Abstract
Staphylococcus aureus is an opportunistic intracellular organism. Although they poorly accumulate in eukaryotic cells, β-lactams show activity against intracellular methicillin (meticillin)-susceptible S. aureus (MSSA) if the exposure times and the drug concentrations are sufficient. Intraphagocytic methicillin-resistant S. aureus (MRSA) strains are susceptible to penicillins and carbapenems because the acidic pH favors the acylation of PBP 2a by these β-lactams through pH-induced conformational changes. The intracellular activity (THP-1 macrophages and keratinocytes) of ceftobiprole, which shows almost similar in vitro activities against MRSA and MSSA in broth, was examined against a panel of hospital-acquired and community-acquired MRSA strains (MICs, 0.5 to 2.0 mg/liter at pH 7.4 and 0.25 to 1.0 mg/liter at pH 5.5) and was compared with its activity against MSSA isolates. The key pharmacological descriptors {relative maximal efficacy (Emax), relative potency (the concentration causing a reduction of the inoculum halfway between E0 and Emax [EC50]), and static concentration (Cs)} were measured. All strains showed sigmoidal dose-responses, with Emax being about a 1 log10 CFU decrease from the postphagocytosis inoculum, and EC50 and Cs being 0.2 to 0.3× and 0.6 to 0.9× the MIC, respectively. Ceftobiprole effectively competed with Bocillin FL (a fluorescent derivative of penicillin V) for binding to PBP 2a at both pH 5.5 and pH 7.4. In contrast, cephalexin, cefuroxime, cefoxitin, or ceftriaxone (i) were less potent in PBP 2a competitive binding assays, (ii) showed only partial restoration of the activity against MRSA in broth at acidic pH, and (iii) were collectively less effective against MRSA in THP-1 macrophages and were ineffective in keratinocytes. The improved activity of ceftobiprole toward intracellular MRSA compared with the activities of conventional cephalosporins can be explained, at least in part, by its greater ability to bind to PBP 2a not only at neutral but also at acidic pH.
Restricted to the hospital setting for many years, the methicillin (meticillin)-resistant Staphylococcus aureus (MRSA) epidemic is now reaching an increasing variety of other environments (12), such as patients in the community in various parts of the world (16, 35, 41) and animals (21, 40). Beyond its spectacular ability to adapt and to develop resistance to most antimicrobial agents (9), including drugs of last resort, such as vancomycin, linezolid, and daptomycin (5, 28, 31), the capacity of S. aureus to invade, sojourn, and thrive intracellularly (8, 23, 34) creates an additional challenge since intracellular forms tend to be poorly susceptible to most available antibiotics (38). Evaluations of new antistaphylococcal agents directed against resistant S. aureus strains must therefore include an assessment of their ability to control intracellular infections. While animal models of staphylococcal infection are being developed (32), models of cultured cells remain useful because they offer the possibility to explore in detail the pharmacological parameters governing the response of the intracellular bacteria to the drug in the absence of host factors (7, 38). In this context, we observed that, contrary to most original assumptions (36), the poor accumulation of β-lactams in phagocytic cells does not preclude the observation of significant activity against intracellular methicillin-susceptible S. aureus (MSSA). This is actually dependent on the time of exposure (12 to 24 h) and whether the extracellular concentration is maintained at a sufficiently large but still clinically pertinent level (7, 20). We previously reported that intraphagocytic MRSA isolates regain almost full susceptibility to penicillins and carbapenems, due to the acidic pH prevailing in phagolysosomes (19). This finding has been rationalized by the observation that acidic pH improves the accessibility to and the acylation of PBP 2a by penicillins within a time frame relevant to the growth rate of MRSA through protein conformational changes (17). This triggered us to study ceftobiprole in this context. Ceftobiprole, also known as BAL9141 and Ro 63-9141 (4), is the first clinically developed cephalosporin that shows almost similar activities against MRSA and MSSA isolates in conventional in vitro tests (15, 42). It has now been approved for clinical use in some countries and has been studied in a large array of preclinical and clinical settings (see references 3 and 43 for recent reviews). Cephalosporins active against MRSA are characterized by the presence of a bulky hydrophobic moiety in position 3 (27) (vinyl pyrrolidinone in the case of ceftobiprole; see the supplemental material for the structural formula), which increases interactions with PBP 2a and induces conformational changes that render the protein more susceptible to acylation by drugs, even at neutral pH (11, 22, 39). In the present study, the intracellular activity of ceftobiprole was examined against a panel of hospital-acquired and community-acquired MRSA strains. We then studied its activity against MRSA and MSSA strains in broth and its properties of binding to PBP 2a at neutral and acidic pHs and compared its activity and binding with those of selected conventional cephalosporins approved for use for the treatment of staphylococcal infections. We also compared the activity of ceftobiprole to the activities of conventional cephalosporins against intracellular MSSA and MRSA in THP-1 macrophages as a model of phagocytic cells, which are known to harbor persisting S. aureus cells for long periods and to help disseminate the organism (1, 23), and in keratinocytes, which may also host S. aureus (24) and which is a model pertinent for the use (and current approval) of ceftobiprole for complicated skin and soft tissue infections (10).
