Abstract
Two hypothetical genes were functionally verified to be a pyrophosphatase and a PAP phosphatase in Thermococcus onnurineus NA1. This is the first report of the pyrophosphatases and the PAP phosphatases being organized in the gene clusters of the sulfate activation system only in T. onnurineus NA1 and “Pyrococcus abyssi.”
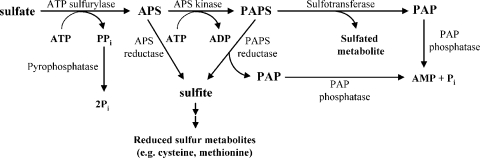
Sulfate is assimilated through reduction to sulfite and incorporation into the sulfur metabolites such as cysteine, methionine, or homocysteine (4) and through sulfation of various metabolites by the action of sulfotransferases (13, 15, 22, 27) (Fig. 1). The sulfate assimilation pathways require the activation of sulfate, forming adenosine 5′-phosphosulfate (APS) by ATP-sulfurylase (EC 2.7.7.4) and 3′-phosphoadenosine-5′-phosphosulfate (PAPS) by APS kinase (EC 2.7.1.25) (11). Pyrophosphatase (EC 3.6.1.1) favors the former reaction by effectively removing inorganic pyrophosphates (PPi) to phosphates (6). Soluble pyrophosphatases from a wide variety of sources have been identified and classified to two superfamilies, the inorganic pyrophosphatase superfamily (family I) and the DHH (Asp-His-His) phosphoesterase superfamily (family II) (5, 29, 32). However, a specific enzyme for the reaction has not been pinpointed yet. In the latter reaction, PAPS enters the reductive sulfate assimilation pathway involving PAPS reductase or is utilized as a sulfate donor for sulfotransferase, yielding 3′-phosphoadenosine-5′-phosphate (PAP). The specific PAP phosphatase has been known to remove the 3′-phosphate of PAP to prevent the intracellular trapping of adenine nucleotides and the inhibition of PAPS reductase, sulfotransferase, and oligoribonuclease by the metabolite (19, 28). A 3′(2′),5′-diphosphonucleoside 3′(2′)-phosphohydrolase from Chlorella species and a 2′(3′),5′-bisphosphate nucleotidase from guinea pig liver could dephosphorylate PAP to AMP (17, 26), and the proteins encoded by cysQ, Rv2131c, HAL2, SAL1, and RHL genes from Escherichia coli, Mycobacterium tuberculosis, yeast, and plants, respectively, and murine bisphosphate nucleotidase were also reported to have phosphomonoesterase activity toward PAP (12, 20, 23, 25, 30).
FIG. 1.
Sulfate assimilation pathways. All intermediates are shown in bold, and enzymes are shown alongside the reaction arrows.
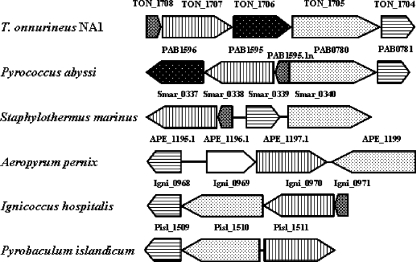
In the sequenced hyperthermophilic archaeal genome of Thermococcus onnurineus NA1 (16), two genes encoding the ATP sulfurylase (TON_1707) and the APS kinase (TON_1704) could be identified by sequence similarity with their counterparts. Those genes flank two open reading frames, TON_1705 and TON_1706, which are annotated as hypothetical proteins. The Sequence Similarity Database gene cluster search (http://www.genome.jp) of the Kyoto Encyclopedia of Genes and Genomes revealed that clustering of all four genes was maintained in “Pyrococcus abyssi,” while Staphylothermus marinus, Aeropyrum pernix, Ignicoccus hospitalis, and Pyrobaculum islandicum showed clustering of only three genes except the TON_1706 ortholog (Fig. 2).
FIG. 2.
Organization of the gene cluster involved in sulfate activation system. Comparison of T. onnurineus NA1 gene cluster and the corresponding region in other genomes. Open reading frames with sequence similarities are outlined using the same pattern.
