Abstract
PhcA positively and negatively regulates many genes responsible for pathogenicity of Ralstonia solanacearum. The type III secretion system-encoding hrp regulon is one of the negatively controlled operons. PhcA bound to the promoter region of prhIR and repressed its expression, demonstrating that PhcA shuts down the most upstream component of a signal transfer system for hrpB activation.
Ralstonia solanacearum, a soilborne phytopathogen, causes wilt disease in a wide range of plant hosts throughout the tropical and subtropical world (14, 25). After invasion through root openings, R. solanacearum colonizes, multiplies in intercellular spaces, and then invades xylem vessels. The bacteria produce extracellular polysaccharide (EPS) in xylem vessels, and this is believed to be responsible for wilt symptom because large amounts of EPS slime reduce sap flow in xylem vessels (8).
Many gram-negative pathogenic bacteria that infect plants and animals use a type III secretion system (TTSS) to interact with their respective hosts (11, 17). Virulence proteins, so-called type III effectors, are injected from pathogens into the host cytosol via the TTSS needle structure (18). Proteins that compose TTSS are encoded by hrp (hypersensitive reaction and pathogenicity) genes, which are clustered and form the hrp regulon (29). TTSS is a key pathogenicity determinant, as R. solanacearum with TTSS mutations cannot cause disease (2).
Growth in a minimal medium or cocultivation with plant cells induces the expression of the R. solanacearum hrp regulon via an AraC-type transcriptional regulator, HrpB (12). Plant signals, which are presumed to reside on the surface of plant cells, are perceived by PrhA, which is located on the outer membrane of R. solanacearum (20). PrhA transduces signals to the periplasmic domain of the inner membrane protein PrhR, which is an anti-sigma factor. Once PrhR receives the signals, an extracytoplasmic function sigma factor, PrhI, is released from PrhR to the cytoplasm and directs RNA polymerase to transcribe prhJ (3). Subsequently, PrhJ, which is a member of the LuxR/UhpA family of transcriptional activators, activates transcription of hrpG, and then HrpG induces expression of hrpB (4). Moreover, it has been suggested that HrpG activity is increased by metabolic signals perceived in the minimal medium through an as-yet-unknown mechanism (28).
PhcA is a LysR-type transcriptional regulator of EPS synthesis and other virulence factors (6), and its activity is controlled by a quorum-sensing system with a unique autoinducer, 3-hydroxy palmitic acid methylester (10). At high cell densities, activated PhcA induces transcription of xpsR, which encodes a positive regulator of EPS production (16). PhcA regulates other virulence-associated activities in addition to EPS production. Synthesis of β-1,4-endoglucanase and pectin methylesterase are positively regulated at high cell densities (27), while synthesis of endopolygalacturonase, a siderophore, and motility by flagella or type IV pili are negatively regulated (1, 5, 9, 26). The expression of the hrp regulon is also negatively regulated by PhcA at high cell densities (13). Therefore, R. solanacearum has two cell density-dependent phenotypes which are regulated by PhcA, and virulence gene expression controlled by PhcA plays a critical role in successful host plant infection (22). The focus of this work was to analyze regulation mechanism of PhcA for the hrp regulon in detail.
PhcA binds to the promoter region of the prhIR operon in the hrp gene regulatory cascade.
The PhcA regulator has been genetically demonstrated to negatively regulate the hrp regulon (13). HrpB, the AraC family transcriptional regulator, positively regulates the entire hrp regulon (7). Plant signals perceived during the pathogen-host plant interaction are speculated to activate hrpB expression through a six-gene regulatory cascade (Fig. 1) (3). We used promoter binding assays to determine the step in the hrpB cascade at which PhcA exerts its negative regulation of the TTSS regulon.
FIG. 1.