MATERIALS AND METHODS
Antibiotics, purified PBP 2a, and other main reagents.
Ceftobiprole (the active form of the compound used clinically [ceftobiprole medocaril]) was obtained as the microbiological standard from Johnson & Johnson Pharmaceutical Research & Development, Raritan, NJ. The comparators, chosen to represent narrow-spectrum, expanded-spectrum, and broad-spectrum cephalosporins (cephalexin, cefuroxime, and ceftriaxone, respectively) and cephamycins (cefoxitin), were obtained from Sigma-Aldrich, St. Louis, MO, or Teva Pharma, Wilrijk, Belgium. Gentamicin, used to control extracellular growth in the absence of another antibiotic (7), was obtained as Geomycin (distributed in Belgium by Glaxo-SmithKline SA, Genval, Belgium); Bocillin FL (a fluorescent derivative of penicillin V [13]) was obtained from Invitrogen Corp., Carlsbad, CA. Staphylococcal PBP 2a (soluble form) was obtained from Escherichia coli Rosetta 2(DE3) transformed with plasmid pET28a carrying the truncated PBP 2a-coding sequence (ΔM1-Y23 PBP 2a). The protein was purified on an S-Sepharose HP column and then on a phenyl-Sepharose column (Amersham plc, Little Chalfont, United Kingdom), and the purity of the working sample was assessed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) with Coomassie blue staining and fluorescence visualization (14). Additional details on the production and purification procedure are given in the supplemental material. Cell culture medium and serum were from Invitrogen or Becton Dickinson, Franklin Lakes, NJ. Unless stated otherwise, all other reagents were obtained from Merck AG (Darmstadt, Germany) or Sigma-Aldrich.
Cell lines.
Experiments were performed with (i) THP-1 cells (ATCC TIB-202; American Type Culture Collection, Manassas, VA), a human myelomonocytic cell line that displays macrophage-like activity and that is maintained in our laboratory, as described previously (7), and (ii) human skin keratinocytes, obtained as primary human keratinocytes (catalog no. 12332-011; Gibco, Invitrogen Corporation, Invitrogen SA, Merelbeke, Belgium) and cultivated in defined keratinocyte-serum-free medium (Invitrogen, Carlsbad, CA).
Bacterial strains and susceptibility testing.
The strains used in this study, their main characteristics, and their origins are listed in Table 1. They were obtained from the American Type Culture Collection, the Network on Antimicrobial Resistance in Staphylococcus aureus (operated by Eurofins Medinet, Inc., Herndon, VA; supported under NIAID/NIH contract no. HHSN2722007 00055C), or clinical collections. MICs were measured by the microdilution method, as described earlier (19). For assays performed at a specified pH, the broth was adjusted to that pH prior to inoculation, and we checked that this pH had been maintained at its original value (±0.1 pH unit) at the end of the experiment. When S. aureus was tested at pH 5, it grew more slowly than it did at higher pHs, but it grew sufficiently to allow the accurate determination of the MIC. The MRSA phenotype of each strain was confirmed by detection of mecA by PCR (19) and the staphylococcal chromosome cassette mec (SCCmec) subgroup of most of the strains was established as described previously (18).
TABLE 1.
Characteristics of strains used in this study
| Phenotypea and strain no. | Origin | SCCmec group | MIC (mg/liter)
|
|
|---|---|---|---|---|
| pH 7.4 | pH 5.5 | |||
| MSSA | ||||
| ATCC 25923 (β-lactamase −) | Laboratory standardb | NAg | 0.5 | 0.25 |
| ATCC 11632 (β-lactamase +) | Laboratory standardb | NA | 0.5-1 | 0.25-0.5 |
| NRS52 (VISA) | Clinical (bile infection)c | NA | 1 | 0.5 |
| Geometric mean | 0.72 | 0.36 | ||
| HA-MRSA | ||||
| ATCC 33591 (inducible) | Laboratory standardb | III | 2 | 0.5 |
| ATCC 33592 | Laboratory standardb | NDh | 2 | 0.5-1 |
| ATCC 43300 | Laboratory standardb | ND | 1 | 0.5 |
| N4120210 | Clinical (wound infection)d | I | 2 | 1 |
| N4112910 | Clinical (nasal swab)d | ND | 0.5-1 | 0.25-0.5 |
| N4120032 | Clinical (urinary tract infection)d | ND | 2 | 0.5 |
| NRS18 (VISA) | Clinical (wound, skin and soft tissue infection)-c | II | 0.5-1 | 0.5 |
| NRS126 (VISA) | Clinical (bloodstream infection)c | II | 1-2 | 0.25-0.5 |
| VRS1 (VRSA) | Clinical (catheter exit site)c | II | 2 | 0.5 |
| VRS2 (VRSA) | Clinical (wound infection)c | II | 1-2 | 0.5 |
| Geometric mean | 1.45 | 0.43 | ||
| CA-MRSA | ||||
| NRS192 (PVL +) | Clinical (pneumonia, septic arthritis)c | IVa | 2 | 1 |
| NRS384 (PVL +) | Clinical (wound, skin and soft tissue infection)c | IVa | 2 | 1 |
| N4090440 (PVL +) | Clinical (wound infection)d | IVa | 1 | 0.5 |
| N4042228 (PVL +) | Clinical (septicemia sec. to soft-tissues abscess)d | IVa | 1 | 0.5 |
| STA 44 (PVL +) | Clinicale | V | 2 | 1 |
| STA 268 (PVL +) | Clinicale | V | 2 | 1 |
| CHU (PVL +) | Clinicale | V | 2 | 1 |
| MEH2225605 (PVL +) | Clinicalf | IVa | 2 | 1 |
| NRS386 (PVL −) | Clinical (bloodstream infection)c | IVa | 2 | 0.5-1 |
| Geometric mean | 1.71 | 0.83 | ||
VISA, vancomycin intermediate S. aureus (MICs, >2 and <8 mg/liter); VRSA, vancomycin-resistant S. aureus (MICs, >8 mg/liter); PVL, Panton-Valentine leucocidin; −, negative; +, positive.
