Abstract
Pseudomonas aeruginosa produces the quorum signal 2-heptyl-3-hydroxy-4-quinolone (Pseudomonas quinolone signal), which is important for stimulating outer membrane vesicle (MV) formation. Here we describe the importance of the 3-hydroxyl and 2-alkyl chain for MV production and the length of the 2-alkyl chain for association with MVs.
It is clear that bacteria do not act as single entities but as communities wherein they communicate to coordinate group behaviors. This phenomenon, known as quorum sensing (QS), utilizes signaling molecules derived from central metabolic intermediates (19). These small molecules exit the bacterial cells and are trafficked to other cells within the population. Upon reaching a threshold concentration, often indicative of achieving a particular cell density, these signals cause changes in gene expression (19).
Many bacteria are thought to utilize QS, frequently producing multiple signaling molecules important for inter- and/or intraspecies communication. Acyl-homoserine lactone (HSL) signals bound by their cognate transcriptional regulators constitute the most widespread intraspecies QS system in gram-negative bacteria, exemplified by the opportunistic pathogen Pseudomonas aeruginosa. P. aeruginosa utilizes two HSL QS systems, referred to as the las and rhl systems. The las system involves production of the signaling molecule N-(3-oxododecanoyl)-l-homoserine lactone (3OC12-HSL) by the LasI synthase and sensing of this molecule by the transcriptional regulator LasR (19). The rhl system of P. aeruginosa is similar to the las system, involving the synthesis of N-butyryl-l-homoserine lactone (C4-HSL) by RhlI and sensing by RhlR (19). C4-HSL is thought to freely diffuse out of bacterial cells (11), whereas export of the more hydrophobic 3OC12-HSL is aided by the efflux pump MexAB-OprM (5, 20). P. aeruginosa also produces a third, non-HSL signaling molecule, 2-heptyl-3-hydroxy-4-quinolone (Fig. 1), termed the Pseudomonas quinolone signal (PQS) (21). Unlike HSLs, PQS is thus far unique to P. aeruginosa. PQS, through binding to the transcriptional regulator PqsR (MvfR), enhances the expression of a number of virulence factors (4, 8); thus, PQS is required for P. aeruginosa virulence in several models of infection (10). Together, 3OC12-HSL, C4-HSL, and PQS constitute a complex signaling network that controls the expression of more than 300 genes (2, 22, 23).
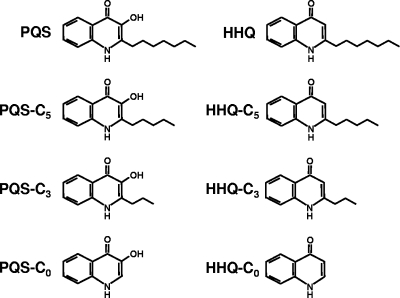
FIG. 1.
Structures of the quinolones used in this study. HHQ-C3 is 2-propyl-4-quinolone; all other derivatives are defined in the text.
Along with PQS, P. aeruginosa produces more than 50 additional 4-alkyl-quinolones (also referred to as 4-alkyl-quinolines), some of which have antimicrobial activities and many whose functions are unknown (3, 14, 15). P. aeruginosa 4-alkyl-quinolones, including the immediate precursor to PQS, 2-heptyl-4-quinolone (HHQ) (Fig. 1), are synthesized by a head-to-head condensation of anthranilic acid and β-keto fatty acid (1, 3). Although the reaction mechanism is not fully elucidated, proteins encoded by the pqsABCD operon are likely involved. HHQ is hydroxylated by the putative monooxygenase PqsH to form PQS (1, 3). Xiao et al. have shown that PQS and HHQ act as signaling molecules through binding to PqsR, although PQS has more potent signaling activity (24).
Due to its hydrophobic nature, PQS is unlikely to freely diffuse out of bacterial cells. In support of this notion, we recently demonstrated that PQS is packaged within membrane vesicles (MVs) liberated from the outer membrane of P. aeruginosa (16). These spherical, bilayered vesicles generally range in size from 50 to 250 nm in diameter and are naturally produced by most gram-negative bacteria (13, 18). Although the mechanism of MV production in P. aeruginosa is not known, we provided evidence that inactivation of pqsH results in significantly reduced MV formation by P. aeruginosa (16). MV formation by the pqsH mutant could be restored to near wild-type levels by the exogenous addition of PQS, although interestingly, PQS signaling was not required (16). Based on these findings, a model was proposed in which PQS initiates MV formation by interacting with the lipid A component of lipopolysaccharide (LPS), the primary lipid in the outer leaflet of the gram-negative bacterial outer membrane. We provided support for this model using a series of biophysical techniques that conclusively demonstrated the interaction of PQS with lipid A (17). This interaction requires the 2-alkyl and 3-hydroxyl groups of PQS, as their removal significantly diminishes PQS-lipid A interactions (17). The goal of the present study is to expand on these initial studies by examining the role of the 2-alkyl and 3-hydroxyl components of PQS in stimulating MV production and packaging into MVs.
