Abstract
Lactococcus lactis, a gram-positive bacterium widely used by the dairy industry to manufacture cheeses, is subject to infection by a diverse population of virulent phages. We have previously determined the structures of three receptor binding proteins (RBPs) from lactococcal phages TP901-1, p2, and bIL170, each of them having a distinct host range. Virulent phages p2 and bIL170 are classified within the 936 group, while the temperate phage TP901-1 is a member of the genetically distinct P335 polythetic group. These RBPs comprise three domains: the N-terminal domain, binding to the virion particle; a β-helical linker domain; and the C-terminal domain, bearing the receptor binding site used for host recognition. Here, we have designed, expressed, and determined the structure of an RBP chimera in which the N-terminal and linker RBP domains of phage TP901-1 (P335) are fused to the C-terminal RBP domain of phage p2 (936). This chimera exhibits a stable structure that closely resembles the parental structures, while a slight displacement of the linker made RBP domain adaptation efficient. The receptor binding site is structurally indistinguishable from that of native p2 RBP and binds glycerol with excellent affinity.
A broad number of products are manufactured by large-scale bacterial fermentation, including the value-added fermented dairy products. Most bacterial fermentation industries have experienced problems with phage contamination. Phage outbreaks are costly and time-consuming because they can slow or arrest the fermentation process and adversely affect product quality (15). For decades, the dairy industry has relied on an array of strategies to control this natural phenomenon, including rotation of their bacterial cultures (11, 24, 25). However, in spite of these efforts, new virulent lactococcal phages keep emerging. A better understanding of the various mechanisms affecting the genetic diversity of the phage population is necessary for optimal phage control strategies (18).
Lactococcal phages are among the most studied bacterial viruses because of the economic importance of their hosts. Hundreds of lactococcal phages have been isolated, and the vast majority of them have a long, contractile tail, thereby belonging to the Siphoviridae family (1). Lactococcus lactis phages are currently classified into 10 genetically distinct groups (10), but only members of 3 of them are highly adapted to multiply in milk, namely, the 936, c2, and P335 groups (11, 24, 25). The first step for such an effective viral infection is host recognition, which necessitates the interaction between the adsorption device located at the distal tail end of the phage and the cell surface receptor (32). Members of the 936 and P335 groups recognize their host through an interaction between their receptor binding protein (RBP) (13) and receptors, probably lipoteichoic acids, at the host cell surface (27, 29-31).
We have previously determined the crystal structures of three RBPs, from the virulent lactococcal phages p2 (30, 31) and bIL170 (936 group) (27) and from the temperate phage TP901-1 (P335 group) (29). The RBPs of these phages have a similar architecture of three protomers related by a threefold axis. Each protomer comprises three domains: the N terminus (named shoulders in p2), the interlaced β-prism linker (the “neck” domain), and the jelly-roll domain (2) at the C terminus (the “head” domain). This last domain harbors a saccharide binding site likely involved in host recognition, as it binds with high affinity to phosphoglycerol, a component of teichoic acid (8, 19, 27, 29-31). We have previously shown that the shoulder and neck domains are highly conserved in the RBPs of 936-like phages (8, 19, 27, 29-31). The individuality of the RBP C-terminal domain sequence likely dictates phage specificity for the receptor, which may specifically recognize different substitutions (H, GlcNAc, or d-Ala) of the phosphoglycerol moieties of the L. lactis teichoic acid polymers. Recently, the complete genomic sequence of the reference virulent phage P335 was determined, and comparative analysis revealed that the C terminus of its RBP showed homology to the RBP of the virulent lactococcal phage P475 of the 936 group (17). Such homology between RBP head domains was surprising because the two lactococcal phage groups rarely shared common genes or domains. This observation suggested that modular shuffling of domains can occur between these otherwise genetically distinct phage groups.
The overall fold of the N-terminal RBP domain is different in 936- and P335-like phages. In the P335 group, the N-terminal domain comprises a unique helix that fits into the rest of the phage baseplate (28, 29) (Fig. 1A), while in the 936 group, this 140-residue domain is a large β-sandwich with an external α-helix (30) (Fig. 1B). Nonetheless, the N-terminal domains of the two RBPs may still be, related because both appear to be built using a coiled coil, although the 936-like phages have an additional β-sandwich. The β-prism linkers (neck domain) of the two phage groups also differ in sequence and in radius, but they have a similar fold, the latter being also close to that of T4 phage short fiber (33). The linker domain of phage TP901-1 is wider than that of p2 and exhibits a repeated motif (G-X-Y-X-Y, where X is polar and Y nonpolar). Finally, the C-terminal domains of both species share the same fold, a jelly-roll motif (2) also found in adenovirus (5) and reovirus (3, 4, 6).
