Abstract
Conjugative plasmids generally encode proteins that block the conjugative entry of identical or similar plasmids into the host cell, a phenomenon known as entry exclusion. Here, we demonstrate that two Ti plasmids of Agrobacterium tumefaciens encode robust entry exclusion functions. Two proteins, TrbJ and TrbK, can each mediate entry exclusion and act synergistically. The trbJ and trbK genes are included within the trb operon, which is tightly regulated by the quorum-sensing regulator TraR and the cognate acylhomoserine lactone. In the absence of quorum-sensing signals, these proteins are not significantly expressed, and cells lacking TrbJ and TrbK are efficient Ti plasmid recipients. In the presence of these signals, these strains block the entry of Ti plasmids and instead become efficient conjugal donors.
Many conjugative plasmids are able to block the entry of identical or closely related types of plasmids by creating a functional barrier at the cell surface. This phenomenon is known as entry (or surface) exclusion. Two different types of exclusion determinants are known to cause this phenomenon. Surface-exposed outer membrane proteins, exemplified by TraT of the F plasmid, are thought to block the formation of stable mating aggregates between two donor cells (24). Other proteins, such as TraS of the F plasmid and TrbK of RP4, are located in the inner membrane and inhibit conjugative DNA transfer (8, 24).
Entry exclusion of Agrobacterium Ti plasmids has not been documented, but it is plausible that they too have such a system (17). These plasmids are capable of efficient conjugation and carry a complete suite of conjugative transfer genes, designated tra and trb genes (1, 5, 17, 25). One of these genes, trbK, resembles the trbK genes of the IncP plasmids RP4, RK2, and R18 (all of which are virtually identical), which mediate entry exclusion of the corresponding plasmids (8, 9, 15, 18). Another Ti plasmid gene, trbJ, resembles the trbJ gene of RP4, which may or may not contribute to entry exclusion. Lessl et al. and Lyras et al. reported that TrbJ proteins from IncPα plasmids mediate low-level entry exclusion (15, 18). Haase et al. presented somewhat conflicting data about the role of TrbJ from RP4 (8, 9). The reasons for these conflicting data are unclear. The trbJ and trbK genes of RP4 and of Ti plasmids lie within operons of genes that direct mating-pair formation (Mpf genes) (1, 17). The structure encoded by Mpf genes is sometimes referred to as a mating bridge and resembles the family of type IV systems that are able to translocate DNA and/or protein into foreign cells (3). TrbK of RP4 is not required for conjugation (9), so its sole function may be in entry exclusion. Similarly, TrbK of pTiC58 is dispensable for conjugation (17). In contrast, the TrbJ proteins of pTiC58 and of RP4 are essential for conjugation (9, 17).
TrbK of RP4 is a lipoprotein that has a lipid attachment motif and is localized mainly to the cytoplasmic membrane (8). Its signal sequence is removed proteolytically, and one or more acyl groups are added to a cysteine residue at the newly created amino terminus. This cysteine is required for wild-type levels of entry exclusion, although residual levels were detectable when this cysteine was altered (8). The alteration of the cysteine residue causes decreased affinity for the cytoplasmic membrane. Significantly, all known Ti plasmid TrbK proteins lack this cysteine residue. They are therefore unlikely to be acylated. Both TrbK and TrbJ proteins are strongly predicted to have cleaved signal sequences (see below), though this prediction has not been experimentally confirmed and the localization patterns of the proteins have not been determined.
All Ti plasmid tra and trb genes are regulated by the TraR and TraI quorum-sensing system (6), and a variety of plasmids of Rhizobium, Mesorhizobium, and Sinorhizobium spp. regulate conjugation genes in similar fashions (7). TraR resembles the transcription factor LuxR of Vibrio fischeri, while TraI resembles the V. fischeri LuxI protein and synthesizes the pheromone 3-oxo-octanoylhomoserine lactone (OOHL). This pheromone binds to and activates TraR. Significantly, both TraR and TraI are encoded on Ti plasmids, and therefore, this system detects a quorum of conjugal donors rather than of conjugal recipients. As this system detects only conjugal donors, it seemed plausible that conjugation in V. fischeri had evolved to occur preferentially between conjugal donors. Although conjugation between donor cells may seem futile, it may have the potentially useful effect of increasing the plasmid copy number, as transfer requires conjugative DNA replication. Furthermore, it has been well established that TraR-OOHL complexes increase the plasmid copy number by enhancing vegetative replication (16, 19). However, the findings of the present study disproved this hypothesis, as we documented that octopine-type and nopaline-type Ti plasmids have entry exclusion systems and that both TrbJ and TrbK can carry out entry exclusion independently and synergistically. In this sense, our findings tend to support the results of the studies of RP4 by the Lessl and Lyras groups (15, 18) rather than those of the studies by Haase et al. (8, 9). However, like all tra and trb genes, trbJ and trbK are tightly regulated by activated TraR (6, 11, 21), and in the absence of activated TraR, neither TrbJ nor TrbK is significantly expressed and host cells exhibit little or no entry exclusion. These cells, therefore, are efficient recipients, despite the fact that they have Ti plasmids.
MATERIALS AND METHODS
Strains, oligonucleotides, and reagents.