TON_1705 orthologs are annotated as hypothetical proteins or type I phosphodiesterase/nucleotide pyrophosphatases, which catalyze the cleavage of phosphodiester and phosphosulfate bonds in NAD, deoxynucleotides, and nucleotide sugars. TON_1705 exhibited 36 to 82% similarity in its amino acid sequence to its orthologs and contained residues involved in metal binding or catalysis which are conserved in the members of the alkaline phosphatase superfamily (8-10). A motif search of the protein sequence of TON_1706 using Pfam suggests that it might belong to a DHH phosphoesterase superfamily including functionally related enzymes such as the family II inorganic pyrophosphatases, prune, a cyclic AMPase and RecJ, a single-stranded DNA exonuclease (1). TON_1706 and its ortholog, P. abyssi PAB1596 (a hypothetical protein with 62% identity), bear the characteristic triplet motif, Asp-His-His, that contributes to the active site and also three other motifs conserved in the DHH phosphoesterase superfamily. The phylogenetic tree computed with the multiple sequence alignment produced by T-Coffee revealed that TON_1706 and PAB1596 formed a cluster distinct from all other members of DHH subfamilies 1 and 2 (data not shown).
Since TON_1705 and TON_1706 genes are flanked by ATP sulfurylase and APS kinase, we predicted their functionalities by considering which activities the enzymes in the respective superfamily could show in connection with the sulfate assimilation pathway. For TON_1705 belonging to the alkaline phosphatase superfamily, it was predicted to be a phosphatase, a nucleotide pyrophosphatase, or a sulfohydrolase, which is required to modulate the concentrations of various adenylate compounds such as PAPS, APS, AMP, or ADP and pyrophosphate. It has been reported that the sulfohydrolytic activities to degrade PAPS and APS in rat liver and human placenta were due to enzymes having a nucleotide pyrophosphatase nature (7, 31). TON_1706 was predicted to function as a PAP phosphatase by the clue that YtqI from Bacillus subtilis belongs to the DHH phosphoesterase superfamily and has PAP phosphatase activity along with oligoribonuclease activity (7, 18, 31).
The TON_1705 and TON_1706 genes were PCR amplified from T. onnurineus NA1 genomic DNA and cloned into the pET-24a(+) vector (Novagen, Madison, WI). Proteins were overexpressed in E. coli Rosetta(DE3)pLysS (Stratagene, La Jolla, CA) in Luria-Bertani medium by induction with 1 mM isopropyl-β-d-thiogalactopyranoside at 37°C. The proteins were purified to homogeneity using Talon metal affinity column chromatography (BD Biosciences Clontech, Palo Alto, CA). The buffer of the proteins was then exchanged with 50 mM Tris-HCl buffer (pH 8.0), which includes 10% glycerol, using Centricon YM-10 (Millipore, Bedford, MA). Each 37-kDa and 56-kDa protein was shown to be the major component of the purified protein samples by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
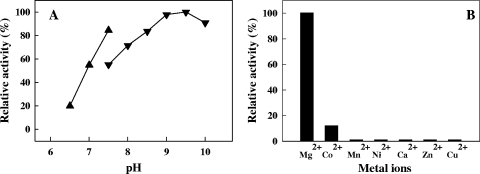
The nucleotide pyrophosphatase, the sulfohydrolase, or the phosphatase activity of the TON_1705-encoded protein was examined using adenylate compounds such as ATP, ADP, AMP, 3′-AMP, PAPS, and APS as substrates (2, 3, 11, 24), but little or no phosphohydrolytic or sulfohydrolytic activity was detected for those substrates (Table 1 and data not shown). However, the protein exhibited very high pyrophosphatase activity, hydrolyzing inorganic pyrophosphate to orthophosphate (Table 1). Thus, this results in expanding the substrate spectrum of the alkaline phosphatase superfamily. The pyrophosphatase activity was pH dependent and evaluated to be maximal in the pH 9.0 to 9.5 range (Fig. 3A). In the absence of metal ions, no activity was detected (data not shown), and the enzyme activity relied on the presence of the Mg2+ ion (Fig. 3B). The result confirms the metalloenzymatic nature of the TON_1705-encoded protein, as all the members of the alkaline phosphatase superfamily have strong dependency on divalent cations. Maximal activity of the enzyme was observed with the Mg2+ ion at the concentration of 0.2 mM (data not shown). The kinetic parameters of the enzyme, Km and kcat, toward pyrophosphate were determined to be 18.8 μM and 2.1 s−1, respectively. The affinity of the enzyme for pyrophosphate was between Km values of smaller than 10 μM for most family I pyrophosphatases and high Km values of 90 to 160 μM for family II pyrophosphatases (21). The kcat/Km value of 1.1 × 105 M−1 s−1 is 1 or 2 orders of magnitude lower than those of the family I pyrophosphatase from Pyrococcus horikoshii (6.6 × 106) (14) and the family II pyrophosphatase of Bacillus subtilis (2.0 × 107) (21).