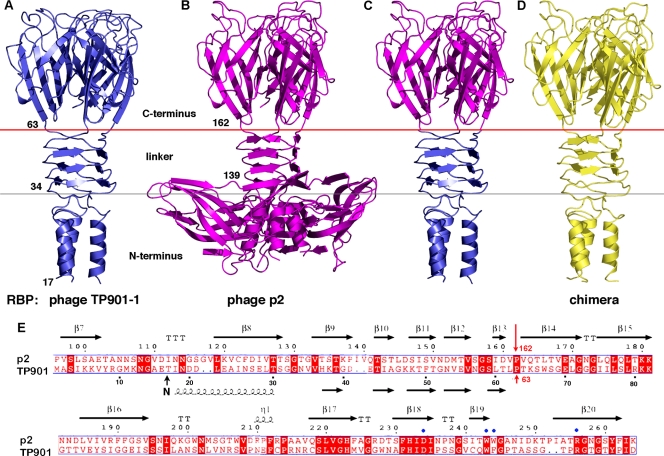
FIG. 1.
Structures and sequences of RBPs from lactococcal phages. (A) Three-dimensional structure of the RBP from phage TP901-1 (P335 group; blue). (B) Three-dimensional structure of the RBP from phage p2 (936 group; magenta). (C) View of a model associating domains of TP901-1 (N terminus and linker domain, below red line, blue) and p2 (head, above red line, magenta) RBPs. (D) Three-dimensional crystal structure of chimera form 1 (yellow) assembled according to the model in panel C. (E) Sequence alignment of the RBPs of p2 (part) and TP901-1. The secondary structure is described above the alignment. The binding residues are shown with blue dots. The hinge proline (Pro 162/63) is identified by a red arrow. The chimera is composed of the N-terminal domain (residues 17 to 33) and the linker domain residues (residues 34 to 63) from phage TP901-1 RBP and the C-terminal domain (residues 163 to 264) from phage p2 RBP.
The question addressed here was whether exchange between the C-terminal domains of two phage groups would lead to a stable protein with conserved binding capacity. To answer this question, we have generated an RBP chimera comprising the N-terminal and linker domains of phage TP901-1 fused to the C-terminal domain of phage p2. We have produced this chimera and determined its crystal structure and its sugar binding capacity. These results indicate that straightforward domain exchange produced a stable chimera with a conserved binding capacity and a structure close to that of each of the parental parts.
MATERIALS AND METHODS
Lactococcal phages and host strains.
Phages p2 and TP901-1 and their L. lactis host strains were obtained from the Felix d'Hérelle Reference Center for Bacterial Viruses (www.phage.ulaval.ca). L. lactis strains were grown in M17 (Oxoid) supplemented with 0.5% glucose (GM17) without shaking at 30°C. When propagating phages, 10 mM CaCl2 was added to the medium. Phage DNA was isolated as previously reported (14).
Cloning and bacterial expression.
A gene coding for a chimeric RBP protein containing the N-terminal/shoulder and linker/neck domains of the lactococcal temperate phage TP901-1 (amino acids 1 to 63) and the C-terminal/head domain of the virulent lactococcal phage p2 RBP (amino acids 162 to 264) was constructed and cloned into the Gateway destination vector pDest17 (Invitrogen). A common proline residue situated at the beginning of the C-terminal domain (proline 63 in TP901-1 and proline 162 in p2) was used to define the boundary between the linker domain and C-terminal domain for constructing the chimera. PCR amplification of DNA sequences was performed using previously described plasmids (29, 30) containing the full-length RBP of phage p2 or TP901-1. Amplification of the individual domains was performed in two steps, with each domain first being amplified, followed by a joining step of amplification. The resulting vector was transformed into the Escherichia coli C41(DE3)/pLysS (Lucigen) strain for expression. Transformed cells were grown in 2× YT medium at 37°C until an optical density at 600 nm of 0.5 was reached. Expression was induced with 0.5 mM isopropyl-β-thiogalactoside (IPTG), and cells were left for 20 h at 17°C. Bacterial cells were recovered by centrifugation at 5,000 × g for 10 min. Bacterial pellets were then resuspended in 40 ml of lysis buffer (50 mM Tris [pH 8.0], 300 mM NaCl, 10 mM imidazole, 0.25 mg/ml lysozyme, EDTA-free antiproteases [Roche]) per liter of cell culture and frozen at −80°C.
