a new toxin-antitoxin system in Escherichia coli. J. Mol. Biol. 372894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senn, M. M., P. Giachino, D. Homerova, A. Steinhuber, J. Strassner, J. Kormanec, U. Fluckiger, B. Berger-Bachi, and M. Bischoff. 2005. Molecular analysis and organization of the σB operon in Staphylococcus aureus. J. Bacteriol. 1878006-8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siboo, I. R., H. F. Chambers, and P. M. Sullam. 2005. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect. Immun. 732273-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki, M., J. Zhang, M. Liu, N. A. Woychik, and M. Inouye. 2005. Single protein production in living cells facilitated by an mRNA interferase. Mol. Cell 18253-261. [DOI] [PubMed] [Google Scholar]
- 31.Takagi, H., Y. Kakuta, T. Okada, M. Yao, I. Tanaka, and M. Kimura. 2005. Crystal structure of archaeal toxin-antitoxin RelE-RelB complex with implications for toxin activity and antitoxin effects. Nat. Struct. Mol. Biol. 12327-331. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, J., Y. Zhang, and M. Inouye. 2003. Characterization of the interactions within the mazEF addiction module of Escherichia coli. J. Biol. Chem. 27832300-32306. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Y., J. Zhang, H. Hara, I. Kato, and M. Inouye. 2005. Insights into the mRNA cleavage mechanism by MazF, an mRNA interferase. J. Biol. Chem. 2803143-3150. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, Y., J. Zhang, K. P. Hoeflich, M. Ikura, G. Qing, and M. Inouye. 2003. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol. Cell 12913-923. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, Y., L. Zhu, J. Zhang, and M. Inouye. 2005. Characterization of ChpBK, an mRNA interferase from Escherichia coli. J. Biol. Chem. 28026080-26088. [DOI] [PubMed] [Google Scholar]
- 36.Zhu, L., S. Phadtare, H. Nariya, M. Ouyang, R. N. Husson, and M. Inouye. 2008. The mRNA interferases, MazF-mt3 and MazF-mt7 from Mycobacterium tuberculosis target unique pentad sequences in single-stranded RNA. Mol. Microbiol. 69559-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu, L., Y. Zhang, J. S. Teh, J. Zhang, N. Connell, H. Rubin, and M. Inouye. 2006. Characterization of mRNA interferases from Mycobacterium tuberculosis. J. Biol. Chem. 28118638-18643. [DOI] [PubMed] [Google Scholar]