From the American Type Culture Collection.
From the Network on Antimicrobial Resistance in Staphylococcus aureus (operated by Eurofins Medinet, Inc., Herndon, VA).
Clinical collection (Y. Glupczynski, Cliniques Universitaires UCL de Mont-Godinne, Yvoir, Belgium).
Clinical collection (Y. C. Huang, Chang Gung Children's Hospital, Taiwan).
Clinical collection (L. Y. Hsu, Singapore General Hospital, Singapore).
NA, not applicable.
ND, not determined.
Cells, cell infection, and assessment of intracellular activity of antibiotics.
Cell infection was performed exactly as described previously (7, 18, 19), and the postphagocytosis inoculum was set at 1.5 × 106 to 3.0 × 106 CFU per mg of cell protein. For both cell types, the intracellular growth of S. aureus within 24 h in the presence of gentamicin at an extracellular concentration of 0.5× its MIC in broth (to fully prevent extracellular growth [7, 33]) was about 1 log10 CFU/mg protein. The changes in CFU from the CFU of the postphagocytosis inoculum was taken as the response to the antibiotics and was plotted as a function of the antibiotic extracellular concentration. As shown earlier (7, 20), a sigmoidal function (Hill function) can be fitted to the data if both coordinates are subjected to logarithmic transformation. The use of logarithmic transformation for concentrations is in line with what is commonly used to describe pharmacological dose-responses when the doses span several orders of magnitude, as is the case here. The change in CFU also needs to be treated logarithmically because chemotherapeutic responses, unlike enzyme inhibition, for instance, progress by fractional and not constant changes upon finite increases in the drug concentration.
PBP 2a binding of ceftobiprole and other cephalosporins.
The PBP 2a binding of ceftobiprole and the other cephalosporins tested was assessed by countermarking experiments by following the general method described for 3H-labeled penicillin G (29, 37) but by using Bocillin FL (a boron-dipyrromethene [bodipy] derivative of penicillin V [13]) as the reporter antibiotic. The purified PBP 2a was incubated at 37°C for 25 min with increasing concentrations of the cephalosporins under study, after which 100 μM Bocillin FL was added to the samples for an additional 25 min at 37°C. The reaction was terminated by the addition of SDS-loading buffer, and the samples were then subjected to SDS-PAGE. Following electrophoresis, Western blotting was performed with an anti-rabbit monoclonal antibody directed against the bodipy moiety of Bocillin FL (antibodipy primary antibody [1/500; catalog no. A5770; Invitrogen, Carlsbad, CA]) and goat anti-rabbit immunoglobulin G labeled with horseradish peroxidase (catalog no. 65-6120; Invitrogen [Zymed, Carlsbad, CA]; this method has been validated against the conventional assay by using the fluorescent properties of Bocillin FL [17]). Bands were revealed with the SuperSignal West Pico chemiluminescence substrate (Pierce, Rockford, IL), and scanned films were subjected to densitometric analysis (Image J software, version 1.3.1; available from the Research Service Branch of the National Institute of Mental Health at http://rsb.info.nih.gov/ij).
Statistical analyses.
Curve-fitting analyses were performed with Prism (version 4.02) software for Windows and statistical analyses with Instat (version 3.06) software (GraphPad Prism Software, San Diego, CA).
RESULTS
Strains and susceptibility to ceftobiprole at neutral and acidic pHs in broth.
Table 1 shows that the MICs of the strains used in this study ranged from 0.5 to 1, 0.5 to 2, and 1 to 2 mg/liter for MSSA, hospital-acquired MRSA (HA-MRSA), community-acquired MRSA (CA-MRSA) strains when they were tested in broth. With the exception of strain NRS18, the MICs were globally 1 twofold dilution (MSSA, CA-MRSA) to 2 twofold dilutions (HA-MRSA) lower when they were measured at pH 5.5.
Susceptibilities of intracellular (THP-1 macrophages) MSSA, HA-MRSA, and CA-MRSA strains to ceftobiprole.