To determine their importance for MV formation, PQS and HHQ derivatives with alkyl chains of various lengths were chemically synthesized (Syntech Solutions, San Diego, CA) (Fig. 1). Aside from 2-pentyl-4-quinolone (HHQ-C5), these molecules are not naturally produced by P. aeruginosa at detectable levels (14). Each molecule was examined for its ability to stimulate MV formation by a P. aeruginosa pqsA pqsH double mutant as outlined previously by Mashburn-Warren et al. (17). This mutant strain is unable to produce or perform known modifications to P. aeruginosa 4-alkyl-quinolones and consequently produces low levels of MVs. Preparation and quantification of MVs from the wild type and the pqsA pqsH mutant grown with and without the addition of quinolones were performed as described previously (17). All quinolones were added at biologically relevant concentrations (50 μM), and methanol, the quinolone solvent, was used as a control. PQS derivatives with alkyl chains of three and five carbons induced MV formation, although to a lesser extent than PQS (Fig. 2A). As expected, the addition of HHQ and HHQ derivatives did not provoke statistically significant increases in MV formation (Fig. 2A and B and data not shown). Although 3-hydroxy-4-quinolone (PQS-C0) did not induce MV formation at 50 μM, it did significantly induce MV formation when the concentration was increased to 100 μM or 200 μM, while increasing the levels of 4-quinolone (HHQ-C0) had no effect (Fig. 2B). Transmission electron microscopy of quinolone-induced MV preparations revealed they were the same size and shape as naturally produced MVs from wild-type P. aeruginosa (data not shown). These data support a model in which the third-position hydroxyl of PQS is absolutely critical to initiate MV formation, while the alkyl group, although important for the potency of the MV-inducing activity of the molecule, is dispensable.
FIG. 2.
The third-position hydroxyl group of PQS is required for P. aeruginosa MV formation, while the length of the second-position alkyl group impacts PQS potency. (A) MV production by the P. aeruginosa pqsA pqsH double mutant in the absence (−) or presence of HHQ (HHQ), PQS (PQS), and PQS derivatives (PQS-C0, PQS-C3, PQS-C5). Quinolones in 50 μM concentrations were used, and MV preparation and quantification were done as previously described (17). MV levels in the presence of each HHQ derivative did not statistically differ from those in the presence of HHQ (data not shown). (B) MV production by the P. aeruginosa pqsA pqsH mutant in the presence of increasing concentrations of PQS-C0. HHQ-C0 at 200 μM was included as a control, and 50 μM PQS-C0 from Mashburn-Warren et al. (14) is provided as a reference. Error bars represent the standard error of the mean for triplicate experiments. *, P of <0.05 by Student's t test, compared to the no-addition control (−). OD600 nm, optical density at 600 nm.
Over 95% of naturally produced PQS has been shown to be associated with MVs and bacterial cells within a planktonic P. aeruginosa culture (16). Based on these experiments, we were interested in examining whether the alkyl chain length affected the localization of PQS. To determine the localization of PQS and its derivatives, thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) were used. P. aeruginosa PA14 was grown in the presence of PQS and its derivatives (HHQ and its derivatives were also used as controls). Exponentially growing cells were pelleted by centrifugation, and supernatants were passed through 0.45-μm filters and ultracentrifuged to collect MVs. This process, specifically filtration and the use of exponentially growing P. aeruginosa PA14, is critical to minimize contamination of MV preparations with lysogenic bacteriophage and extracellular flagella. Volumes of 500 μl of the resuspended cell pellet and cell-free supernatant were extracted with 1.5 ml acidified ethyl acetate, while concentrated MVs (250 μl) were extracted with 1 ml of acidified ethyl acetate. Extracts were then evaporated under a continuous stream of N2. After the various extracts were concentrated two- to sixfold by resuspension in methanol, 10-μl samples were analyzed by TLC. TLC was performed as described previously (21), with a 95:5 dichloromethane-methanol solvent system (6). TLC plates were analyzed under UV excitation with a Syngene G:box (Syngene, Frederick, MD). Percentages of PQS and PQS derivatives in each fraction were calculated with the Syngene Genetools software (Syngene, Frederick, MD), using synthetic quinolones as standards. Because of the difficulties in analyzing HHQ by TLC, HPLC was used to determine the localization of HHQ and its derivatives within the cell culture. Dried extracts were resuspended in 250 μl of methanol, of which 50 μl was injected into a Varian Pro Star HPLC system fitted with a Varian Pursuit 5 C8 ChromSep HPLC column. Elution was carried out with a gradient of 30 to 100% methanol over a 50-min period with a 1-ml/min flow rate. Percentages of quinolones were determined by measuring peak absorbancies at 233 and 338 nm. The results revealed that around 90% of the PQS derivatives with C0 and C3 alkyl chain lengths were present in the MV-free supernatant, while 85% of PQS and 52% of the 2-pentyl-3-hydroxy-4-quinolone (PQS-C5) derivative were associated with the cells and MVs (Table 1). It is of note that there appears to be enrichment of PQS and PQS-C5 in MVs, as essentially equal amounts of these molecules are found associated with MVs and cells despite the fact that MVs likely comprise less than 1% of the total outer membrane of planktonic cultures (9, 12, 13). As expected, more than 90% of the HHQ derivatives were present in the MV-free supernatants, whereas HHQ was found in the supernatants (69%) or in association with bacterial cells (31%). Collectively, these data indicate that the length of the PQS alkyl chain and the presence of the third-position hydroxyl are critical for localization with bacterial cells and MVs.