Bacterial strains and plasmids used in this study are described in Table 1, while oligonucleotides used for PCR amplification and site-directed mutagenesis and for nuclease S1 protection assays are described in Table 2. Antibiotics and ONPG (o-nitrophenyl-β-d-galactopyranoside) were purchased from Sigma-Aldrich. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was purchased from Gold Biotechnologies. Restriction endonucleases, T4 DNA ligase, and T4 polynucleotide kinase were purchased from New England Biolabs. Taq polymerase was purchased from Promega, and [γ-32P]ATP was purchased from Perkin Elmer.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid(s) | Descriptiona | Source and/or reference |
|---|---|---|
| Strains | ||
| WCF5 | R10 traR traR-lacZ Kmr | 6 |
| R10 | Octopine type strain; pTiR10 | S. K. Farrand |
| C58 | Nopaline type strain; pTiC58 | S. K. Farrand |
| C58C1RS | Ti plasmid-less derivative of C58; Rifr Smr | S. K. Farrand |
| HC158 | C58 containing pHC320 inserted into the nopaline-type Ti plasmid pTiC58 by Campbell-type integration; traR traR-lacZ Kmr | This study |
| HC159(pYDH902) | R10 cured of pTiR10 and containing cosmid pYDH902 | This study |
| HC161 | Strain with polar mutation of trbD by the insertion of pHC327 | This study |
| HC162 | Strain with polar mutation of trbJ by the insertion of pHC328 | This study |
| HC163 | Strain with polar mutation of trbK by the insertion of pHC329 | This study |
| HC164 | Strain with polar mutation of trbF by the insertion of pHC330 | This study |
| Plasmids | ||
| pCF218 | PtetR-traR Tcrrep-RP4 | 6 |
| pBBR1MCS5 | Broad-host-range vector; rep-pBRR1 Gmr | 14 |
| pVIK111 | Carries promoterless lacZ; oriR6K Kmr | 12 |
| pKNG101 | sacB+ Smrrep-R6K oriT-RP4 | 13 |
| pPR1068 | pMAL2 derivative with NdeI site at ATG codon of malE; Ptac-MBP-lacZα lacIq Apr ori-ColE1 | Paul Riggs (20) |
| pPZP200 and pPZP201 | Broad-host-range vectors; rep-pVS1 Spr | 10 |
| pJZ335 | traR from pTiA6NC cloned into pPZP201 | 26 |
| pJZ381 | EcoRI fragment containing traR cloned into pBBR1MCS5 | 2 |
| pYDH902 | Cosmid containing rep and traI-trb operons; rep-RP4 Tcr | 4 |
| pHC011 | pPR1068 digested with NdeI and SacI, with replacement by a linker containing NdeI-KpnI-SacI sites to delete the malE gene; Ptac is fused to NdeI-KpnI-SacI-AvaI-XmnI-EcoRI-BamHI-XbaI-SalI-PstI-HindIII; rep-ColE1 Apr | This study |
| pHC012 | pHC011 digested with EcoRV and KpnI and ligated to pBBRMCS5 after digestion with SphI and KpnI, with 3′-end fill-in of the SphI site with the Klenow fragment of DNA polymerase I; Ptac is fused to NdeI-KpnI-ApaI-XhoI-SalI-Bsp106I-ClaI-HindIII-EcoRI-PstI-SmaI- BamHI-SpeI-XbaI-BstXI-SacI; rep-pBBR1 Gmr | This study |
| pHC320 | pVIK111 containing an EcoRI-XbaI fragment including the 5′ end of traR and upstream sequences; rep-R6K Kmr | This study |
| pHC327 | PCR fragment of trbD made using oligonucleotides TDF and TDR and cloned into pKNG101 for Campbell recombination mutagenesis; rep-R6K Smr | This study |
| pHC328 | PCR fragment of trbJ made using oligonucleotides TJF and TJR and cloned into pKNG101 for Campbell recombination mutagenesis; rep-R6K Smr | This study |
| pHC329 | PCR fragment of trbK made using oligonucleotides TKF and TKR and cloned into pKNG101 for Campbell recombination mutagenesis; rep-R6K Smr | This study |
| pHC330 | PCR fragment of trbF made using oligonucleotides TFF and TFR and cloned into pKNG101 for Campbell recombination mutagenesis; rep-R6K Smr | This study |
| pHC335 | pJZ335 digested with BamHI and ligated to remove a small BamHI fragment between Plac and traR; carries traR cloned into pPZP201; rep-pVS1 Spr | This study |
| pHC361 | PCR fragment containing trbJK made using oligonucleotides TrbJKF-N and R10-trbKJK3, digested with BamHI, and cloned into pHC012; Ptac-trbJK rep-pBBR1 Gmr | This study |
| pHC364 | trbBCDEJK cloned as a HindIII-BamHI fragment into pHC012; Ptac-trbBCDEJK rep-pBBR1 Gmr | This study |
| pHC368 | PCR fragment containing trbK made using oligonucleotides R10-trbKF-N and R10-trbKJK3, digested with BamHI, and cloned into pHC012; Ptac-trbK rep-pBBR1 Gmr | This study |
| pUP200 | 1,236-nucleotide DNA fragment made by PCR amplification using pHC012 as the template and oligonucleotides MfeI-For and NsiI-Rev as primers, cloned into the EcoRI-PstI gap of pPZP200, with the Ptac promoter upstream of the multiple-cloning site of pHC012; rep-pVS1 Gmr | This study |
| pUP402 | PCR fragment containing trbJ made using oligonucleotides TrbJKF-N and TrbJR-N and cloned into pHC012; Ptac-trbJ rep-pVS1 Gmr | This study |
| pUP403 | PCR fragment containing trbJ made using oligonucleotides TrbJKF-N and TrbJR-N and cloned into pUP200; Ptac-trbJ rep-pVS1 Gmr | This study |
| pUP404 | trbK from pHC368 cloned into pUP200; Ptac-trbK rep-pVS1 Gmr | This study |
| pUP405 | Derivative of pHC368 lacking the 3′ codon of trbK | This study |
| pUP406 | Derivative of pUP404 lacking the 3′ codon of trbK | This study |
| pUP407 | Derivative of pUP402 lacking the 3′ codon of trbJ | This study |
| pUP408 | Derivative of pUP403 lacking the 3′ codon of trbJ | This study |
MBP, maltose-binding protein.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequencea |
|---|---|
| Oligonucleotides used to make polar mutations in trb genes | |
| TDF | 5′-GCTGGATCCATCGCTGGTGCGATGCTG-3′ |
| TDR | 5′-GCTTCTAGAACCATCTCGACCCCTTCAG-3′ |
| TJF | 5′-GCTGGATCCAATGGGCAATGTCGAAGATG-3′ |
| TJR | 5′-GCTTCTAGAGCGCGAGAATGACGATCAG-3′ |
| TKF | 5′-GCTTCTAGAGCAGGCACAAAAGGATCTG-3′ |
| TKR | 5′-CGGCGATACCGACCTCGATG-3′ |
| TFF | 5′-GCTGGATCCTCATCCCCTACATCGTTGAG-3′ |
| TFR | 5′-GCTTCTAGACCATGCCTTTCAAAGCTGTG-3′ |
| Oligonucleotides used to subclone trb genes | |
| TrbJKF-N | 5′-GCTGAATTCGCAAAGGGGGATCGCCCATG-3′ |
| TrbJR-N | 5′-GTCGGATCCGAGAATGACGATCAGACGCG-3′ |
| R10-trbKF-N | 5′-GCTGAATTCGACGATGGAGCCACGCTGGTG-3′ |
| R10-trbKJK3 | 5′-ATGAACATGATGCGTTTGAC-3′ |
| Oligonucleotides used to clone Ptac-lacZ on pPZP200 | |
| MfeI-For | 5′-GTCCAATTGTATACGCAAGGCGACAAGGTG-3′ |
| NsiI-Rev | 5′-GTCATGCATACTTATTCAGGCGTAGCACCA-3′ |
| Oligonucleotides used for mutagenesis | |
| pJ_W269Stop-F | 5′-GAGCCACGCTGATGAGCTCGC-3′ |
| pJ_W269Stop-R | 5′-GCGAGCTCATCAGCGTGGCTC-3′ |
| pK_W75Stop-F | 5′-GAAACCGAGATGATAGTTCACC-3′ |
| pK_W75Stop-R | 5′-GGTGAACTATCATCTCGGTTTC-3′ |
| pTacR1 | 5′-ACGACGTTGTAAAACGACGGC-3′ |
| pTacR2 | 5′-GCCATTCAGGCTGCGCAACTG-3′ |
| Oligonucleotides used for nuclease S1 protection assay | |
| trbKS1 | 5′-GGCTGGACAGTAATCCAGGTGCCGATGCCTGCACTACGAC-3′ |
| 23SRNAS1 | 5′-AGGCTCGGGCTCCGACTGTTTGTAGGCATCCGGTTTCAG-3′ |
Italics indicate restriction endonuclease cleavage sites used in plasmid construction.