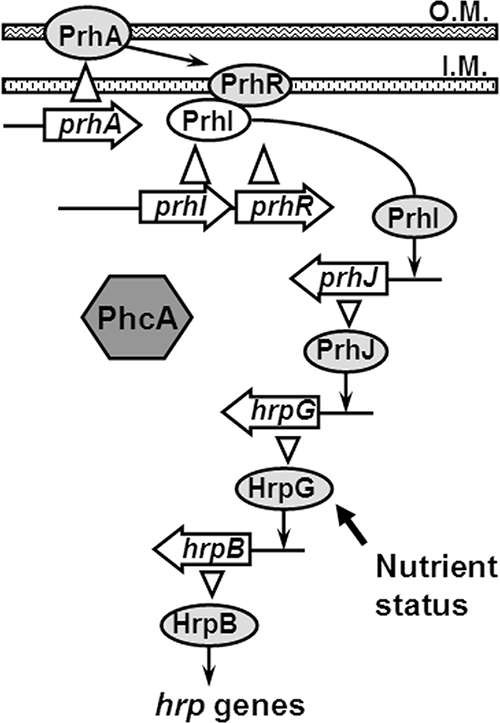
Model of hrp gene regulatory cascade in R. solanacearum induced in response to bacterium-plant cell contact. PhcA is a negative regulator of hrp regulon. Lines with arrows represent positive regulation of gene expression. Open arrowheads represent protein translation. A solid arrow indicates cell nutrient status related to growth conditions. O.M., outer membrane; I.M., inner membrane. (Modified from reference 4 with permission of the publisher.)
An ∼1-kb DNA fragment containing phcA was amplified from chromosomal DNA of R. solanacearum OE1-1 (race 1, biovar 3 [19]) grown in BG medium at 28°C (2) by using PCR with a pair of primers, phcAA5 and phcAB4 (see Table S1 in the supplemental material). The amplified fragment was cloned into pET22b(+) (Novagen, Madison, WI) to construct pphcA5. Escherichia coli BL21(DE3) cells transformed with pphcA5 were incubated at 37°C in 500 ml of fresh LB medium supplemented with ampicillin. When the cell optical density at 600 nm reached 0.6, IPTG (isopropyl-β-d-thiogalactopyranoside; final concentration, 1 mM) was added, and incubation was continued for two more hours at 28°C. PhcA was purified from these cells using the His tag on its C terminus with Ni-nitrilotriacetic acid agarose (Qiagen, Valencia, CA) and HiTrap Q FF (5 ml; GE Healthcare Bio-Sciences, Piscataway, NJ) columns by using ÄKTA prime (GE Healthcare Bio-Sciences). At the final step, PhcA was eluted with a linear gradient of 0.5 M Na2SO4 in 10 mM Tris-HCl (pH 7.5), concentrated, and dialyzed against a dialysis buffer (10 mM Tris-HCl, pH 7.5, 50 mM KCl, 1 mM EDTA, 10% glycerol). Three proteins with molecular mass of ca. 40 kDa were observed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2). Purified proteins were transferred to a polyvinylidene difluoride membrane. Each of three protein bands was cut out from the membrane and subjected to a Procise 492 protein sequencer (Applied Biosystems, Foster City, CA). The N-terminal amino acid sequences of all three proteins were MVNVDTKLLV, which were identical to the sequence for PhcA. Since PhcA is activated in proportion to cell density in the quorum-sensing system, these proteins might reflect the modified forms of PhcA, although it was heterologously expressed and purified from E. coli.
FIG. 2.
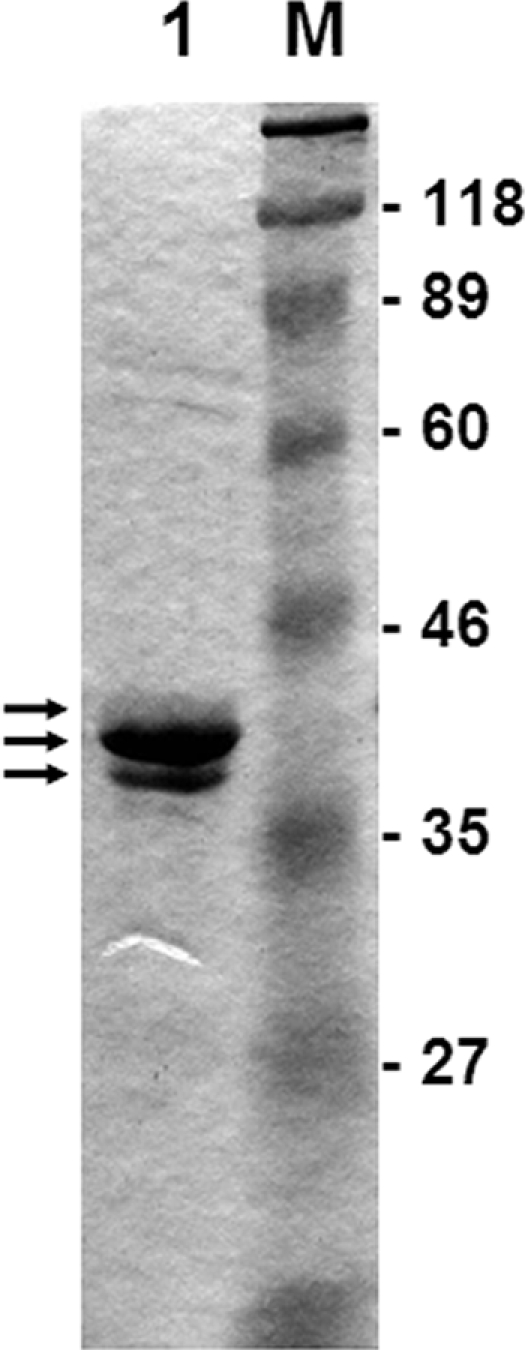
Purification of PhcA. Overexpressed His-tagged proteins were separated by 12.5% SDS-PAGE. Arrows indicate PhcA identified by N-terminal amino acid sequence analysis. Lane 1, purified PhcA; lane M, molecular mass marker. Molecular masses are on the right in kDa.