Protein purification.
Cells were thawed with shaking in the presence of 20 mM MgSO4 and 10 μg/ml of DNase. The lysate was then sonicated and cleared by a 30-min centrifugation at 21,400 × g. After filtration on a 0.45-μm filter, supernatant was loaded on a 5 ml HiTrap nickel affinity column (GE Healthcare) equilibrated in imidazole buffer (50 mM Tris [pH 8.0], 300 mM NaCl, 10 mM imidazole). The column was washed and eluted with the same buffer containing increased imidazole concentrations of 50 mM and 250 mM, respectively. The eluted protein was further purified on a HiLoad 26/60 Superdex 200 (GE Healthcare) gel filtration column in a buffer containing 1.8 mM KH2PO4, 10.1 mM Na2HPO4, 2.7 mM KCl, and 137 mM NaCl, pH 7.2. Purified material was concentrated to appropriate crystallization concentrations on an Amicon Ultra-15 centrifugal filter unit with a 30-kDa cutoff.
Protein crystallization and crystal structure determination.
Crystallization trials were performed using a sitting-drop technique implemented on a nanodrop-dispensing robot (PixSys; Cartesian) in Greiner 96-well plates. At 291 K, initial crystals were obtained under Stura footprint screen (Molecular Dimensions Limited) conditions E3 (0.1 M sodium cacodylate [pH 5.5], 18% polyethylene glycol [PEG] 2000 monomethyl ether) and F4 (0.2 M imidazole malate [pH 6.0], 15% PEG 4000). Optimization of these conditions yielded two different crystal morphologies with best-diffracting crystals under modified condition E3 (0.1 M sodium cacodylate [pH 5.5], 12% PEG 2000 monomethyl ether) with a protein concentration of 7.5 g/liter (form 1) and under modified condition F4 (0.2 M imidazole malate [pH 6.1], 17% PEG 4000) with a protein concentration of 6.3 g/liter (form 2). Data were collected at the European synchrotron radiation facility (ESRF, Grenoble, France) on line ID14-EH1. Crystals were directly flash-frozen from their mother liquor in the nitrogen gas flow. Both data sets were integrated and reduced using MOSFLM and SCALA (7). Molecular replacement was performed using the automated molecular replacement server BALBES (20) for form 1 and using PHASER (22) with the p2 RBP C terminus trimer as a search model (Protein Data Bank number 1BSD; residues 162 to 264) for form 2. Refinement was performed using REFMAC (26), while rebuilding was done using COOT (16). Structure validation was performed using MolProbity (21). The data collection and refinement results are summarized in Table 1. Structure superimpositions were performed with turbo-Frodo, and figures were generated with Pymol (9).
TABLE 1.
Data collection and refinement statistics for both forms of the chimerical RBP
| Parameter | Valuea for form:
|
|
|---|---|---|
| 1 (residues 17-165) | 2 (residues 34-165) | |
| Data collection | ||
| PDB access code | 3d8m | 3da0 |
| Space group | P213 | P21 |
| Unit cell (Å) | 85.8 | 59.0, 44.0, 79.7, 90.0, 101.6, 90.0 |
| Beamline | ID14-EH1 | ID14-EH1 |
| Detectors | ADSC Q210 | ADSC Q210 |
| Wavelength (Å) | 0.934 | 0.934 |
| Rotation range (°) | 182 | 150 |
| Resolution range (Å) | 85.75-3.35 (3.53-3.35) | 39.01-1.65 (1.74-1.65) |
| No. of observations | 63,688 (9,216) | 142,064 (21,202) |
| No. of unique reflections | 3,206 (450) | 46,882 (6,921) |
| Completeness | 100 (100) | 98.3 (99.9) |
| Redundancy | 19.9 (20.5) | 3.0 (3.1) |
| I/σI | 15.9 (6.6) | 7.7 (2.3) |
| Rsym (%) | 24.5 (50.9) | 16.6 (39.4) |
| Refinement | ||
| Resolution range (Å) | 60.75-3.35 (3.44-3.35) | 30.0-1.65 (1.69-1.65) |
| No. of unique reflections | 2,744 | 44,350 |
| No. of atoms | ||
| Protein | 1,137 | 3,129 |
| Water | 293 | |
| R /Rfree | ||
| All | 22.8/26.9 | 16.5/20.1 |
| Last shell | 26.9/34.3 | 20.2/26.7 |
| RMSD | ||
| Bonds (Å) | 0.015 | 0.018 |
| Angles (°) | 1.147 | 1.699 |
| Mean B value (Å2) | ||
| Protein | 27.93 | 15.5 |
| Ramachandran (%) | ||
| Favored region | 93.20 | 97.97 |
| Allowed regions | 97.96 | 100.00 |
Values in parentheses belong to the last shell.