TABLE 1.
Localization of quinolones
| Quinolone | % Quinolone present ina:
|
||
|---|---|---|---|
| Cells | Supernatant | MVs | |
| PQS-C0 | 3.2 ± 4.4 | 91.8 ± 0.1 | 5.0 ± 4.5 |
| PQS-C3 | 4.5 ± 5.8 | 89.8 ± 3.2 | 5.7 ± 2.5 |
| PQS-C5 | 29.9 ± 5.3 | 47.8 ± 0.7 | 22.3 ± 4.6 |
| PQS | 31.7 ± 13.5 | 15.1 ± 0.1 | 53.2 ± 13.6 |
| HHQ-C0 | <1 | 95.8 ± 0.1 | 4.2 ± 0.1 |
| HHQ-C3 | 1.4 ± 0.5 | 97.7 ± 0.6 | 0.8 ± 0.1 |
| HHQ-C5 | 2.8 ± 1.6 | 94.2 ± 3.3 | 3.0 ± 1.7 |
| HHQ | 31.0 ± 2.9 | 69.0 ± 2.9 | <1 |
Quinolones were added to the P. aeruginosa pqsA pqsH mutant, and localization was determined as described previously (17). Data represent the average and standard deviation of the results of triplicate experiments.
The observation that PQS-C0 and 2-propyl-3-hydroxy-4-quinolone (PQS-C3) show reduced association with bacterial cells and MVs correlates well with recent fluorescence resonance energy transfer (FRET) studies from our laboratory demonstrating that PQS readily integrates into LPS while HHQ and PQS-C0 do not (17). Based on these studies and the localization data in Table 1, we hypothesized that PQS-C3 incorporation into LPS would be reduced, while PQS-C5 would incorporate into LPS in a manner similar to PQS. To test incorporation into P. aeruginosa LPS, FRET analysis was performed as previously described (17). The results indicated that PQS-C3 integration into LPS was significantly reduced compared to that of PQS (Fig. 3), similar to what was observed for PQS-C0 (17). In contrast, PQS-C5 demonstrated increased integration compared to that of PQS-C3, nearly to the same level as that of PQS (Fig. 3). These results suggest that the length of the alkyl chain is critical for stable integration into LPS and establish a five-carbon alkyl chain as the minimum requirement for notable association with cells and MVs and for integration into LPS.
FIG. 3.
The length of the PQS alkyl chain is critical for intercalation into P. aeruginosa LPS. The FRET ID/IA spectroscopic signals (intensity of donor/intensity of acceptor) versus time for LPS aggregates with addition of PQS, PQS-C3, and PQS-C5 are shown. LPS with incorporated donor and acceptor labels was added at 0 s, and the quinolones were added after 50 s. The signal was then measured for an additional 250 s. FRET is used here as a probe dilution assay; thus, intercalation of quinolones causes an increase in the distance between acceptor and donor (i.e., reduced energy transfer, increases in ID/IA). For a control, methanol was added without quinolones. Data represent triplicate measurements, and the PQS data reported by Mashburn-Warren et al. (16) are provided as a reference.