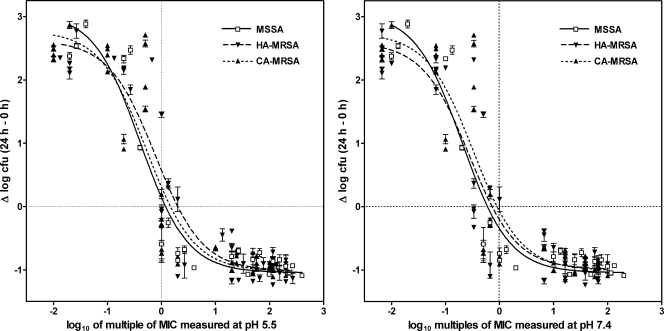
In a first series of experiments, 24-h dose-responses studies were performed with 15 strains chosen from among the isolates listed in Table 1 (MSSA, n = 3; HA-MRSA, n = 6; CA-MRSA, n = 6) and with a wide range of extracellular concentrations of antibiotics. The data were used to fit sigmoidal functions (Hill's equation [see reference 7 for details]), yielding for each strain the values of the two key pharmacological descriptors of antibiotic activity defined previously (7), namely, the relative maximal efficacy (Emax) and the relative potency (the concentration causing a reduction of the inoculum halfway between E0 and Emax [EC50]) of ceftobiprole, together with an important characteristic of the model, that is, bacterial growth in the absence of antibiotic [E0]). The data were plotted as multiples of the MIC to allow comparison of the activity of ceftobiprole at equipotent concentrations. As shown in the supplemental material, sigmoid dose-responses were obtained for each strain, and the values of Emax (∼1 log10 CFU decrease), EC50 (close to the corresponding MICs, as determined in broth at pH 5.5), and E0 (∼2.5 log10 CFU increase) were similar or closely similar. The data for all strains were then pooled by phenotype (MSSA, HA-MRSA, or CA-MRSA), and the results are graphically shown in Fig. 1 (with numerical data and the results of the statistical analyses presented in Table 2). This shows that ceftobiprole globally exerted similar activity against all strains, regardless of their resistance phenotypes, although its relative potency was slightly, albeit statistically significantly, higher (lower EC50) against MSSA than against HA-MRSA and, to some extent, CA-MRSA.
FIG. 1.
Dose-response curves of ceftobiprole against MSSA (strains ATCC 25293, ATCC 11632, and NRS52), HA-MRSA (strains ATCC 33591, ATCC 33592, ATCC 433000, NRS18, NRS126, and VRS1), and CA-MRSA (strains NRS192, N4090440, N4042228, STA44, STA228, and MEH22256-05) phagocytized by human THP-1 macrophages after 24 h of incubation of the cells in the presence of increasing concentrations of the antibiotic. The ordinate shows the change in the number of CFU (means ± standard deviations; n = 3; several standard deviation bars are smaller than the symbols) per mg of cell protein. The abscissa is the multiple of the MIC (in log10 units) obtained for each strain when it was tested in broth at pH 5.5 or pH 7.4 (for strains for which different MICs were obtained [Table 1], calculations were made on the basis of the means of these values). Data from dose-response experiments performed with each strain (see the supplemental material) of each of the three phenotypes indicated above were pooled and used to fit one single sigmoidal function. The equation used, the goodness of fit, the pertinent pharmacological descriptors, and a statistical analysis of their differences (between groups and between mode of plots) are shown in Table 2.
TABLE 2.
Pharmacological descriptors, goodness of fit, and statistical analysis of studies of the dose-response of ceftobiprole against MSSA, HA-MRSA, and CA-MRSA strainsa
| Data plot function and resistance pattern | Pharmacological descriptorb
|
Goodness of fit (R2) | |||
|---|---|---|---|---|---|
| E0c (95% CId) | Emaxe (95% CI) | EC50f (95% CI) | Csg | ||
| Data plotted as a function of the MIC measured at pH 5.5 | |||||
| MSSA | 3.06 (2.49 to 3.64) AC,a | −1.04 (−1.27 to −0.81) A,a | 0.41 (0.23 to 0.70) AC,a | 1.19 | 0.93 |
| HA-MRSA | 2.63 (2.27 to 2.98) B,a | −1.01 (−1.20 to −0.82) A,a | 0.77 (0.51 to 1.17) B,a | 1.97 | 0.91 |
| CA-MRSA | 2.78 (2.34 to 3.211) BC,a | −1.02 (−1.27 to −0.77) A,a | 0.54 (0.33 to 0.88) C,a | 1.45 | 0.87 |
| Data plotted as a function of the MIC measured at pH 7.4 | |||||
| MSSA | 3.06 (2.49 to 3.64) AC,a | −1.04 (−1.27 to −0.81) A,a | 0.20 (0.12 to 0.35) A,b | 0.60 | 0.92 |
| HA-MRSA | 2.60 (2.19 to 3.01) B,a | −0.99 (−1.21 to −0.77) A,a | 0.28 (0.17 to 0.45) AB,b | 0.74 | 0.88 |
| CA-MRSA | 2.74 (2.27 to 3.21) BC,a | −1.01 (−1.29 to −0.73) A,a | 0.33 (0.19 to 0.56) BC,b | 0.91 | 0.84 |
Data are for 24 h of incubation and are from Fig. 1.