TABLE 1.
Substrate specificity of the phosphatase activities of the TON_1705- and TON_1706-encoded proteins
| Substratea | Activityb
|
|
|---|---|---|
| TON_1705 | TON_1706 | |
| Pyrophosphate | 100 | <1 |
| PAP | ND | 100 |
| PAPS | ND | 23 |
| ADP | 5 | <1 |
| 3′-AMP | 4 | <1 |
| ATP | 4 | <1 |
| AMP | 1 | <1 |
| Polyphosphate | <1 | <1 |
| 3′-CMP | ND | <1 |
| Fructose 1,6-bisphosphate | ND | <1 |
| d-myo-Inositol 1-monophosphate | ND | <1 |
Substrates were used at concentrations of 1.0 mM and 0.5 mM for TON_1705 and TON_1706, respectively.
Activity is indicated by considering the relative activities of TON_1705 and TON_1706 toward pyrophosphate and PAP to be 100%, which are equivalent to specific activities of 0.16 μmol min−1 mg−1 and 483.6 μmol min−1 mg−1, respectively. ND, not determined.
FIG. 3.
Effects of pH (A) and divalent metal ions (B) on the pyrophosphatase activity of a TON_1705-encoded protein. (A) pH dependence reactions were run with the following buffers (each at 50 mM) chosen on the basis of pH measured at room temperature: MOPS (4-morpholinepropanesulfonic acid) (triangles) and Tris-HCl (upside-down triangles). (B) Various divalent metal ions were added at a concentration of 1 mM.
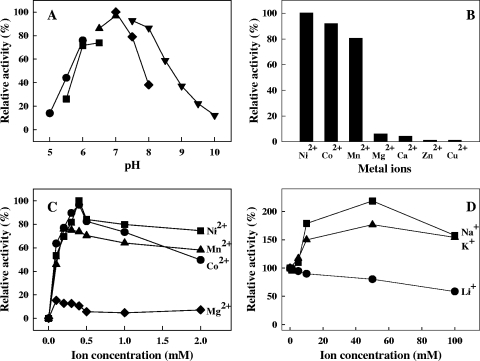
The purified TON_1706-encoded protein displayed phosphatase activity toward PAP as predicted, proving the functionality. The PAP phosphatase activity of the TON_1706-encoded protein was investigated as a function of pH and various metal ions (Fig. 4). The optimum pH was found to be 7.0, and the enzyme retained 50% of its activity between pHs 6 and 8.5 (Fig. 4A). The enzyme was most active in the presence of a Ni2+ ion and Co2+ or Mn2+ could replace Ni2+, affording 92% and 80% of the activity observed with Ni2+. Less than 10% activity was observed with the other metal ions (Fig. 4B). Maximal activity of the enzyme was observed with NiCl2 and CoCl2 at a concentration of 0.4 mM (Fig. 4C). The enzyme activity was very weakly inhibited by Li+ with 58% activity remaining in the presence of 100 mM Li+ while it was activated by 50 mM each of Na+ and K+ by 2.2-fold and 1.8-fold, respectively (Fig. 4D). The enzyme showed high activity toward PAP (100%) and PAPS (23%) but not toward PAP analogues, including 3′-phosphate (3′-AMP and 3′-CMP) or other tested phosphorylated compounds (Table 1), indicating that the enzyme is a very specific PAP phosphatase. The kinetic parameters of the enzyme, Km and kcat, toward PAP were determined to be 288 μM and 509 s−1, respectively. The kcat/Km value of 1.8 × 106 M−1 s−1 is comparable to that of other PAP phosphatases, E. coli CysQ (2.3 × 107 M−1 s−1), yeast HAL2 (2.8 × 107 M−1 s−1), murine bisphosphate nucleotidase (6.9 × 107 M−1 s−1), and M. tuberculosis Rv2131c (7 × 105 M−1 s−1) (12, 30).