Fluorescence-quenching experiments.
Fluorescence experiments were carried out on a Varian Eclipse spectrofluorimeter using a quartz cuvette in a right-angle configuration; the light paths were 0.4 and 1 cm for the excitation and emission, respectively. The interaction with RBP was monitored by recording the quenching of the intrinsic protein fluorescence upon addition of ligand aliquots. The excitation wavelength was 290 nm, and emission spectra were recorded in the range from 320 to 380 nm. The excitation slit was 5 nm, while the emission slit was 10 nm. Titrations were carried out at 20°C in a phosphate buffer [1 μM protein in 10 mM (Na/Na2) phosphate, 50 mM NaCl, pH 7.5]. The fluorescence intensities at the maximum of emission (354 nm) were corrected for the buffer contribution before plotting and further analysis. The affinity was estimated by plotting the decrease of fluorescence intensity at the emission maximum as [100 − (Ii − Imin)/(I0 − Imin)·100] against the quencher concentration, where I0 is the maximum fluorescence intensity of the protein alone, Ii is the fluorescence intensity after the ith addition of quencher, and Imin is the fluorescence intensity at the saturating concentration of quencher. The Kdiss value was estimated using Prism 3.02 (GraphPad Software Inc.) by nonlinear regression for a single binding site with the equation Y = Bmax·X/(Kdiss + X), where Bmax is the maximal binding, Y is the fluorescence intensity, X is the concentration of ligand added, and Kdiss is the concentration of ligand required to reach half-maximal binding.
RESULTS AND DISCUSSION
Structure of the RBP chimera.
The chimera construct used in this study encoded 165 residues from lactococcal phages p2 and TP901-1, an N terminus of 21 residues from the cloning vector, and the His6 tag. Specifically, residues 1 to 63 were from the RBP of the temperate phage TP901-1 (P335 species) and were fused to residues 163 to 264 from the RBP of the virulent phage p2 (936 species) (Fig. 1C and E), resulting in a RBP chimera (Fig. 1D) that could be produced and purified. The junction point, Pro 162 (p2 numbering) or 63 (TP901-1 numbering), is located at the linker/head domain junctions. It interrupts the last β-strand of the linker and redirects the amino acid chain to the top (Fig. 1C and E).
Crystallization of this RBP chimera led to two crystal forms (Table 1). Form 1 diffracts to a relatively low resolution (3.35 Å), and the electron density starts at the same residue number as the native RBP from the temperate phage TP901-1 (27, 29-31). It contains a cleaved protomer in the asymmetric unit, comprising residues 17 to 165 (chimera numbering). The trimer, found in native wild-type lactococcal RBPs, was generated using the crystallographic threefold axis of the P213 space species (Fig. 2A). On the other hand, form 2 diffracts with a better resolution (1.65 Å) but is cleaved at the beginning of the β-helical linker domain and starts at residue 34. Form 2 contains the expected trimer in the asymmetric unit, with each protomer comprising residues 34 to 164 (Fig. 1 and 2A).
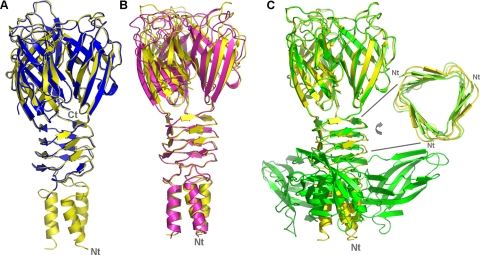
FIG. 2.