The equation for the sigmoidal dose-response is as follows: 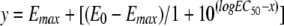 , where x is the concentration (in mg/liter). Statistical analyses were performed as follows: (i) data for the parameters (E0, Emax, and EC50) between MSSA, HA-MRSA, and CA-MRSA for pH 5.5 (upper half of table) or 7.4 (lower half of table) were compared by one-way analysis of variance with the Tukey-Kramer multiple-comparisons test (data with different uppercase letters were significantly different from each other [P < 0.05]) and (ii) data for the same parameters but between values observed at pH 5.5 (upper half of table) and with those observed at pH 7.4 (lower half of table) were compared by unpaired t test, two tailed (data with different lowercase letters are significantly different from each other [P<0.05]).
, where x is the concentration (in mg/liter). Statistical analyses were performed as follows: (i) data for the parameters (E0, Emax, and EC50) between MSSA, HA-MRSA, and CA-MRSA for pH 5.5 (upper half of table) or 7.4 (lower half of table) were compared by one-way analysis of variance with the Tukey-Kramer multiple-comparisons test (data with different uppercase letters were significantly different from each other [P < 0.05]) and (ii) data for the same parameters but between values observed at pH 5.5 (upper half of table) and with those observed at pH 7.4 (lower half of table) were compared by unpaired t test, two tailed (data with different lowercase letters are significantly different from each other [P<0.05]).
Change in log10 CFU per mg of cell protein from the original postphagocytosis inoculum for an infinitely low ceftobiprole extracellular concentration.
CI, confidence interval.
Change in log10 CFU per mg of cell protein from the original postphagocytosis inoculum for an infinitely large ceftobiprole extracellular concentration.
Ceftobiprole concentration (in multiples of the MIC [Table 1; for strains for which two different values were obtained, the mean value was used]) giving a response halfway between E0 and Emax.
Apparent static concentration (in multiples of the MIC [Table 1; for strains for which two different values were obtained, the mean value was used]), as determined by graphical intrapolation of the corresponding function.
Susceptibilities of MSSA and MRSA to conventional cephalosporins compared with that to ceftobiprole at neutral and acidic pH in broth and after phagocytosis by THP-1 macrophages and keratinocytes.
We previously found that acidic pH allowed the almost complete recovery of the activities of penicillins and carbapenems against MRSA strains when they were tested at acidic pH (pH 5.5) in broth or after phagocytosis by macrophages or keratinocytes (18, 19). This was therefore investigated in the present study with conventional cephalosporins, and the results were compared with those obtained with ceftobiprole.
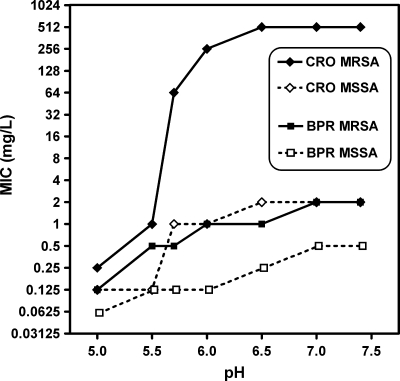
Table 3 shows that acidic pH (pH 5.5) also markedly reduced the MICs of conventional cephalosporins for MRSA in broth but that the MIC of ceftobiprole remained the lowest of all drugs tested at that pH. To ensure that this was not related to differences in the thresholds at which a significant change in activity would occur, full pH dependence curves were made over the pH 5 to pH 7.5 range. These showed an abrupt decrease in the MICs of conventional cephalosporins between pH 6.5 and pH 5.5 for MRSA but a shallow decrease in the MICs ceftobiprole over the whole pH range that paralleled that seen for all drugs when their activities against MSSA were tested (see a typical example for the results for ceftriaxone versus those for ceftobiprole in Fig. 2; see Fig. SP2 in the supplemental material for data for each individual cephalosporin tested).
TABLE 3.
MICs of cephalosporins against selected MSSA and MRSA strains at pH 7.4 and 5.5 in broth and pH-induced decreases in MICs
| Cephalosporin | MSSA ATCC 25923
|
MRSA ATCC 33591
|
Decrease in MRSA MIC/MSSA MIC ratio | MRSA MIC/MSSA MIC ratio at pH 5.5 | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter)
|
pH-induced MIC decrease (fold) | MIC (mg/liter)
|
pH-induced MIC decrease (fold) | |||||
| pH 7.4 | pH 5.5 | pH 7.4 | pH 5.5 | |||||
| Cephalexin | 2 | 1 | 2 | 256 | 16 | 16 | 8 | 16 |
| Cefuroxime | 2 | 0.125 | 16 | 128 | 1 | 128 | 8 | 4 |
| Cefoxitin | 1 | 0.25 | 4 | 512 | 1 | 512 | 128 | 4 |
| Ceftriaxone | 2 | 0.125 | 16 | 512 | 1 | 512 | 32 | 4 |
| Ceftobiprole | 0.5 | 0.25 | 2 | 2 | 0.5 | 4 | 2 | 2 |
FIG. 2.
Influence of pH on MICs of ceftobiprole (BPR) and ceftriaxone (CRO) for MSSA ATCC 25923 and MRSA ATCC 33591, as measured in broth.