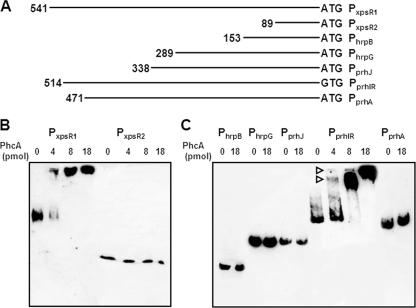
PhcA is demonstrated to bind to the xpsR promoter (16). Two biotinylated xpsR promoter fragments were used. Biotin-labeled DNA fragments were prepared with a 5′-biotinylated oligonucleotide primer, xpsRB3B (see Table S1 in the supplemental material). PCR amplification was performed at 95°C for 4 min followed by 35 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 2 min. Purified PhcA (4 to 18 pmol) was mixed with biotinylated DNA fragments, and binding to DNA was analyzed using the LightShift chemiluminescent electrophoretic mobility shift assay kit (Pierce, Rockford, IL) according to the manufacturer's instructions. The larger xpsR fragment contained the promoter region to which PhcA can bind, and a smaller fragment lacked this region (Fig. 3A) (30). As expected, the purified PhcA bound only to the larger fragment in a concentration-dependent manner (Fig. 3B), indicating that the His-tagged PhcA indeed showed the expected promoter-binding activity. Biotinylated DNA fragments containing promoter regions of prhA, prhIR, prhJ, hrpG, and hrpB were prepared with primer pairs prhAB2B/prhAA5, prhIB2B/prhIA1, prhJB2B/prhRA1, hrpGB2B/hpaBB1, and hrpBB4B/hrpCA1, respectively (Fig. 3A; see Table S1 in the supplemental material). Even when an excess amount of PhcA was used, no PhcA binding to promoter regions of hrpB, hrpG, prhJ, and prhA was observed. PhcA bound only to the promoter region of prhIR in a concentration-dependent manner (Fig. 3C). This result clearly demonstrated that PhcA targets the prhIR operon in the hrp regulatory cascade. Since the phcA mutation had no effect on expression of prhA (13) and since prhA is constitutively expressed (unpublished data), this conclusion makes sense. PrhIR are the first proteins receiving the host cell signal from PrhA. Once prhIR expression is repressed, expression of all the downstream genes, including hrpB, could be shut down (Fig. 1), resulting in the absence of hrp regulon expression.
FIG. 3.
Gel mobility shift assay for PhcA. (A) Biotinylated promoter DNA fragments are as follows: PxpsR1, xpsR containing the PhcA binding region; PxpsR2, xpsR lacking this region; PhrpB, hrpB; PhrpG, hrpG; PprhJ, prhJ; PprhIR, prhIR; PprhA, prhA. The length of the fragment (bp) is shown at the left end of each line. Next to the 3′ end is a start codon marked ATG or GTG. (B) Biotinylated xpsR promoter fragments were mixed with purified PhcA with C-terminal His tag (0 to 18 pmol), separated on a polyacrylamide gel, and transferred onto a positively charged nylon membrane. The membrane was treated with streptavidin-horseradish peroxidase conjugate, and labeled DNA fragments were visualized with Chemi-Lumi One (Nakarai Tesque, Kyoto, Japan). One microgram of poly(dI·dC) was added as nonspecific competitor DNA. (C) Biotinylated promoter fragments of genes belonging to the hrp gene regulatory cascade were incubated with His-tagged PhcA. Promoters PhrpB, PhrpG, PprhJ, PprhIR, and PprhA are shown on the top. Open triangles indicate the DNA fragments forming complexes with PhcA.