Superimposition and comparison of the RBPs from lactococcal phages p2 and TP901-1 as well as their chimera. (A) Superimposition of chimera form 1 (yellow) and form 2 (blue). (B) Superimposition, using the N-terminal and linker domains, of form 2 (yellow) on the wild-type RBP of phage TP901-1 (pink). (C) Superimposition, using the C-terminal domains, of form 2 (yellow) on the wild-type RBP of phage p2 (green). Inset, 90° view of the β-prism linker domains of p2 (green) and the chimera (yellow), illustrating the larger size of the latter.
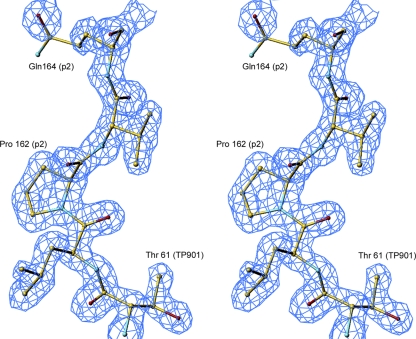
In both crystal forms, the electron density map is well defined, considering the respective resolutions. In particular, the stretch containing Pro 162 (p2) or 63 (TP901-1) at the linker/head junction of the RBP chimera is perfectly defined in the electron map density (Fig. 3). Superimposition of the form 1 reconstituted trimer onto the trimer of form 2 yields a good root mean square deviation (RMSD) value of 0.49 Å for the 393 Cα carbons in common (Fig. 2A).
FIG. 3.
2fo-fc 1.65-Å resolution electron density (stereo view) contoured at 1σ of the stretch of residues linking the swapped domains of the chimera, including the hinge proline.
Comparison of the chimera structures with the wild-type structures.
We have superimposed the RBP structures of the chimera and of the wild type from TP901-1, using the common domains, N terminus, and β-prism. The superimposition is excellent with both forms, yielding RMSD values of 0.69 and 0.30 Å between their Cα atoms for forms 1 and 2, respectively (Fig. 2B; Table 2). Similarly, when superimposing the RBP C-terminal domains of the chimera and phage p2, the RMSD values are even better, at 0.47 Å and 0.28 Å for forms 1 and 2, respectively (Fig. 2C; Table 2). These two comparisons indicate that the structures of the individual domains are rigid enough to keep close structures, independently of the domains upon which they are grafted or the different space groups to which they belong. In the latter case, the superimposition of the wild-type linker domains indicates that the linker domain of phage TP901-1 is slightly larger than that of phage p2 (Fig. 2C, inset). Indeed, when superimpositions are applied to the whole RBP structures, the RMSD values become worse, due to the differences in domain orientations (Table 2). The RMSD values still remain smaller than that calculated between the Cα atoms of the RBPs of p2 and TP901-1, which is 1.53 Å. To adapt to the linker difference between phages p2 and TP901-1, a stretch of ∼10 residues (starting at the hinge proline of the linker/C terminus junction of the chimera) adopts a different track from that in the TP901-1 structure, leading to a C terminus domain rotation of ∼8 degrees. Finally, the receptor binding sites of the wild-type RBP of phage p2 and the binding domain of the chimera are very similar.
TABLE 2.
Summary of superimposition results for both forms of the chimerical protein
| Crystal form | RMSD (Å)a
|
|||
|---|---|---|---|---|
| TP901-1
|
p2
|
|||
| N terminus/ linker domain | All | C terminus | All | |
| 1 (long) | 0.69 (136) | 1.30 (436) | 0.47 (309) | 1.17 (416) |
| 2 (short) | 0.30 (89) | 1.20 (389) | 0.28 (303) | 0.97 (389) |
RMSD values after superimposition. Values in parentheses represent the numbers of Cα carbons used in superimposition.
Interpreting the cleavage sites within a structural context.
As indicated above, the analyses of the two crystal forms of the RBP chimera revealed protease cleavages during the course of crystallization (Fig. 4). The reasons for the cleavages are unknown, although traces of contaminating proteases from the expression organisms cannot be ruled out. Interestingly, the cleavage site in form 1 was also previously reported in the structure of the native RBP from phage TP901-1 (29). The freshly prepared protein is intact, but it is cleaved in a few days. We can see the amino acid chain from threonine 17 in the electron map density (Fig. 4A). Since the structure of the intact RBP protein of TP901-1 is still unknown, we cannot interpret this cleavage in terms of residue accessibility.