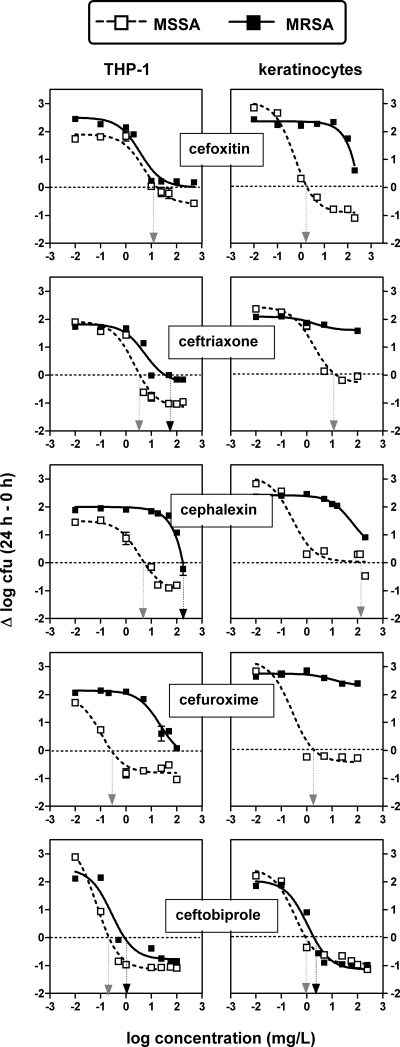
The antibacterial activity of ceftobiprole was then tested against the intracellular forms of MSSA (ATCC 25923) and MRSA (ATCC 33591) phagocytized by professional phagocytes (THP-1 macrophages) and nonprofessional phagocytes (skin keratinocytes) and was compared with the activities of conventional cephalosporins. The results are shown graphically in Fig. 3, with numerical data and the results of the statistical analyses presented in Table 4. For THP-1 macrophages and MSSA, all cephalosporins showed similar or nearly similar relative efficacies (Emaxs) and 50% effective concentrations (EC50s). Moreover, all Cs values were within the limits of the concentrations clinically achievable in the serum of humans. Against MRSA, cefoxitin and, to a lesser extent, ceftriaxone showed activities roughly similar to those obtained against MSSA, whereas cephalexin and cefuroxime were largely ineffective. In MSSA-infected keratinocytes, only cefoxitin and ceftobiprole showed activities similar to those observed in THP-1 macrophages. For MRSA, ceftobiprole was the only effective antibiotic (and had activity against MRSA similar to that against MSSA), whereas none of the conventional cephalosporins could prevent the intracellular growth of the bacteria.
FIG. 3.
Dose-response curves of cephalosporins against MSSA (ATCC 25293) and MRSA (ATCC 33591) phagocytized by human THP-1 macrophages or skin keratinocytes after 24 h of incubation of the cells in the presence of increasing concentrations of the antibiotics. The ordinate shows the change in the number of CFU (means ± standard deviations; n = 3; several standard deviation bars are smaller than the symbols) per mg of cell protein. The vertical arrows point to the Cs for each condition (gray arrow, MSSA; black arrow, MRSA). The goodness of fit, the pertinent pharmacological descriptors, and a statistical analysis of their differences (between antibiotics and between MSSA and MRSA) are shown in Table 4.
TABLE 4.
Pharmacological descriptors, goodness of fit, and statistical analysis of the dose-response studies of cephalosporins against MSSA and MRSAa
| Cell line and antibioticb | MSSA ATCC 25923
|
MRSA ATCC 33591
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E0c (95% CId) | Emaxe (95% CI) | EC50f (95% CI) | Csg | R2 | E0 (95% CI) | Emax (95% CI) | EC50 (95% CI) | Cs | R2 | |
| THP-1 macrophages | ||||||||||
| FOX | 1.90 (1.47-2.33) ad,A | −0.59 (−1.13-−0.06) ac,A | 5.30 (1.47-19.1) | 16.4 | 0.971 | 2.50 (1.98-3.01) abc,B | −0.001 (−0.49-0.49) a,B | 3.64 (1.10 to 12.0) | >200 | 0.951 |
| CRO | 1.91 (1.30-2.53) a,A | −1.17 (−1.67-−0.67) b,A | 2.13 (0.70-6.46) | 0.26 | 0.956 | 1.82 (1.35-2.30) c,A | −0.28 (−0.79-0.23) ab,B | 5.45 (1.44 to 20.7) | 35.5 | 0.934 |
| CFX | 1.50 (1.17-1.84) ad,A | −1.00 (−1.39-−0.61) ab,A | 3.49 (1.25-9.71) | 5.50 | 0.984 | 1.99 (1.75-2.23) ac,B | (1)h B | (1) | ∼ 170 | 0.955 |
| CXM | 1.97 (1.02-2.92) ad,A | −0.80 (−1.14-−0.47) abce,A | 0.11 (0.03-0.43) | 0.26 | 0.959 | 2.14 (1.90-2.38) ac,A | (1) B | (1) | >100 | 0.968 |
| BPR | 3.49 (2.84-4.14) c,A | −1.15 (−1.34-−0.95) b,A | 0.07 (0.04-0.12) | 0.21 | 0.991 | 2.47 (1.26-3.68) b,A | −0.78 (−1.37-−0.20) c,B | 0.27 (0.05 to 1.43) | 0.89 | 0.939 |
| Keratinocytes | ||||||||||
| FOX | 3.06 (2.530-3.588) bc,A | −0.91 (−1.24-−0.59) ae,A | 0.55 (0.27-1.13) | 1.84 | 0.989 | 2.36 (2.12-2.61) abc,B | (1) B | (1) | >200 | 0.946 |
| CRO | 2.44 (1.75-3.13) bc,A | −0.30 (−1.03-0.43) ce,A | 1.83 (0.39-8.52) | 14.8 | 0.967 | 2.09 (1.86-2.33) ac,A | (2)i B | (2) | >200 | 0.947 |
| CFX | 3.09 (1.82-4.36) bc,A | 0.035 (−0.57-0.64) d,A | 0.27 (0.03-2.19) | ∼ 200 | 0.928 | 2.42 (2.33-2.50) b,A | (1) B | (1) | ∼ 200 | 0.993 |
| CXM | 3.23 (1.52-4.93) bc,A | −0.42 (−1.37-0.52) e,A | 0.27 (0.02-2.96) | 0.26 | 0.943 | 2.75 (2.51-2.99) b,A | (1) B | (1) | ∼ 200 | 0.773 |
| BPR | 2.48 (1.82-3.14) be,A | −0.94 (−1.25-−0.63) ab,A | 0.35 (0.13-0.95) | 0.91 | 0.975 | 2.04 (1.44-2.65) ac,A | −1.14 (−1.60-−0.69) c,A | 1.08 (0.42 to 2.77) | 1.95 | 0.964 |