Quantitative conjugation assays.
Conjugative donors and recipients were cultured in AT minimal broth (24a) at 27°C for 5 h, concentrated by centrifugation, combined in a ratio of 50 recipients per donor, spotted onto AT agar medium, and incubated for 2.5 h for R10-derived donor strains or 18 h for C58-derived donor strains. Mating was stopped by resuspending the cells from the agar in 1× AT buffer, and then the cells were serially diluted and plated onto selective AT defined agar medium containing the appropriate antibiotics.
Site-directed mutagenesis in trbJ and trbK.
Site-directed mutagenesis in trbJ and trbK was performed by using a synthetic overlap extension PCR (23). For the mutation of trbJ, a 1,100-bp fragment of pUP404 including a unique EcoRI site located upstream of trbJ and a unique BamHI site located downstream of trbJ was amplified using Platinum Taq Hi Fi DNA polymerase (Invitrogen). For the mutation of trbK, a 530-bp fragment of pHC368 including the same restriction sites listed above was amplified. All oligonucleotides used in this study are listed in Table 2 and were obtained from Integrated DNA Technologies (Coralville, IA). For trbJ, the flanking primers TrbJKF-N and pTacR2 were used in separate reactions with two complementary mutagenic primers, with pUP404 as the template. For trbK, the flanking primers were R10-trbKF-N and pTacR1 and the template was pHC368. In both cases, the two PCR products were combined and used as the template in a second round of PCR with the same flanking primers to generate the complete trbJ or trbK gene. The second set of PCR products was digested with EcoRI and BamHI and ligated into pUP404 or pHC368, digested with the same enzymes. These mutations caused a one-codon deletion at the 3′ ends of both genes. Mutated sequences were confirmed by automated DNA sequencing.
Nuclease S1 protection assays.
RNA was isolated from cells cultured to late log phase and harvested in the presence of 2 volumes of RNAprotect bacterial reagent (Qiagen) per volume of culture. Cell pellets were frozen at −80°C. Lysozyme (200 μl of a 10-mg/ml solution) and 700 μl of buffer RLT (Qiagen) were added to the frozen cell pellets, and the tubes were subjected to a vigorous vortex. Lysates were clarified by centrifugation for 2 min, and RNA was precipitated from the supernatant by the addition of 500 μl of ethanol. Samples were applied to RNeasy spin columns (Qiagen) and centrifuged for 15 s at 10,000 rpm. Buffer RW1 (350 μl) was added to each column, and columns were centrifuged for 15 s at 10,000 rpm. DNase I was diluted eightfold in RDD buffer (Qiagen), and 80 μl per column was added. After 15 min of incubation, columns were washed successively with 350 μl of buffer RW1 and 500 μl of buffer RPE, and RNA was eluted using 40 μl of RNase-free water.
Oligonucleotides were radiolabeled with [γ-P32]ATP and T4 DNA kinase. A 500-pg sample of radiolabeled oligonucleotides was hybridized with 20 μg of total RNA for 10 h at 42°C and then digested with 250 U of nuclease S1 for 1 h at 37°C. Reaction mixtures were then ethanol precipitated and suspended in 5 μl of 0.1 M NaOH, and 5 μl of formamide loading dye was added. Five microliters of each sample was size fractionated using 18% denaturing Tris-borate-EDTA polyacrylamide gels and quantified using a Storm PhosphorImager (model 840; Molecular Dynamics). A 2.5-pg aliquot of 32P-labeled nondigested oligonucleotide was added to one lane of each gel.
RESULTS
Two Ti plasmids encode functional and tightly regulated entry exclusion systems.
The overexpression of TraR in strains containing the native traI gene causes constitutive expression of all genes of the quorum-sensing regulon (6). We reasoned that any entry exclusion gene may also be regulated by TraR and, if so, would most likely be expressed constitutively in strains overexpressing TraR. The overexpression of TraR during conjugation also relieves the requirement for octopine, which is otherwise needed to induce the transcription of the native traR gene (6) and therefore tends to make conjugation data more reproducible. We measured the efficiency of Ti plasmid transfer from R10 derivative WCF5(pJZ381), which overexpresses TraR (Table 1), to two recipient strains: R10(pHC335), which also overexpresses TraR, and R10(pPZP201), which does not. Both recipient strains carried the Ti plasmid pTiR10, which is virtually identical to other so-called octopine-type Ti plasmids (25). The former strain gave rise to 300-fold fewer transconjugants than the latter strain (Table 3, first two lines), indicating that either TraR itself or, more likely, the product of a TraR-regulated gene mediated a robust level of entry exclusion.
TABLE 3.