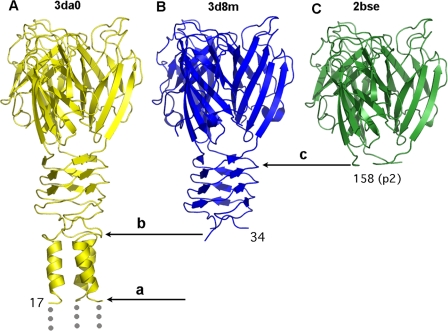
FIG. 4.
Identification of the protease cleavage sites on RBPs and their correlation with domain junctions. (A) Chimera form 1 and its cleavage after Glu16. (B) Chimera form 2 and its cleavage after Thr30, between the N terminus and linker domains. (C) RBP head domain of phage p2 from the complex with llama VHHs.
In contrast, the cleavage observed in chimera form 2 occurs in a large accessible loop (ELTSG∧GN) between two glycine residues (Fig. 4B). We never observed this cleavage in solution, but crystal packing clearly indicates that the absent density cannot be attributed to flexibility due to packing constraints. Another cleavage site was previously observed in the RBP structure of phage p2 while in complex with llama VHH domains (30) as well as in the RBP structure of the lactococcal phage bIL170 (936 group) (27). This cleavage occurs in a loop (T∧VSGSIDVP162) at the end of the last β-strand of the linker domain, just before the hinge proline 162. Interestingly, the cleavages in form 2 as well as in the wild-type RBPs of phages p2 and bIL170 correspond to structural domain boundaries, with high solvent accessibility. It is noteworthy that small additions of protease have been used to induce cleavage at domain boundaries, thus facilitating crystallization of individual domains (12).
Interaction with glycerol.
We have shown previously that the RBPs of phages p2 (27, 29-31), TP901-1 (29), and bIL170 (27) bind glycerol and phosphoglycerol with high affinity. Glycerol was found in the putative receptor binding site, close to a Trp or Phe residue, and establishing strong hydrogen bonds with Arg, Glu, and His. These data led us to postulate that the phage receptor at the bacterial cell surface might be teichoic or lipoteichoic acids, which are polymers of substituted phosphoglycerol. In the RBP of the virulent lactococcal phage p2, a tryptophan is part of the binding site in the head domain, which makes it possible to measure the affinity of the RBP chimera for glycerol using fluorescence quenching (27, 29-31). When glycerol is added in increasing concentrations to an RBP chimera solution of 1 μM, the maximum fluorescence is quenched by ∼30% (see Fig. S1 in the supplemental material). Analysis of the decrease curve yields a Kdiss value of 80 nM, a value slightly better than that measured for wild-type RBP of phage p2 and also for the RBP head domain of p2 expressed alone (27, 29-31), which indicates that the chimeric RBP is able to bind to glycerol.
In conclusion, we have shown here that an artificial RBP chimera can be designed in a straightforward manner and is capable of binding activities. The RBP domain grafting resulted in stable chimerical structures in which the domains closely resemble the wild-type structures. Such conserved structure was possible due to adaptation through small displacements of the linkers. From a phage evolution standpoint, it suggests that lactococcal RBPs are built to efficiently exchange a head domain, which may lead to host range variation. Module shuffling also likely helps them to persist in a man-made environment containing distinct bacterial cells.
Supplementary Material
Acknowledgments
This work was supported in part by the Marseille-Nice Genopole and by a grant from the Agence Nationale de la Recherche (BLAN07-1_191968). S.M. acknowledges support from the Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic Program.
Footnotes
Published ahead of print on 13 March 2009.
Supplemental material for this article is available at http://jb.asm.org/.