Two shifted bands were observed depending on the amount of PhcA, indicating that two possible PhcA binding sites could exist in the promoter region of prhIR. The xpsR promoter region also contains two PhcA binding sites, one main site and another very weak site (16, 30). Judging from the band density, the two binding sites in the prhIR promoter appeared to have identical affinities to PhcA. PhcA regulates several target promoters, including the xpsR and prhIR promoters (9), and its regulatory actions on each might be different.
Determination of the PhcA binding site in the prhIR promoter region.
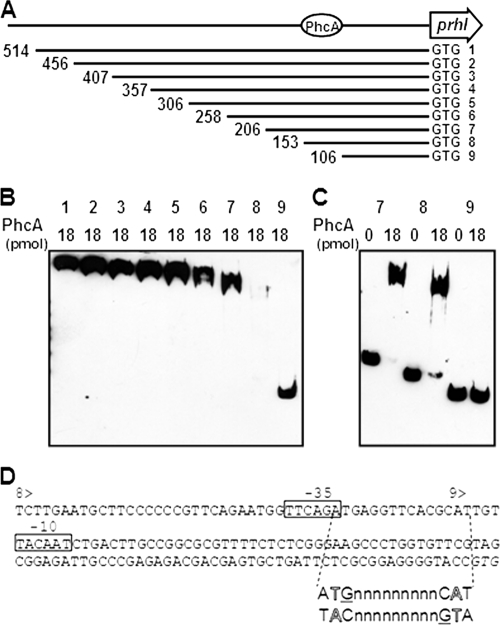
Although we have not determined the precise transcriptional start site, we demonstrated that PhcA bound to a 514-bp fragment upstream from the start codon of prhIR. In order to narrow down the PhcA binding site in the prhIR promoter region, a series of fragments that had 50-bp deletions and were biotinylated on the 3′ end were constructed with primer pairs prhIB2B/prhIA3, prhIB2B/prhIA4, prhIB2B/prhIA5, prhIB2B/prhIA6, prhIB2B/prhIA7, prhIB2B/prhIA8, prhIB2B/prhIA9, and prhIB2B/prhIA10 (Fig. 4A; see Table S1 in the supplemental material), and PhcA binding was analyzed by electrophoretic mobility shift assay. While PhcA bound to prhIR promoter fragments ranging from 514 bp (PprhIR) to 153 bp (PprhIR8), no binding to the DNA fragment 106 bp in size (PprhIR9) was observed (Fig. 4B), indicating that the PhcA binding site in the prhIR promoter region is located at least between nucleotides −153 and −106 upstream from the start codon (Fig. 4A). We found that more free 153-bp fragment (PprhIR8) remained than free 206-bp fragment (PprhIR7) when these fragments were mixed with PhcA (Fig. 4C). This may reflect, as mentioned before, a possible second PhcA binding site or that the PhcA binding site extends beyond the 153-bp fragment.
FIG. 4.
Gel mobility shift assay for PhcA binding to the prhIR promoter region. (A) Schematic drawing of prhIR promoter deletion. 1, PprhIR; 2, PprhIR2; 3, PprhIR3; 4, PprhIR4; 5, PprhIR5; 6, PprhIR6; 7, PprhIR7; 8, PprhIR8; 9, PprhIR9. The length of fragment (bp) is shown at the left end of each line. Next to the 3′ end is a start codon marked GTG. The putative PhcA binding site is indicated by a circle. (B) Biotinylated prhIR fragments with several deletions were mixed with 18 pmol of purified His-tagged PhcA and separated by SDS-PAGE. DNA bands were visualized as described for Fig. 3. Numbers above the lanes indicate the promoter fragments represented in panel A. All DNA fragments were incubated with 18 pmol of PhcA. (C) Biotinylated prhIR fragments PprhIR7, PprhIR8, and PprhIR9 were mixed with either 0 or 18 pmol of purified His-tagged PhcA. Numbers above the lanes indicate the promoter fragments represented in panel A. (D) Nucleotide sequence of the prhIR promoter region. 8> and 9> indicate the beginnings of PprhIR8 and PprhIR9, respectively. Putative −35 and −10 sequences of σ70 are boxed. A hypothetical recognition site sequence is shown below to illustrate the interrupted dyadic sequence and T-N11-A motif (outlined letters); guanines expected to be directly involved in binding are underlined. The initiation codon is shown in italics.