FIG. 4.
Effects of pH (A), metal ions (B), divalent metal ion concentrations (C), and monovalent metal ion concentrations (D) on the PAP phosphatase activity of a TON_1706-encoded protein. (A) pH dependence reactions were run with the following buffers (each at 50 mM) chosen on the basis of pH measured at room temperature: sodium acetate (circles), MES (4-morpholineethanesulfonic acid) (squares), MOPS (4-morpholinepropanesulfonic acid) (triangles), HEPES (diamonds), and Tris-HCl (upside-down triangles). (B) Various metal ions were added at a concentration of 1 mM. (C) Divalent metal ion (Ni2+, Co2+, Mn2+, and Mg2+) dependence over a concentration range of 0 to 2 mM. (D) Monovalent metal ion (Li+, Na+, and K+) dependence over a concentration range of 0 to 100 mM in the presence of 0.4 mM NiCl2. Activities are expressed as percentages of the activity observed in the absence of monovalent cations.
In this study, two hypothetical genes, TON_1705 and TON_1706, were identified as functioning as a pyrophosphatase and a PAP phosphatase, respectively. Although various enzymatic functions such as alkaline phosphatase, phosphonoacetate hydrolase, phosphonate monoesterase, phosphoglycerate mutase, and sulfatases have been reported for the alkaline phosphatase superfamily, inorganic pyrophosphatase activity was for the first time demonstrated among that the superfamily in this study. Furthermore, this is the first time that the pyrophosphatases and the PAP phosphatases have been shown to be organized in the gene clusters of the sulfate activation system only in T. onnurineus NA1 and P. abyssi.
It is not clear whether PAPS, formed by the sulfate activation system, serves as a sulfate donor for the formation of sulfated metabolites or is enzymatically reduced to sulfite, which enters the cysteine biosynthetic pathway, in T. onnurineus NA1. Genes encoding a sulfotransferase and a PAPS reductase (EC 1.8.4.8) have not been detected in the T. onnurineus NA1 genome by bioinformatic analysis.
Nucleotide sequence accession numbers.
The TON_1705 and TON_1706 sequences have been deposited in GenBank under accession number CP000855.
Acknowledgments
This work was supported by a KORDI in-house program (PE98402) and the Marine and Extreme Genome Research Center program of the Ministry of Land, Transport, and Maritime Affairs, Republic of Korea, and by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund; KRF-2006-532-C00011).
Footnotes
Published ahead of print on 13 March 2009.
REFERENCES
- 1.Aravind, L., and E. V. Koonin. 1998. A novel family of predicted phosphoesterases includes Drosophila prune protein and bacterial RecJ exonuclease. Trends Biochem. Sci. 2317-19. [DOI] [PubMed] [Google Scholar]
- 2.Baykov, A. A., O. A. Evtushenko, and S. M. Avaeva. 1988. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 171266-270. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff, E., T.-A. Tran-Thi, and K. F. A. Decker. 1975. Nucleotide pyrophosphatase of rat liver. A. Comparative study on the enzymes solubilized and purified from plasma membrane and endoplasmic reticulum. Eur. J. Biochem. 51353-361. [DOI] [PubMed] [Google Scholar]
- 4.Brunold, C. 1993. Regulatory interactions between sulfate and nitrate assimilation, p. 61-75. In L. J. De Kok, I. Stulen, H. Rennenberg, C. Brunold, and W. E. Rauser (ed.), Sulfur nutrition and sulfur assimilation in higher plants. SPB Academic Publishing, The Hague, The Netherlands.