Entry exclusion of octopine-type and nopaline-type Ti plasmids by homologous and heterologous recipients
| Recipient | Donor | Relevant protein(s) expressed in recipient | No. of transconjugantsa per donor (SD) | Exclusion coefficientb |
|---|---|---|---|---|
| Homologous recipients | ||||
| R10(pPZP201) | WCF5(pJZ381)c | None | 0.94 (0.2) | 1 |
| R10(pHC335) | WCF5(pJZ381) | TraR, TraI, TraA to TraH, TrbB to TrbL | 0.003 (0.001) | 313 |
| C58(pPZP201) | HC158(pJZ381)d | None | 0.22 (0.03) | 1 |
| C58(pHC335) | HC158(pJZ381) | TraR, TraI, TraA to TraH, TrbB to TrbL | 0.0008 (0.0004) | 275 |
| Heterologous recipients | ||||
| C58(pPZP201) | WCF5(pJZ381) | None | 0.032 (0.01) | 1 |
| C58(pHC335) | WCF5(pJZ381) | TraR, TraI, TraA to TraH, TrbB to TrbL | 0.005 (0.002) | 64 |
| R10(pPZP201) | HC158(pJZ381) | None | 0.001 (0.001) | 1 |
| R10(pHC335) | HC158(pJZ381) | TraR, TraI, TraA to TraH, TrbB to TrbL | <0.00001e | >100 |
| Recipients lacking a Ti plasmid | ||||
| C58C1RS(pPZP201) | WCF5(pJZ381) | None | 0.47 (0.22) | 1 |
| C58C1RS(pHC335) | WCF5(pJZ381) | TraR | 0.55 (0.08) | 0.85 |
| C58C1RS(pPZP201) | HC158(pJZ381) | None | 0.35 (0.035) | 1 |
| C58C1RS(pHC335) | HC158(pJZ381) | TraR | 0.42 (0.13) | 0.8 |
Transconjugants were selected using the Kmr gene of the Ti plasmid and the Spr gene of pPZP201 or pHC335. In mock conjugations, we did not detect spontaneous resistance to either kanamycin or spectinomycin. The data are the averages of results from three independent experiments, with the standard deviations shown in parentheses.
The exclusion coefficient is the number of transconjugants per donor for the no-exclusion control (lines with exclusion coefficients of 1) divided by the number of transconjugants of the tested recipient strain per donor.
R10-derived strain containing a Kmr gene on the octopine-type Ti plasmid.
C58-derived strain containing a Kmr gene on the nopaline-type Ti plasmid.
No transconjugants were detected in an assay mixture containing 100,000 donor bacteria.
Similar experiments were carried out using strains harboring the nopaline-type Ti plasmid pTiC58. Here, strain HC158(pJZ381) was used as a Ti plasmid donor. This strain contains a nopaline-type Ti plasmid that has a kanamycin resistance gene to facilitate the selection of transconjugants. This strain also overexpresses TraR from pJZ381. Strains C58(pHC335) and C58(pPZP201) were used as recipients. The former recipient yielded approximately 300-fold fewer transconjugants than the latter (Table 3, third and fourth lines). We conclude that TraR or a TraR-regulated gene in pTiC58 can exclude the conjugal entry of the same type of plasmid.
We also tested the abilities of nopaline-type Ti plasmids to exclude octopine-type Ti plasmids and vice versa. The octopine-type Ti plasmid present in WCF5(pJZ381) conjugated approximately 60-fold less efficiently into a strain containing a nopaline-type Ti plasmid and expressing TraR than into a congenic strain not expressing TraR [Table 3, lines for heterologous recipients C58(pPZP201) and C58(pHC335)]. Similar results were obtained with the reciprocal cross (Table 3, last two lines for heterologous recipients). In the first cross, entry exclusion appeared to be slightly weaker than that in either homologous cross [Table 3, compare line for heterologous recipient C58(pHC335) with lines for homologous recipients R10(pHC335) and C58(pHC335)], suggesting that entry exclusion determinants of the nopaline-type Ti plasmid may function more effectively in blocking a homologous donor than in blocking a heterologous one. For the second cross [Table 3, line for heterologous recipient R10(pHC335)], no such conclusion is possible. No transconjugant colonies were detected, suggesting very strong entry exclusion. However, relatively few transconjugants were detected with the negative control [Table 3, line for heterologous recipient R10(pPZP201)], suggesting either that TraR-independent entry exclusion acted in the recipient to block entry or that the plasmid from the donor conjugated inefficiently into this recipient.
To confirm that TraR mediates entry exclusion indirectly, we measured conjugation using recipient strains lacking Ti plasmids. Strains C58C1RS(pHC335) and C58C1RS(pPZP201) lack Ti plasmids, and the former strain expresses TraR while the latter one does not. Neither strain excluded the entry of either Ti plasmid (Table 3, lines for recipients lacking a Ti plasmid), indicating that TraR is not sufficient for entry exclusion and that it functions by activating one or more entry exclusion genes.
These data also allow us to compare a strain containing a Ti plasmid but lacking TraR with a strain lacking a Ti plasmid. Strains C58(pPZP201) and C58C1RS(pPZP201) are identical except for the presence or absence of a Ti plasmid. Neither strain overexpresses TraR. These two strains showed little if any difference in their inability to exclude either Ti plasmid (Table 3, compare the first line with the fourth-to-last line and the third line with the second-to-last line). This finding indicates that entry exclusion determinants are not significantly expressed in the absence of active TraR.
Identification of the entry exclusion determinants encoded in the Ti plasmid.
As described above, plasmid RP4 has a trb operon that resembles those of Ti plasmids (Fig. 1). Within the RP4 operon, the trbK gene encodes a product required for entry exclusion (8, 9, 15, 18), while the trbJ product may (15, 18) or may not (8, 9) play an accessory role. TrbK of RP4 is 23.5 and 18.2% identical to the TrbK proteins of octopine- and nopaline-type Ti plasmids, respectively, while TrbJ of RP4 is 20.7% identical to both Ti plasmid TrbJ proteins. TrbK proteins of Ti plasmids lack the acylation site of TrbK of RP4, suggesting that they may be nonfunctional or weakly functional. Both TrbJ and TrbK were strongly predicted by the program SignalP-HMM to have cleaved signal sequences (probability, 1.0).
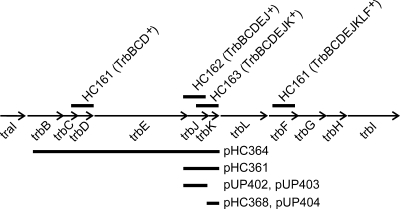
FIG. 1.