REFERENCES
- 1.Ackermann, H. W. 2007. 5500 phages examined in the electron microscope. Arch. Virol. 152227-243. [DOI] [PubMed] [Google Scholar]
- 2.Argos, P., T. Tsukihara, and M. G. Rossmann. 1980. A structural comparison of concanavalin A and tomato bushy stunt virus protein. J. Mol. Evol. 15169-179. [DOI] [PubMed] [Google Scholar]
- 3.Benson, S. D., J. K. Bamford, D. H. Bamford, and R. M. Burnett. 2004. Does common architecture reveal a viral lineage spanning all three domains of life? Mol. Cell 16673-685. [DOI] [PubMed] [Google Scholar]
- 4.Benson, S. D., J. K. Bamford, D. H. Bamford, and R. M. Burnett. 1999. Viral evolution revealed by bacteriophage PRD1 and human adenovirus coat protein structures. Cell 98825-833. [DOI] [PubMed] [Google Scholar]
- 5.Burmeister, W. P., D. Guilligay, S. Cusack, G. Wadell, and N. Arnberg. 2004. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 787727-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell, J. D., A. E. Prota, T. S. Dermody, and T. Stehle. 2002. Crystal structure of reovirus attachment protein sigma1 reveals evolutionary relationship to adenovirus fiber. EMBO J. 211-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborative Computational Project, Number 4. 1994. The CCP4 suite: programs for crystallography. Acta Crystallogr. D 50760-766. [DOI] [PubMed] [Google Scholar]
- 8.De Haard, H. J., S. Bezemer, A. M. Ledeboer, W. H. Muller, P. J. Boender, S. Moineau, M. C. Coppelmans, A. J. Verkleij, L. G. Frenken, and C. T. Verrips. 2005. Llama antibodies against a lactococcal protein located at the tip of the phage tail prevent phage infection. J. Bacteriol. 1874531-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLano, W. The PyMOL molecular graphics system (http://www.pymol.org). DeLano Scientific LLC, San Carlos, CA.
- 10.Deveau, H., S. J. Labrie, M. C. Chopin, and S. Moineau. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 724338-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deveau, H., M. R. Van Calsteren, and S. Moineau. 2002. Effect of exopolysaccharides on phage-host interactions in Lactococcus lactis. Appl. Environ. Microbiol. 684364-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong, A., X. Xu, A. M. Edwards, C. Chang, M. Chruszcz, M. Cuff, M. Cymborowski, R. Di Leo, O. Egorova, E. Evdokimova, E. Filippova, J. Gu, J. Guthrie, A. Ignatchenko, A. Joachimiak, N. Klostermann, Y. Kim, Y. Korniyenko, W. Minor, Q. Que, A. Savchenko, T. Skarina, K. Tan, A. Yakunin, A. Yee, V. Yim, R. Zhang, H. Zheng, M. Akutsu, C. Arrowsmith, G. V. Avvakumov, A. Bochkarev, L. G. Dahlgren, S. Dhe-Paganon, S. Dimov, L. Dombrovski, P. Finerty, Jr., S. Flodin, A. Flores, S. Graslund, M. Hammerstrom, M. D. Herman, B. S. Hong, R. Hui, I. Johansson, Y. Liu, M. Nilsson, L. Nedyalkova, P. Nordlund, T. Nyman, J. Min, H. Ouyang, H. W. Park, C. Qi, W. Rabeh, L. Shen, Y. Shen, D. Sukumard, W. Tempel, Y. Tong, L. Tresagues, M. Vedadi, J. R. Walker, J. Weigelt, M. Welin, H. Wu, T. Xiao, H. Zeng, and H. Zhu. 2007. In situ proteolysis for protein crystallization and structure determination. Nat. Methods 41019-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont, K., F. K. Vogensen, H. Neve, J. Bresciani, and J. Josephsen. 2004. Identification of the receptor-binding protein in 936-species lactococcal bacteriophages. Appl. Environ. Microbiol. 705818-5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emond, E., B. J. Holler, I. Boucher, P. A. Vandenbergh, E. R. Vedamuthu, J. K. Kondo, and S. Moineau. 1997. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl. Environ. Microbiol. 631274-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emond, E., and S. Moineau. 2007. Bacteriophages and food fermentations, p. 93-124. In S. McGrath and D. van Sinderen (ed.), Bacteriophage: genetics and molecular biology. Horizon Scientific Press/Caister Academic Press, Norwich, United Kingdom.