Typical LysR-type transcriptional regulators usually repress transcription from their own promoter and activate transcription from a divergent primer. They generally need coinducers, which include extracellular signaling molecules, metabolites, and ions, and are highly specific for a particular LysR-type regulator (24). On the other hand, PhcA does not autoregulate its own promoter and may not require a coinducer. PhcA instead requires an additional activator, the two-component regulator VsrD in the case of positive regulation of xpsR (16). A consensus sequence for the binding site of LysR-type regulators, a T-N11-A motif, is located in the promoter region of xpsR (15). PhcA is unique among LysR-type regulator proteins because it controls multiple and unlinked genes positively and negatively (25). In this study, we demonstrated for the first time PhcA binding to the promoter of negatively regulated genes. Although no significant overall similarity between the promoter sequences of positively regulated xpsR and negatively regulated prhIR was found, the hypothetical recognition site sequence (the interrupted dyadic sequence and T-N11-A motif) was found in the prhIR promoter (Fig. 4D). Symmetrical guanines in the two dyad arms that are expected to be directly involved in binding (24) were also identified. Putative −35 and −10 sequences of σ70 were found in the promoter region of prhIR. However, unlike the positively regulated xpsR, the prhIR T-N11-A motif overlapped with the −35 sequence (Fig. 4D), suggesting that PhcA binding may prevent formation of active complexes between RNA polymerase and promoters.
The PhcA binding region of prhIR promoter is required for inhibition of gene expression.
Since PhcA bound to the promoter region of prhIR operon in vitro, we investigated whether binding of PhcA to the promoter region would repress prhIR gene expression in vivo. First, a phcA deletion mutant, RK5035, was constructed as follows. A ∼1.7-kb DNA fragment containing the phcA open reading frame was amplified from OE1-1 chromosomal DNA by using a pair of primers, phcAA4 and phcAB5 (see Table S1 in the supplemental material). The resulting amplified fragment was cloned into a pK18mobsacB vector (23) to construct pphcA21. Since two PstI sites were located in the phcA gene, pphcA21 was digested and religated to delete the internal PstI fragment, creating plasmid pphcA22, which was used to construct a phcA mutant by using allelic replacement double crossover. DNA fragments containing different amounts of the prhIR promoter region, PprhIR, PprhIR8, and PprhIR9 (Fig. 4A), were cloned into a broad-host-range lacZ transcriptional fusion vector, pHRP309 (21), to construct plasmids pprhIR1, pprhIR8, and pprhIR9, respectively. These plasmids, along with the empty vector pHRP309, were put into R. solanacearum strains OE1-1 and RK5035 (phcA), and promoter activities were measured (Table 1) . Background β-galactosidase activities with and without phcA were similar (pHRP309; Table 1). When cells carried pprhIR1 or pprhIR8, prhIR promoter activity was derepressed only in the phcA mutant and increased two- to threefold. In contrast, the promoter activity of pprhIR9-carrying cells was derepressed even in the wild-type background, and no increase in expression was observed for RK5035 (Table 1). This is in good agreement with our in vitro result showing that PhcA bound to promoter fragments in pprhIR1 and pprhIR8 but did not bind to the fragment in prhIR9 (Fig. 4B). Taken together, these two results suggest that PhcA binds to the promoter region of prhIR operon and represses prhIR expression in R. solanacearum.
TABLE 1.
prhIR promoter activity in the wild type and the phcA mutant
| Plasmid | Promoter size (bp)a | β-Galactosidase activity (Miller units)b
|
|||
|---|---|---|---|---|---|
| OE1-1
|
RK5035c
|
||||
| Mean | Standard error | Mean | Standard error | ||
| pprhIR1 | 514 | 3,047 | 689 | 8,061 | 1,316 |
| pprhIR8 | 153 | 3,272 | 854 | 5,726 | 1,614 |
| pprhIR9 | 106 | 9,140 | 3,561 | 7,638 | 1,919 |
| pHRP309 | None | 1,429 | 310 | 1,482 | 361 |
Size refers to the region upstream from the start codon. The promoter region was fused to lacZ.