- 5.Cooperman, B. S., A. A. Baykov, and R. Lahti. 1992. Evolutionary conservation of the active site of soluble inorganic pyrophosphatase. Trends Biochem. Sci. 17262-266. [DOI] [PubMed] [Google Scholar]
- 6.Fauque, G., J. LeGall, and L. L. Barton. 1991. Sulfate-reducing and sulfur-reducing bacteria, p. 271-337. In J. M. Shively and L. L. Barton (ed.), Variations in autotrophic life. Academic Press, London, United Kingdom.
- 7.Fukui, S., H. Yoshida, and I. Yamashina. 1981. Sulfohydrolytic degradation of 3′-phosphoadenosine 5′-phosphosulfate (PAPS) and adenosine 5′-phosphosulfate (APS) by enzymes of a nucleotide pyrophosphatase nature. J. Biochem. 901537-1540. [DOI] [PubMed] [Google Scholar]
- 8.Galperin, M. Y., and M. J. Jedrzejas. 2001. Conserved core structure and active site residues in alkaline phosphatase superfamily enzymes. Proteins 45318-324. [DOI] [PubMed] [Google Scholar]
- 9.Galperin, M. Y., A. Bairoch, and E. V. Koonin. 1998. A superfamily of metalloenzymes unifies phosphopentomutase and cofactor-independent phosphoglycerate mutase with alkaline phosphatases and sulfatases. Protein Sci. 71829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gijsbers, R., H. Ceulemans, W. Stalmans, and M. Bollen. 2001. Structural and catalytic similarities between nucleotide pyrophosphatases/phosphodiesterases and alkaline phosphatases. J. Biol. Chem. 2761361-1368. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg, A. E., R. R. Trussel, and L. S. Clesceri. 1985. Standard methods for the examination of water and wastewater, 16th ed. American Public Health Association, American Water Works Association, Water Pollution Control Federation, Washington, DC.
- 12.Hatzios, S. K., A. T. Iavarone, and C. R. Bertozzi. 2008. Rv2131c from Mycobacterium tuberculosis is a CysQ 3′-phosphoadenosine-5′-phosphatase. Biochemistry 475823-5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakoby, W. B., and D. M. Ziegler. 1990. The enzymes of detoxication. J. Biol. Chem. 26520715-20718. [PubMed] [Google Scholar]
- 14.Jeon, S. J., and K. Ishikawa. 2005. Characterization of the Family I inorganic pyrophosphatase from Pyrococcus horikoshii OT3. Archaea 1385-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaassen, C. D., and J. W. Boles. 1997. Sulfation and sulfotransferases 5: the importance of 3′-phosphoadenosine 5′-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J. 11404-418. [DOI] [PubMed] [Google Scholar]
- 16.Lee, H. S., S. G. Kang, S. S. Bae, J. K. Lim, Y. Cho, Y. J. Kim, J. H. Jeon, S. S. Cha, K. K. Kwon, H. T. Kim, C. J. Park, H. W. Lee, S. I. Kim, J. Chun, R. R. Colwell, S.-J. Kim, and J.-H. Lee. 2008. The complete genome sequence of Thermococcus onnurineus NA1 reveals a mixed heterotrophic and carboxydotrophic metabolism. J. Bacteriol. 1907491-7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lik-Shing Tsang, M., and J. A. Schiff. 1976. Properties of enzyme fraction A from Chlorella and copurification of 3′(2′),5′-bisphosphonucleoside 3′(2′)-phosphohydrolase, adenosine 5′-phosphosulfate sulfohydrolase and adenosine-5′-phosphosulfate cyclase activities. Eur. J. Biochem. 65113-121. [DOI] [PubMed] [Google Scholar]
- 18.Mechold, U., G. Fang, S. Ngo, V. Ogryzko, and A. Danchin. 2007. YtqI from Bacillus subtilis has both oligoribonuclease and pAp-phosphatase activity. Nucleic Acids Res. 354552-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mechold, U., V. Ogryzko, S. Ngo, and A. Danchin. 2006. Oligoribonuclease is a common downstream target of lithium-induced pAp accumulation in Escherichia coli and human cells. Nucleic Acids Res. 342364-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murguía, J. R., J. M. Bellés, and R. Serrano. 1995. A salt-sensitive 3′(2′),5′-bisphosphate nucleotidase involved in sulfate activation. Science 267232-234. [DOI] [PubMed] [Google Scholar]