PQS is a unique signaling molecule in that it is not only involved in cell signaling but also stimulates MV formation and remains associated within MVs. This investigation has provided new insight into the multifunctional nature of PQS, specifically the importance of the hydroxyl group and alkyl chain for stimulating MV formation. While both constituents are important for MV formation, our results suggest that the third-position hydroxyl is absolutely critical for stimulating MV formation and for association with MVs. This is not surprising, as our previous studies provided proof that this hydroxyl, along with the alkyl side chain, was critical for interaction with LPS (17). However, this is in contrast to the importance of these constituents for PQS signaling, in which the alkyl chain is absolutely critical to induce PQS-controlled genes (7). Although the manner by which PQS-LPS interactions stimulate MV formation is not fully elucidated, these studies support a model in which interactions governed by the PQS alkyl chain and hydroxyl group are critical for this process.
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (M.W.). M.W. is a Burroughs Wellcome Investigator in Pathogenesis of Infectious Disease.
We also thank members of the Whiteley lab for critical reading of the manuscript.
Footnotes
Published ahead of print on 13 March 2009.
REFERENCES
- 1.Bredenbruch, F., M. Nimtz, V. Wray, M. Morr, R. Muller, and S. Haussler. 2005. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J. Bacteriol. 1873630-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deziel, E., S. Gopalan, A. P. Tampakaki, F. Lepine, K. E. Padfield, M. Saucier, G. Xiao, and L. G. Rahme. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-l-homoserine lactones. Mol. Microbiol. 55998-1014. [DOI] [PubMed] [Google Scholar]
- 3.Deziel, E., F. Lepine, S. Milot, J. He, M. N. Mindrinos, R. G. Tompkins, and L. G. Rahme. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA 1011339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diggle, S. P., K. Winzer, S. R. Chhabra, K. E. Worrall, M. Camara, and P. Williams. 2003. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 5029-43. [DOI] [PubMed] [Google Scholar]
- 5.Evans, K., L. Passador, R. Srikumar, E. Tsang, J. Nezezon, and K. Poole. 1998. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 1805443-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher, M. P., S. P. Diggle, M. Camara, and P. Williams. 2007. Biosensor-based assays for PQS, HHQ and related 2-alkyl-4-quinolone quorum sensing signal molecules. Nat. Protoc. 21254-1262. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher, M. P., S. P. Diggle, S. A. Crusz, S. R. Chhabra, M. Camara, and P. Williams. 2007. A dual biosensor for 2-alkyl-4-quinolone quorum-sensing signal molecules. Environ. Microbiol. 92683-2693. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher, L. A., S. L. McKnight, M. S. Kuznetsova, E. C. Pesci, and C. Manoil. 2002. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J. Bacteriol. 1846472-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoekstra, D., J. W. van der Laan, L. de Leij, and B. Witholt. 1976. Release of outer membrane fragments from normally growing Escherichia coli. Biochim. Biophys. Acta 455889-899. [DOI] [PubMed] [Google Scholar]
- 10.Jander, G., L. G. Rahme, and F. M. Ausubel. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 1823843-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 1631210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesty, N. C., and M. J. Kuehn. 2004. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J. Biol. Chem. 2792069-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehn, M. J., and N. C. Kesty. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 192645-2655. [DOI] [PubMed] [Google Scholar]
- 14.Lepine, F., S. Milot, E. Deziel, J. He, and L. G. Rahme. 2004. Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa. J. Am. Soc. Mass Spectrom. 15862-869. [DOI] [PubMed] [Google Scholar]
- 15.Machan, Z. A., G. W. Taylor, T. L. Pitt, P. J. Cole, and R. Wilson. 1992. 2-Heptyl-4-hydroxyquinoline N-oxide, an antistaphylococcal agent produced by Pseudomonas aeruginosa. J. Antimicrob. Chemother. 30615-623. [DOI] [PubMed] [Google Scholar]
- 16.Mashburn, L. M., and M. Whiteley. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437422-425. [DOI] [PubMed] [Google Scholar]
- 17.Mashburn-Warren, L., J. Howe, P. Garidel, W. Richter, F. Steiniger, M. Roessle, K. Brandenburg, and M. Whiteley. 2008. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Mol. Microbiol. 69491-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mashburn-Warren, L. M., and M. Whiteley. 2006. Special delivery: vesicle trafficking in prokaryotes. Mol. Microbiol. 61839-846. [DOI] [PubMed] [Google Scholar]
- 19.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 978789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson, J. P., C. Van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 1811203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 9611229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 1852066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 1852080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao, G., E. Deziel, J. He, F. Lepine, B. Lesic, M. H. Castonguay, S. Milot, A. P. Tampakaki, S. E. Stachel, and L. G. Rahme. 2006. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol. Microbiol. 621689-1699. [DOI] [PubMed] [Google Scholar]