Genetic organization of the trb operon of an octopine-type Ti plasmid. Short thick lines above the genetic map represent DNA fragments that were used in suicide plasmids to create transcriptionally polar mutations upon Campbell-type integration. Fragments of the trb region overexpressed by fusion to the Ptac promoter are shown beneath the genetic map.
The cleavage of TrbJ was predicted to remove 33 residues, while the cleavage of TrbK was predicted to remove 21 residues.
We sought to determine whether TrbK and/or TrbJ of an octopine-type Ti plasmid plays a role in entry exclusion. To address this question, we compared strain R10(pHC335), which contains a native octopine-type Ti plasmid and overexpresses TraR, with strain HC159(pYDH902)(pHC335), which lacks the Ti plasmid, overexpresses TraR, and contains a cosmid (pYDH902) that carries the trb and rep operons (4). The donor strain in this experiment was WCF5(pJZ381). Both recipient strains exhibited entry exclusion, and in both cases, TraR overexpression was required (Table 4, first four lines). A similar strain, HC159(pYDH902)(pPZP201), which does not express TraR, showed a low but detectable level of exclusion [Table 4, lines for R10(pPZP201) and HC159(pYDH902)(pPZP201)], due possibly to elevated basal expression of entry exclusion determinants from the multicopy plasmid pYDH902. These data indicate that all genes essential for entry exclusion lie within pYDH902 and probably within the trb operon.
TABLE 4.
Mapping of the entry exclusion locus of the Ti plasmid by using polar insertion mutations within the trb operon
| Recipienta | Relevant protein(s) expressed in recipient | Conjugation efficiencyb (SD) | Exclusion coefficientc |
|---|---|---|---|
| Recipients without trb mutations | |||
| R10(pPZP201) | None (vector control) | 0.90 (0.1) | 1 |
| R10(pHC335) | TraR, TraI, TraA to TraH, TrbB to TrbL | 0.006 (0.0007) | 150 |
| HC159(pYDH902)(pPZP201) | None | 0.11 (0.015) | 8.2 |
| HC159(pYDH902)(pHC335) | TraR, TraI, Trb | 0.0005 (0.00008) | 1800 |
| Recipients with trb genes mutated using transcriptionally polar insertion mutations | |||
| HC161(pHC335) | TraR, TrbBCD | 0.84 (0.1) | 1.07 |
| HC162(pHC335) | TraR, TrbBCDEJ | 0.19 (0.02) | 4.7 |
| HC163(pHC335) | TraR, TrbBCDEJK | 0.007 (0.001) | 129 |
| HC164(pHC335) | TraR, TrbBCDEJKLF | 0.007 (0.001) | 129 |
| Recipients with Trb proteins expressed from a multicopy plasmid via a tac promoter | |||
| R10(pHC012) | None (vector control) | 0.18 (0.02) | 1 |
| R10(pJZ381) | TraR, TraI, TraA to TraH, TrbB to TrbL | 0.0004 (0.0002) | 450 |
| R10(pHC364) | TrbBCDEJK | 0.008 (0.004) | 22.5 |
| R10(pHC361) | TrbJK | 0.0012 (0.0005) | 150 |
| R10(pHC368) | TrbK | 0.025 (0.006) | 7.2 |
The donor strain in each experiment was WCF5(pJZ358), which overexpresses TraR and has an octopine-type Ti plasmid marked with a Kmr determinant (6). Transconjugants were selected using the Kmr gene of the Ti plasmid and the Spr gene of pPZP201 or pHC335 (first eight lines) or using the Gmr gene of pHC012 and its derivatives (last four lines).
Number of transconjugants per donor.
The exclusion coefficient is the number of transconjugants per donor for the no-exclusion control (lines with exclusion coefficients of 1) divided by the number of transconjugants of the tested recipient strain per donor.
To more closely localize the genes responsible for entry exclusion, we constructed four insertion mutations in the trb operon that are predicted to exert strong transcriptional polarity effects on downstream genes. We used derivatives of the suicide plasmid pKNG101 containing various trb fragments. The insertion in HC161 expresses TrbB, TrbC, and TrbD but not TrbE, TrbJ, TrbK, TrbL, TrbF, TrbG, TrbH, or TrbI (Fig. 1). This mutation blocked virtually all entry exclusion [Table 4, line for HC161(pHC335)]. Strain HC162(pHC335) expresses TrbB, TrbC, TrbD, TrbE, and TrbJ and showed approximately fourfold fewer transconjugants than the negative control (Table 4), suggesting a role for TrbJ and/or TrbE in entry exclusion. Strain HC163(pHC335), which expresses TrbB, TrbC, TrbD, TrbE, TrbJ, and TrbK (Fig. 1), strongly expressed entry exclusion (Table 4), indicating that TrbK plays a major role. This strain expressed entry exclusion levels similar to those expressed by HC164(pHC335), which expresses two additional Trb proteins, and by R10(pHC335), which expresses all Trb proteins (Table 4), suggesting that the genes downstream of trbK do not have any role in entry exclusion.
We also tested the expression of Trb proteins from a Ptac promoter of a multicopy plasmid. Plasmid pHC364 expresses TrbB, TrbC, TrbD, TrbE, TrbJ, and TrbK (Fig. 1) and expressed entry exclusion, albeit at a reduced level [Table 4, lines for R10(pJZ381) and R10(pHC364)] compared to that caused by overexpression of TraR on pJZ381. Plasmid pHC361, which expresses only TrbJ and TrbK (Fig. 1), expressed high levels of entry exclusion, while plasmid pHC368, which expresses only TrbK, expressed a low level of entry exclusion (Table 4, last two lines).
To further measure the effects of TrbJ and TrbK on entry exclusion, we expressed these proteins using separate, compatible plasmids in recipient strains. We made a series of fusions using plasmids pHC012 and pUP200, both of which have Ptac promoters and lacZα genes. Ptac-trbJ fusions were constructed in such a way that the lacZα gene was translationally fused to the stop codon of trbE (which lies immediately upstream of trbJ in the native Ti plasmid). This was done to mimic any possible translational coupling between trbE and trbJ. Similarly, Ptac-trbK fusions were made in such a way that the lacZα gene was translationally fused to the stop codon of trbJ.