- 16.Emsley, P., and K. Cowtan. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 602126-2132. [DOI] [PubMed] [Google Scholar]
- 17.Labrie, S. J., J. Josephsen, H. Neve, F. K. Vogensen, and S. Moineau. 2008. Morphology, genome sequence, and structural proteome of type phage P335 from Lactococcus lactis. Appl. Environ. Microbiol. 744636-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labrie, S. J., and S. Moineau. 2007. Abortive infection mechanisms and prophage sequences significantly influence the genetic makeup of emerging lytic lactococcal phages. J. Bacteriol. 1891482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledeboer, A. M., S. Bezemer, J. J. de Hiaard, I. M. Schaffers, C. T. Verrips, C. van Vliet, E. M. Dusterhoft, P. Zoon, S. Moineau, and L. G. Frenken. 2002. Preventing phage lysis of Lactococcus lactis in cheese production using a neutralizing heavy-chain antibody fragment from llama. J. Dairy Sci. 851376-1382. [DOI] [PubMed] [Google Scholar]
- 20.Long, F., A. A. Vagin, P. Young, and G. N. Murshudov. 2008. BALBES: a molecular-replacement pipeline. Acta Crystallogr. D 64125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovell, S. C., I. W. Davis, W. B. Arendall III, P. I. de Bakker, J. M. Word, M. G. Prisant, J. S. Richardson, and D. C. Richardson. 2003. Structure validation by Calpha geometry: phi, psi and Cbeta deviation. Proteins 50437-450. [DOI] [PubMed] [Google Scholar]
- 22.McCoy, A. J., R. W. Grosse-Kunstleve, L. C. Storoni, and R. J. Read. 2005. Likelihood-enhanced fast translation functions. Acta Crystallogr. D 61458-464. [DOI] [PubMed] [Google Scholar]
- 23.McGrath, S., H. Neve, J. F. Seegers, R. Eijlander, C. S. Vegge, L. Brondsted, K. J. Heller, G. F. Fitzgerald, F. K. Vogensen, and D. van Sinderen. 2006. Anatomy of a lactococcal phage tail. J. Bacteriol. 1883972-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moineau, S., M. Borkaev, B. J. Holler, S. A. Walker, J. K. Kondo, E. R. Vedamuthu, and P. A. Vandenbergh. 1996. Isolation and characterization of lactococcal bacteriophages from cultured buttermilk plants in the United States. J. Dairy Sci. 792104-2111. [Google Scholar]
- 25.Moineau, S., D. Tremblay, and S. Labrie. 2002. Phages of lactic acid bacteria: from genomics to industrial applications. ASM News 68388-393. [Google Scholar]
- 26.Murshudov, G., A. A. Vagin, and E. J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53240-255. [DOI] [PubMed] [Google Scholar]
- 27.Ricagno, S., V. Campanacci, S. Blangy, S. Spinelli, D. Tremblay, S. Moineau, M. Tegoni, and C. Cambillau. 2006. Crystal structure of the receptor-binding protein head domain from Lactococcus lactis phage bIL170. J. Virol. 809331-9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sciara, G., S. Blangy, M. Siponen, S. Mc Grath, D. van Sinderen, M. Tegoni, C. Cambillau, and V. Campanacci. 2008. A topological model of the baseplate of lactococcal phage Tuc2009. J. Biol. Chem. 2832716-2723. [DOI] [PubMed] [Google Scholar]
- 29.Spinelli, S., V. Campanacci, S. Blangy, S. Moineau, M. Tegoni, and C. Cambillau. 2006. Modular structure of the receptor binding proteins of Lactococcus lactis phages. The RBP structure of the temperate phage TP901-1. J. Biol. Chem. 28114256-14262. [DOI] [PubMed] [Google Scholar]
- 30.Spinelli, S., A. Desmyter, C. T. Verrips, H. J. de Haard, S. Moineau, and C. Cambillau. 2006. Lactococcal bacteriophage p2 receptor-binding protein structure suggests a common ancestor gene with bacterial and mammalian viruses. Nat. Struct. Mol. Biol. 1385-89. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay, D. M., M. Tegoni, S. Spinelli, V. Campanacci, S. Blangy, C. Huyghe, A. Desmyter, S. Labrie, S. Moineau, and C. Cambillau. 2006. Receptor-binding protein of Lactococcus lactis phages: identification and characterization of the saccharide receptor-binding site. J. Bacteriol. 1882400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valyasevi, R., W. E. Sandine, and B. L. Geller. 1990. The bacteriophage kh receptor of Lactococcus lactis subsp. cremoris KH is the rhamnose of the extracellular wall polysaccharide. Appl. Environ. Microbiol. 561882-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Raaij, M. J., G. Schoehn, M. R. Burda, and S. Miller. 2001. Crystal structure of a heat and protease-stable part of the bacteriophage T4 short tail fibre. J. Mol. Biol. 3141137-1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.