Cells were grown in BG medium up to a cell optical density at 600 nm of 1, and the enzyme activity was measured with SDS-chloroform-treated cells. Mean values and standard errors are in Miller units.
RK5035 is a phcA deletion mutant of OE1-1.
Overexpression of phcA inhibits TTSS function (9) and reduces hrp regulon expression (unpublished data). hrp regulon expression and TTSS formation is necessary for the early stage of R. solanacearum infection (9), at which point PhcA is inactive because of low pathogen cell density in the intercellular spaces. With the aid of effector proteins secreted by the TTSS, R. solanacearum can proliferate and increase cell numbers. At that time, TTSS is no longer required, and in turn, EPS is synthesized. Active PhcA, depending on the quorum-sensing system (10), upregulates EPS production and concomitantly represses the hrp regulon. Expression of prhA, whose product is speculated to sense signals, is likely to be constitutive (unpublished data). As we have demonstrated in this study, the most efficient way to stop the signal cascade and repress the hrp regulon is to reduce the early components PrhIR. This is consistent with the observation that strains with mutations in prhIR have no detectable expression of hrpB and severely reduced virulence on host plants (3).
Nucleotide sequence accession numbers.
The sequences of strain OE1-1 phcA and prhIR, including the promoter regions, have been deposited in DDBJ under accession numbers AB450919 and AB450920, respectively.
Supplementary Material
Acknowledgments
This work was supported in part by KAKENHI (Grant-in-Aid for Scientific Research) from Japan Society for the Promotion of Science (16658020 to Y.H. and 17380031 to K.O.).
Footnotes
Published ahead of print on 5 December 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Bhatt, G., and T. P. Denny. 2004. Ralstonia solanacearum iron scavenging by the siderophore staphyloferrin B is controlled by PhcA, the global virulence regulator. J. Bacteriol. 237896-7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher, C., P. Barberis, A. Trigalet, and D. Démery. 1985. Transposon mutagenesis of Pseudomonas solanacearum: isolation of Tn5-induced avirulent mutants. J. Gen. Microbiol. 1312449-2457. [Google Scholar]
- 3.Brito, B., D. Aldon, P. Barberis, C. Boucher, and S. Genin. 2002. A signal transfer system through three compartments transduces the plant cell contact-dependent signal controlling Ralstonia solanacearum hrp genes. Mol. Plant-Microbe Interact. 15109-119. [DOI] [PubMed] [Google Scholar]
- 4.Brito, B., M. Marenda, P. Barberis, C. Boucher, and S. Genin. 1999. prhJ and hrpG, two new components of the plant signal-dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum. Mol. Microbiol. 31237-251. [DOI] [PubMed] [Google Scholar]
- 5.Brown, D. G., and C. Allen. 2004. Ralstonia solanacearum genes induced during growth in tomato: an inside view of bacterial wilt. Mol. Microbiol. 531641-1660. [DOI] [PubMed] [Google Scholar]
- 6.Brumbley, S. M., B. F. Carney, and T. P. Denny. 1993. Phenotype conversion in Pseudomonas solanacearum due to spontaneous inactivation of PhcA, a putative LysR transcriptional activator. J. Bacteriol. 1755477-5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunnac, S., C. Boucher, and S. Genin. 2004. Characterization of the cis-acting regulatory element controlling HrpB-mediated activation of the type III secretion system and effector genes in Ralstonia solanacearum. J. Bacteriol. 1862309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denny, T. P. 1995. Involvement of bacterial polysaccharides in plant pathogenesis. Annu. Rev. Phytopathol. 33173-197. [DOI] [PubMed] [Google Scholar]
- 9.Denny, T. P. 2006. Plant pathogenic Ralstonia species, p. 573-644. In S. S. Gnanamanickam (ed.), Plant-associated bacteria. Springer, Dordrecht, The Netherlands.