Expressing TrbJ alone from a derivative of pBBRMCS5 (pUP402) decreased conjugation approximately ninefold, while expressing it from a derivative of pPZP200 (pUP403) caused a fourfold decrease (Table 5, third and fourth lines). This difference is most likely attributable to a difference in copy number, as the Ptac-trbJ fusions of the two plasmids are identical in sequence. The expression of TrbK alone in these two vectors caused similar decreases in conjugation (Table 5, fifth and sixth lines). Most importantly, coexpressing these two proteins from compatible plasmids caused a strong additional decrease in conjugation (Table 5, seventh and eighth lines). We conclude that TrbJ and TrbK make independent contributions to entry exclusion and that the presence of both proteins has a synergistic effect.
TABLE 5.
Expression of TrbJ and TrbK in recipients of multicopy plasmidsa
| pBBRMCS5 derivative (description or genotype) | pPZP200 derivative (description or genotype) | Conjugation efficiencyb (SD) | Exclusion coefficientc |
|---|---|---|---|
| pHC012 (vector) | pUP200 (vector) | 0.69 (0.2) | 1 |
| pJZ381 (traR)d | pPZP200 (vector) | 0.002 (0.001) | 345 |
| pUP402 (Ptac-trbJ) | pUP200 (vector) | 0.08 (0.009) | 8.6 |
| pHC012 (vector) | pUP403 (Ptac-trbJ) | 0.18 (0.03) | 3.8 |
| pHC368 (Ptac-trbK) | pUP200 (vector) | 0.10 (0.04) | 6.9 |
| pHC012 (vector) | pUP404 (Ptac-trbK) | 0.21 (0.04) | 3.3 |
| pUP402 (Ptac-trbJ) | pUP404 (Ptac-trbK) | 0.007 (0.002) | 98.6 |
| pHC368 (Ptac-trbK) | pUP403 (Ptac-trbJ) | 0.022 (0.004) | 31.4 |
| pHC361 (Ptac-trbJK) | pPZP200 (vector) | 0.002 (0.0008) | 345 |
The donor strain in each experiment was WCF5(pCF218), which overexpresses TraR and has an octopine-type Ti plasmid marked with a Kmr determinant. Transconjugants were selected using the Kmr gene of the Ti plasmid, the Spr gene of pUP200 or its derivatives, and the Gmr gene of pHC012 or its derivatives.
Number of transconjugants per donor.
The exclusion coefficient is the number of transconjugants per donor for the no-exclusion control (top line) divided by the number of transconjugants of the tested recipient strain per donor.
The overexpression of TraR by pJZ381 induces the expression of all tra and trb genes (6).
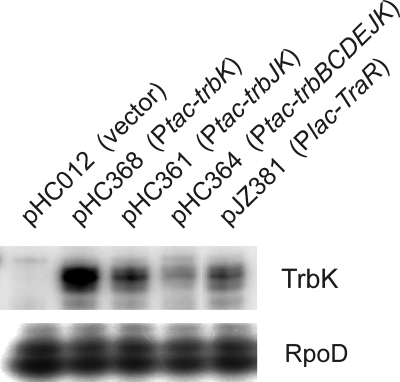
Interestingly, a strain expressing TrbJ and TrbK from separate plasmids showed less entry exclusion than a strain expressing these proteins from a single plasmid (Table 5, last three lines). To ensure that TrbK was expressed at similar levels in these strains, we assayed for the accumulation of TrbK mRNA. Plasmid pHC368, which has a Ptac-trbK fusion, expressed considerably more TrbK mRNA than pHC361, which has a Ptac-trbJK fusion (Fig. 2). Despite this result, the former plasmid expressed entry exclusion more weakly than the latter plasmid. This finding underscores the importance of TrbJ in this process and supports the conclusion that these proteins act preferentially in cis.
FIG. 2.
Results of nuclease S1 protection assays showing trbK transcript levels in recipients containing fusions between Ptac and the indicated trb genes (top) and rpoD transcript levels for each strain (bottom). All strains are derivatives of A. tumefaciens strain R10, which contains pTiR10. Plasmid pJZ381 carries the trbK gene in the Ti plasmid background.
In the course of searching for proteins homologous to TrbK, we fortuitously noticed sequence similarity between TrbK and TrbJ. The C-terminal 12 amino acid residues of these proteins are identical or similar (Fig. 3). This similarity is found among a variety of plasmids of Agrobacterium, Rhizobium, and Sinorhizobium and in two plasmids found in Nitrobacter hamburgensis and Oligotropha carboxidovorans (Fig. 3). The latter two bacteria express TrbJ and TrbK proteins that are strongly similar to those of A. tumefaciens and its close relatives. The last amino acid residues of the proteins show remarkable conservation, even in more distantly related proteins from the IncP-type plasmids. A small number of other cognate TrbJ and TrbK proteins also show sequence similarities at their C termini, but the majority do not (Fig. 3 and data not shown).
FIG. 3.
Alignment of the C termini of TrbJ and TrbK proteins of selected conjugation systems. Sequence similarities between TrbJ and TrbK pairs were obtained using the MegAlign program (DNASTAR). Colons indicate conservative substitutions. R. leguminosarum, Rhizobium leguminosarum; A. rhizogenes, Agrobacterium rhizogenes; R. etli, Rhizobium etli; E. coli, Escherichia coli.
Since the last amino acid residues of TrbJ and TrbK, both tryptophans, are completely conserved not only in all Ti plasmid proteins but also in more distantly related proteins from other plasmids, it seemed plausible that these residues may play a crucial role in protein function. To test the functional importance of the similar C termini of TrbK and TrbJ, we deleted the last amino acid residue by using site-directed mutagenesis and tested the mutated proteins for their roles in entry exclusion. A truncated TrbK protein had a virtually null phenotype when expressed alone and had little if any synergistic effect when coexpressed with TrbJ (Table 6). The corresponding mutation on TrbJ also had a strong impact on the ability of the protein to mediate entry exclusion, though the mutant TrbJ still mediated a low level of exclusion when expressed together with wild-type TrbK (Table 6, 2nd, 3rd, 6th, 7th, 11th, and 12th lines). When both mutant proteins were expressed together in the cell, entry exclusion was negligible compared to that mediated by wild-type proteins (Table 6, last four lines). Overall, these results confirm the prediction that the C termini of the two proteins play a crucial role in entry exclusion.
TABLE 6.