Data are from Fig. 4 for 24 h of incubation. See footnote b of Table 2 for the equation used for modeling. Statistical analysis was as follows: for analysis of the data in each column (one-way analysis of variance with the Tukey test for multiple comparisons), data with different lowercase letters are significantly different from each other (P < 0.01); for analysis of the data in each row (unpaired, two-tailed t test between corresponding parameters for MSSA and MRSA), data with different uppercase letters are significantly different from each other (P < 0.01). No statistical analysis was performed for the parameters EC50 and Cs, as these are related to weight concentrations that cannot be directly compared between antibiotics (see Discussion for the correlation with clinically achievable concentrations in serum).
FOX, cefoxitine; CRO, ceftriaxone; CFX, cephalexin; CXM, cefuroxime; BPR, ceftobiprole. See Table 3 for the MICs.
CFU increase (in log10 units) at 24 h from the corresponding original inoculum, as extrapolated for an infinitely low concentration of cephalosporin.
CI, confidence interval.
CFU decrease (in log10 units) at 24 h from the corresponding original inoculum, as extrapolated for the antibiotic concentration at an infinitely high concentration.
Concentration (mg/liter; total drug) causing a reduction of the inoculum halfway between E0 and Emax, as obtained from the Hill equation (by using a slope factor of 1).
Concentration (mg/liter; total drug) resulting in no apparent bacterial growth (the number of CFU was identical to that of the original inoculum), as determined by graphical interpolation.
(1), no meaningful calculation was possible since data points were obtained for the upper part of the sigmoidal function only.
(2), since there was only a minimal decrease in CFU within the limits of the experiment, the Emax, EC50, and Cs descriptors, as calculated from the Hill equation, become meaningless in the context of antimicrobial activity.
Influence of pH on the binding of ceftobiprole and conventional cephalosporins to PBP 2a.
To determine the influence of pH on the binding of ceftobiprole and conventional cephalosporins to PBP 2a, we looked for the impairment of Bocillin FL binding to PBP 2a after exposure to cephalosporins. We first characterized our system by running experiments in which increasing concentrations of ceftobiprole were added before countermarking was done with a fixed concentration of Bocillin FL (100 μM) at pH 5.5 and 7.4. Ceftobiprole exerted a marked impairment of Bocillin FL binding and had 50% inhibitory concentrations of about 8 μM at pH 7.4 and 1.9 μM at pH 5.5. Conventional cephalosporins were less effective (25 to 50% impairment only at 10 μM, but also with an enhancement at pH 5.5; however, this was not consistently seen for all cephalosporins) (see Fig. SP3 in the supplemental material).
DISCUSSION
The present work extends our knowledge concerning the activities of β-lactams against intracellular S. aureus isolates in two main directions. First, we confirmed that cephalosporins have limited although significant activity against MSSA in THP-1 macrophages, as was previously found for penicillins and carbapenems (7, 20). Second, we showed that the intracellular activity of ceftobiprole is only very modestly affected by the methicillin resistance phenotype (MSSA versus MRSA; differences in MICs of about 1 to 2 log2 dilutions persist, however, between the two types of strains). This is not the case for the other cephalosporins tested, especially when experiments are conducted with keratinocytes. This lower level of activity or even a lack of activity of conventional cephalosporins against intracellular MRSA is surprising at first glance. Earlier studies indeed show that acidic pH favors the activities of penicillins and carbapenems not only in broth (19, 30) but also in cells (in THP-1 macrophages and keratinocytes) to the point of making them equally active against MRSA and MSSA (18, 19).