- 10.Flavier, A. B., S. J. Clough, M. A. Schell, and T. P. Denny. 1997. Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol. Microbiol. 26251-259. [DOI] [PubMed] [Google Scholar]
- 11.Galán, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 2841322-1328. [DOI] [PubMed] [Google Scholar]
- 12.Genin, S., C. L. Gough, C. Zischek, and C. A. Boucher. 1992. Evidence that the hrpB gene encodes a positive regulator of pathogenicity genes from Pseudomonas solanacearum. Mol. Microbiol. 63065-3076. [DOI] [PubMed] [Google Scholar]
- 13.Genin, S., B. Brito, T. P. Denny, and C. Boucher. 2005. Control of the Ralstonia solanacearum type III secretion system (Hrp) genes by the global virulence regulator PhcA. FEBS Lett. 5792077-2081. [DOI] [PubMed] [Google Scholar]
- 14.Hayward, H. C. 1991. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 2965-87. [DOI] [PubMed] [Google Scholar]
- 15.Huang, J., B. F. Carney, T. P. Denny, A. K. Weissinger, and M. A. Schell. 1995. A complex network regulates expression of eps and other virulence genes of Pseudomonas solanacearum. J. Bacteriol. 1771259-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, J., W. Yindeeyoungyeon, R. P. Garg, T. P. Denny, and M. A. Schell. 1998. Joint transcriptional control of xpsR, the unusual signal integrator of the Ralstonia solanacearum virulence gene regulatory network, by a response regulator and a LysR-type transcriptional activator. J. Bacteriol. 1802736-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, Q., and S. Y. He. 2001. Role of the Hrp pilus in type III protein secretion in Pseudomonas syringae. Science 2942556-2558. [DOI] [PubMed] [Google Scholar]
- 19.Kanda, A., M. Yasukohchi, K. Ohnishi, A. Kiba, T. Okuno, and Y. Hikichi. 2003. Ectopic expression of Ralstonia solanacearum effector protein PopA early in invasion results in loss of virulence. Mol. Plant-Microbe Interact. 16447-455. [DOI] [PubMed] [Google Scholar]
- 20.Marenda, M., B. Brito, D. Callard, S. Genin, P. Barberis, C. A. Boucher, and M. Arlat. 1998. PrhA controls a novel regulatory pathway required for the specific induction of Ralstonia solanacearum hrp genes in the presence of plant cells. Mol. Microbiol. 27437-453. [DOI] [PubMed] [Google Scholar]
- 21.Parales, R. E., and C. S. Harwood. 1993. Construction and use of a new broad-host-range lacZ transcriptional fusion vector, pHRP309, for gram− bacteria. Gene 13323-30. [DOI] [PubMed] [Google Scholar]
- 22.Poussier, S., P. Thoquet, D. Trigalet-Demery, S. Barthet, D. Meyer, M. Arlat, and A. Trigalet. 2003. Host plant-dependent phenotypic reversion of Ralstonia solanacearum from non-pathogenic to pathogenic forms via alterations in the phcA gene. Mol. Microbiol. 49991-1003. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 14569-73. [DOI] [PubMed] [Google Scholar]
- 24.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47597-626. [DOI] [PubMed] [Google Scholar]
- 25.Schell, M. A. 2000. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu. Rev. Phytopathol. 38263-292. [DOI] [PubMed] [Google Scholar]
- 26.Tans-Kersten, J., D. Brown, and C. Allen. 2004. Swimming motility, a virulence trait of Ralstonia solanacearum, is regulated by FlhDC and the plant host environment. Mol. Plant-Microbe Interact. 17686-695. [DOI] [PubMed] [Google Scholar]
- 27.Tans-Kersten, J., Y. Guan, and C. Allen. 1998. Ralstonia solanacearum pectin methylesterase is required for growth on methylated pectin but not for bacterial wilt virulence. Appl. Environ. Microbiol. 644918-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valls, M., S. Genin, and C. Boucher. 2006. Integrated regulation of the type III secretion system and other virulence determinants in Ralstonia solanacearum. PLoS Pathog. 2798-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Gijsegem, F., C. Gough, C. Zischek, E. Niqueux, M. F. Arlat, and C. A. Boucher. 1995. The hrp locus of Pseudomonas solanacearum, which controls the production of a type III secretion system, encodes eight proteins related to components of the bacterial flagellar biogenesis complex. Mol. Microbiol. 151095-1114. [DOI] [PubMed] [Google Scholar]
- 30.Yindeeyoungyeon, W., and M. A. Schell. 2000. Footprinting with an automated capillary DNA sequencer. BioTechniques 291034-1041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.