Effects of deleting the C-terminal Trp residues of TrbJ and TrbKa
| pBBRMCS5 derivative (description or genotype) | pPZP200 derivative (description or genotype) | Conjugation efficiencyb (SD) | Exclusion coefficient |
|---|---|---|---|
| pHC012 (vector) | pUP200 (vector) | 2.62 (0.88) | 1 |
| pHC012 (vector) | pUP403 (Ptac-trbJ) | 0.81 (0.68) | 3.2 |
| pHC012 (vector) | pUP408 (Ptac-trbJ*) | 1.66 (0.51) | 1.6 |
| pHC012 (vector) | pUP404 (Ptac-trbK) | 0.26 (0.06) | 10.0 |
| pHC012 (vector) | pUP406 (Ptac-trbK*) | 1.55 (0.69) | 1.7 |
| pUP402 (Ptac-trbJ) | pUP200 (vector) | 0.35 (0.23) | 7.4 |
| pUP407 (Ptac-trbJ*) | pUP200 (vector) | 0.63 (0.22) | 4.2 |
| pHC368 (Ptac-trbK) | pUP200 (vector) | 0.31 (0.14) | 8.5 |
| pUP405 (Ptac-trbK*) | pUP200 (vector) | 1.58 (0.68) | 1.7 |
| pUP402 (Ptac-trbJ) | pUP406 (Ptac-trbK*) | 0.17 (0.07) | 15.2 |
| pUP407 (Ptac-trbJ*) | pUP404 (Ptac-trbK) | 0.09 (0.04) | 28.4 |
| pHC368 (Ptac-trbK) | pUP408 (Ptac-trbJ*) | 0.21 (0.08) | 12.7 |
| pUP405 (Ptac-trbK*) | pUP403 (Ptac-trbJ) | 0.44 (0.12) | 5.9 |
| pUP407 (Ptac-trbJ*) | pUP406 (Ptac-trbK*) | 0.52 (0.26) | 5.1 |
| pUP405 (Ptac-trbK*) | pUP408 (Ptac-trbJ*) | 1.36 (0.07) | 1.9 |
| pUP402 (Ptac-trbJ) | pUP404 (Ptac-trbK) | 0.018 (0.008) | 143.9 |
| pHC368 (Ptac-trbK) | pUP403 (Ptac-trbJ) | 0.055 (0.018) | 47.6 |
The donor strain in each experiment was WCF5(pCF218), which overexpresses TraR and has an octopine-type Ti plasmid marked with a Kmr determinant. Transconjugants were selected using the Kmr gene of the Ti plasmid, the Spr gene of pUP200 or its derivatives, and the Gmr gene of pHC012 or its derivatives. The symbol * denotes a deletion of the last residue of the corresponding protein.
Number of transconjugants per donor.
DISCUSSION
It is well established that strains lacking active TraR do not conjugate or conjugate at extremely low levels (6, 11, 21). We now show that such strains also do not express entry exclusion functions and therefore readily act as conjugative recipients of Ti plasmids. The fact that C58(pPZP201) and C58C1(pPZP201) were virtually identical in their abilities to receive a Ti plasmid, even though the former has a Ti plasmid while the latter lacks it, indicates that entry exclusion genes are tightly regulated. Strains containing conjugal plasmids but not expressing conjugation or entry exclusion functions are sometimes referred to as “female phenocopies” (22). Female phenocopies are generally detected after long-term culturing of a strain at stationary phase. In the case of A. tumefaciens, cultures that do not express active TraR are female phenocopies, even when actively growing, a consequence of the extremely tight regulation of the tra-trb regulon.
The finding that TrbJ and TrbK mediate entry exclusion was initially surprising. On the one hand, TrbJ and TrbK of RP4 have been described previously as mediating this property. However, as described above, there is considerable controversy about the role of TrbJ (8, 9, 15, 18). Furthermore, A. tumefaciens TrbK lacks a cysteine residue that is critical for the normal function of the RP4 protein, suggesting that trbK of A. tumefaciens may be a pseudogene. It seemed plausible that A. tumefaciens might not exhibit entry exclusion, as described above. Finally, it seemed counterintuitive for entry exclusion functions to be encoded within a tightly regulated operon. One may imagine a priori that exclusion genes may be needed even when the Tra-Trb regulon is not expressed, and it would seem a simple evolutionary step for these genes to be expressed constitutively.
As described above, pHC368, which expresses just TrbK, makes considerably more TrbK mRNA than pHC361, which expresses TrbJ and TrbK (Fig. 2). Despite this fact, the former plasmid expresses the entry exclusion phenotype more weakly than the latter. This finding highlights the importance of TrbJ in entry exclusion. However, pHC368 expresses entry exclusion more weakly than pHC361 even in the presence of a second plasmid expressing TrbJ (Table 5, lines for pHC368 and pHC361). The Ptac-trbJ fusions in pHC361, pUP402, and pUP403 are identical, making it unlikely that TrbJ is expressed at greatly different levels by these three plasmids. The most likely interpretation is that TrbJ and TrbK function more effectively when expressed in cis than in trans. An alternative interpretation is that TrbJ and TrbK interact and do so more effectively if expressed at the same location.
As noted earlier, we found a curious sequence similarity between the C termini of TrbJ and TrbK. Mature TrbK proteins are predicted to be quite small, approximately 50 amino acid residues in length, and the C-terminal 15 residues therefore constitute a rather large fraction of the entire protein. The C termini of TrbK proteins are also far more conserved than other parts of these proteins (data not shown), suggesting that the C-terminal residues may be crucial for protein function. In some cases, a TrbK protein from one plasmid may resemble TrbJ from the same plasmid more strongly than it resembles TrbK proteins from other plasmids (Fig. 3). This pattern suggests that the TrbJ and TrbK proteins encoded by a particular plasmid may coevolve by a process resembling gene conversion. In light of the overlapping functions of TrbJ and TrbK, it seemed tempting to speculate that the C termini of both proteins may play a crucial role in entry exclusion. In fact, the results of deleting the last amino acid residues of both proteins confirmed this hypothesis (Table 6). It may be noteworthy that the C-terminal five amino acid residues of TrbK of RP4 are essential for activity (8). Interestingly, our results show that TrbK protein cannot tolerate a truncation eliminating its last amino acid residue. However, TrbJ can still function in the presence of wild-type TrbK, albeit rather poorly. These results also suggest that these two proteins may interact for proper function, but this hypothesis remains to be tested.