- 21.Parfenyev, A. N., A. Salminen, P. Halonen, A. Hachimori, A. A. Baykov, and R. Lahti. 2001. Quaternary structure and metal ion requirement of family II pyrophosphatases from Bacillus subtilis, Streptococcus gordonii, and Streptococcus mutans. J. Biol. Chem. 27624511-24518. [DOI] [PubMed] [Google Scholar]
- 22.Pasqualini, J. R., B. Schatz, C. Varin, and B. L. Nguyen. 1992. Recent data on estrogen sulfatases and sulfotransferases activities in human breast cancer. J. Steroid Biochem. Mol. Biol. 41323-329. [DOI] [PubMed] [Google Scholar]
- 23.Peng, Z., and D. P. Verma. 1995. A rice HAL2-like gene encodes a Ca2+-sensitive 3′(2′),5′-diphosphonucleoside 3′(2′)-phosphohydrolase and complements yeast met22 and Escherichia coli cysQ mutations. J. Biol. Chem. 27029105-29110. [DOI] [PubMed] [Google Scholar]
- 24.Proudfoot, M., E. Kuznetsova, G. Brown, N. N. Rao, M. Kitagawa, H. Mori, A. Savchenko, and A. F. Yakunin. 2004. General enzymatic screens identify three new nucleotidases in Escherichia coli. Biochemical characterization of SurE, YfbR, and YjjG. J. Biol. Chem. 27954687-54694. [DOI] [PubMed] [Google Scholar]
- 25.Quintero, F. J., B. Garciadeblás, and A. Rodríguez-Navarro. 1996. The SAL1 gene of Arabidopsis, encoding an enzyme with 3′(2′),5′-bisphosphate nucleotidase and inositol polyphosphate 1-phosphatase activities, increases salt tolerance in yeast. Plant Cell 8529-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramaswamy, S. G., and W. B. Jakoby. 1987. (2′)3′,5′-Bisphosphate nucleotidase. J. Biol. Chem. 26210044-10047. [PubMed] [Google Scholar]
- 27.Roche, P., F. Debellé, F. Maillet, P. Lerouge, C. Faucher, G. Truchet, J. Dénarié, and J. C. Promé. 1991. Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell 201131-1143. [DOI] [PubMed] [Google Scholar]
- 28.Roth, J. A., A. J. Rivett, and K. J. Renskers. 1982. Properties of human brain phenolsulfotransferase and its role in the inactivation of catecholamine neurotransmitters, p. 107-114. In G. J. Mulder, J. Caldwell, G. M. J. Van Kempen, and R. J. Vonk (ed.), Sulfate metabolism and sulfate conjugation. Taylor and Francis, London, United Kingdom.
- 29.Shintani, T., T. Uchiumi, T. Yonezawa, A. Salminen, A. A. Baykov, R. Lahti, and A. Hachimori. 1998. Cloning and expression of a unique inorganic pyrophosphatase from Bacillus subtilis: evidence for a new family of enzymes. FEBS Lett. 439263-266. [DOI] [PubMed] [Google Scholar]
- 30.Spiegelberg, B. D., J. P. Xiong, J. J. Smith, R. F. Gu, and J. D. York. 1999. Cloning and characterization of a mammalian lithium-sensitive bisphosphate 3′-nucleotidase inhibited by inositol 1,4-bisphosphate. J. Biol. Chem. 27413619-13628. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida, H., S. Fukui, I. Funakoshi, and I. Yamashina. 1983. Substrate specificity of a nucleotide pyrophosphatase responsible for the breakdown of 3′-phosphoadenosine 5′-phosphosulfate (PAPS) from human placenta. J. Biochem. 931641-1648. [DOI] [PubMed] [Google Scholar]
- 32.Young, T. W., N. J. Kuhn, A. Wadeson, S. Ward, D. Burges, and G. D. Cooke. 1998. Bacillus subtilis ORF yybQ encodes a manganese-dependent inorganic pyrophosphatase with distinctive properties: the first of a new class of soluble pyrophosphatase. Microbiology 1442563-2571. [DOI] [PubMed] [Google Scholar]