The finding that a bacterium having a Ti plasmid but not expressing the Tra-Trb regulon is a female phenocopy may imply interesting ecological consequences. For example, one could imagine a situation in which two strains of A. tumefaciens, one containing an octopine-type Ti plasmid similar to pTiA6 and the other having a nopaline-type Ti plasmid similar to pTiC58, colonize the same crown gall tumor. One could imagine furthermore that there is an abundance of octopine but very little or no agrocinopines A and B (the conjugal opines for pTiC58). Conjugal opines are required for conjugation, as they are required for the transcription of both traR genes (6, 21). In such a scenario, the octopine-type Ti plasmid would both conjugate and block the entry of a nopaline-type Ti plasmid, while the nopaline-type Ti plasmid would do neither. If an octopine-type Ti plasmid conjugated into a strain already containing a nopaline-type Ti plasmid, the transconjugants would contain both Ti plasmids. These plasmids are incompatible at the level of DNA replication and would segregate into different daughter cells upon cell division. As a result, new combinations of host strains and Ti plasmids may appear. Thus, an active entry exclusion system would prevent the futile transfer of Ti plasmids between identical strains but would allow the reassortment of Ti plasmids and heterologous host strains even if those strains already contained heterologous Ti plasmids.
Acknowledgments
We thank the members of our laboratory for helpful discussions and for critical reviewing of the text.
This work was supported by a grant from the National Institute of General Medical Sciences (GM42893). U.M.P. acknowledges the financial support of the Brazilian government through a fellowship grant from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes).
Footnotes
Published ahead of print on 20 March 2009.
REFERENCES
- 1.Alt-Morbe, J., J. L. Stryker, C. Fuqua, P. L. Li, S. K. Farrand, and S. C. Winans. 1996. The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J. Bacteriol. 1784248-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho, H., and S. C. Winans. 2007. TraA, TraC and TraD autorepress two divergent quorum-regulated promoters near the transfer origin of the Ti plasmid of Agrobacterium tumefaciens. Mol. Microbiol. 631769-1782. [DOI] [PubMed] [Google Scholar]
- 3.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59451-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dessaux, Y., J. Tempe, and S. K. Farrand. 1987. Genetic analysis of mannityl opine catabolism in octopine-type Agrobacterium tumefaciens strain 15955. Mol. Gen. Genet. 208301-308. [DOI] [PubMed] [Google Scholar]
- 5.Farrand, S. K., I. Hwang, and D. M. Cook. 1996. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J. Bacteriol. 1784233-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 1762796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez, J. E., and M. M. Marketon. 2003. Quorum sensing in nitrogen-fixing rhizobia. Microbiol. Mol. Biol. Rev. 67574-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haase, J., M. Kalkum, and E. Lanka. 1996. TrbK, a small cytoplasmic membrane lipoprotein, functions in entry exclusion of the IncPα plasmid RP4. J. Bacteriol. 1786720-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase, J., R. Lurz, A. M. Grahn, D. H. Bamford, and E. Lanka. 1995. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J. Bacteriol. 1774779-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajdukiewicz, P., Z. Svab, and P. Maliga. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25989-994. [DOI] [PubMed] [Google Scholar]
- 11.Hwang, I., P. L. Li, L. Zhang, K. R. Piper, D. M. Cook, M. E. Tate, and S. K. Farrand. 1994. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc. Natl. Acad. Sci. USA 914639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalogeraki, V. S., and S. C. Winans. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 18869-75. [DOI] [PubMed] [Google Scholar]
- 13.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109137-141. [DOI] [PubMed] [Google Scholar]
- 14.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166175-176. [DOI] [PubMed] [Google Scholar]
- 15.Lessl, M., V. Krishnapillai, and W. Schilf. 1991. Identification and characterization of two entry exclusion genes of the promiscuous IncP plasmid R18. Mol. Gen. Genet. 227120-126. [DOI] [PubMed] [Google Scholar]
- 16.Li, P. L., and S. K. Farrand. 2000. The replicator of the nopaline-type Ti plasmid pTiC58 is a member of the repABC family and is influenced by the TraR-dependent quorum-sensing regulatory system. J. Bacteriol. 182179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, P. L., I. Hwang, H. Miyagi, H. True, and S. K. Farrand. 1999. Essential components of the Ti plasmid trb system, a type IV macromolecular transporter. J. Bacteriol. 1815033-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyras, D., A. W. Chan, J. McFarlane, and V. A. Stanisich. 1994. The surface exclusion system of RP1: investigation of the roles of trbJ and trbK in the surface exclusion, transfer, and slow-growth phenotypes. Plasmid 32254-261. [DOI] [PubMed] [Google Scholar]
- 19.Pappas, K. M., and S. C. Winans. 2003. A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol. Microbiol. 481059-1073. [DOI] [PubMed] [Google Scholar]
- 20.Peng, W. T., L. M. Banta, T. C. Charles, and E. W. Nester. 2001. The chvH locus of Agrobacterium encodes a homologue of an elongation factor involved in protein synthesis. J. Bacteriol. 18336-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piper, K. R., S. Beck von Bodman, and S. K. Farrand. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362448-450. [DOI] [PubMed] [Google Scholar]
- 22.Press, R., N. Glansdorff, P. Miner, J. De Vries, R. Kadner, and W. K. Maas. 1971. Isolation of transducing particles of phi-80 bacteriophage that carry different regions of the Escherichia coli genome. Proc. Natl. Acad. Sci. USA 68795-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., and D. W. Russel. 2001. Molecular cloning: a laboratory manual, 3rd ed., vol. 1-3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Sukupolvi, S., and C. D. O'Connor. 1990. TraT lipoprotein, a plasmid-specified mediator of interactions between gram-negative bacteria and their environment. Microbiol. Rev. 54331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Tempe, J., A. Petit, M. Holsters, M. Van Montagu, and J. Schell. 1977. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: possible relation to transformation in crown gall. Proc. Natl. Acad. Sci. USA 742848-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu, J., P. M. Oger, B. Schrammeijer, P. J. Hooykaas, S. K. Farrand, and S. C. Winans. 2000. The bases of crown gall tumorigenesis. J. Bacteriol. 1823885-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu, J., and S. C. Winans. 2001. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc. Natl. Acad. Sci. USA 981507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]