Restoration of the susceptibility of MRSA to β-lactams has been ascribed to a conformational change in PBP 2a consistent with the opening of its active site from a closed conformation when it is exposed to these antibiotics at acidic pH (17). Although acidic pH also improves the activities of conventional cephalosporins against MRSA, (i) these molecules actually poorly compete with Bocillin FL (the microbiologically active part of which is penicillin V) for binding to PBP 2a, and (ii) at least one of them (cephalexin) keeps fairly elevated MICs for MRSA compared to those for MSSA at pH 5.5. This strongly suggests that conventional cephalosporins are collectively less able than penicillins or carbapenems to cooperate with acidic pH to induce the necessary conformational change in PBP 2a for effective acylation. This possibility needs to be examined experimentally, but it is consistent with the observations made in the present study as well as in our previous studies (18, 19). Conversely, ceftobiprole, like other anti-MRSA cephalosporins (11), causes a conformational change consistent with the opening of the PBP 2a active site even at neutral pH (22). Ceftobiprole may therefore be expected to behave at neutral pH somewhat as penicillins and carbapenems do at acidic pH, i.e., to display MICs for MRSA close to those observed for MSSA, as reported by the discoverers of ceftobiprole (4, 15) and as confirmed here for various HA-MRSA and CA-MRSA isolates. The acidic pH may further facilitate this process, since the MICs of ceftobiprole for MRSA are still further lowered when pH is brought from 7.4 to 5.5. These MICs nevertheless remain slightly higher than those observed for MSSA, which can be interpreted either (i) as corresponding to the energy required to induce the conformational change in PBP 2a from its closed to its open state or (ii) as a competition between other PBPs for binding, given that it is known that only binding to PBP 2a is effective for impairing bacterial growth. In all cases, however, the MICs of ceftobiprole for the strains studied here remain in the range of those for which eradication was observed in clinical trials (2, 26), equal to or less than those corresponding to a target attainment rate of 100% in pharmacokinetic/pharmacodynamic evaluation (25), and less than the clinical breakpoints (4 mg/liter) approved so far for skin and skin structure infections.
Modulation of the activity against MRSA by acidic pH also probably explains the observations made with infected THP-1 macrophages for cefoxitin and ceftriaxone, since these cephalosporins eventually display low MICs when they are tested at acidic pH (only fourfold higher than those for MSSA). Conversely, the failure of cephalexin to control MRSA infections in the same cells can be explained by the fact that its MIC remains elevated even at acidic pH. There is, however, some inconsistency for cefuroxime, since it showed low a MIC for MRSA at acidic pH in broth but was nevertheless unable to control MRSA infection in THP-1 macrophages. More extensive structure-activity relationship studies are probably needed in this context.
The situation is quite different for infected keratinocytes, in which all conventional cephalosporins tested almost totally failed to control infection with MRSA (which ceftobiprole does) but showed a response similar to that of ceftobiprole against MSSA. Potential reasons may include (i) a lower level of acidification of the phagolysosomes in infected keratinocytes than in infected THP-1 macrophages, which would then affect all cephalosporins except ceftobiprole; (ii) the differential handling of ceftobiprole compared with that of the other cephalosporins by cells, especially keratinocytes; and (iii) the higher levels of susceptibility of cephalexin and cefuroxime to the β-lactamase of MRSA ATCC 33591 compared to the susceptibilities of the other cephalosporins and ceftobiprole when they are exposed to the intracellular milieu (but a simple effect of pH can be ruled out, since cefuroxime has an MIC as low as that of ceftobiprole at pH 5.5). These hypotheses could not be tested in the present work, as they represent major undertakings requiring the availability of radiolabeled compounds to track the intracellular fate of the drugs and their degradation products.
The cell culture models used in the present study suffer from many limitations that have been analyzed in previous studies (7, 18-20). We may also need to expand our models to other cell types, such as endothelial and epithelial cells, which could handle S. aureus in a different fashion. Yet, the models, as designed so far, allow the objective comparison of meaningful pharmacological properties between antibiotics against intracellular infections, which is an important step for the proper design and interpretation of the results of more elaborate in vitro and in vivo studies. Thus, despite all the uncertainties mentioned above, the data reported here clearly demonstrate and rationalize the superiority of ceftobiprole over conventional cephalosporins for controlling intracellular infections caused by MRSA in two cell types that are probably important for consideration when clinicians are dealing with persistent staphylococcal infections. Of note, however, is the fact that in all cases the reduction of the intracellular CFU load over the postphagocytosis inoculum never exceeded about 1 log unit. The reason for this limited intracellular efficacy, which has been observed for all β-lactams studied so far, has no simple explanation that can be offered at this stage. The model used indeed allows the observation of the reduction of the intracellular inoculum down to 2 to 3 log CFU achieved with other antibiotics against S. aureus, including MRSA (6). Further studies will need to establish whether it represents an intrinsic limitation of β-lactams with clinical significance.
Supplementary Material
Acknowledgments
We thank Y.C. Huang and L. Y. Hsu for the kind gifts of CA-MRSA strains from their clinical collections. M.-C. Cambier provided dedicated technical assistance.
S.L. was boursière of the Belgian Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture and is presently chargé de recherches of the Belgian Fonds de la Recherche Scientifique (FRS-FNRS). F.V.B. is maître de recherches of the FRS-FNRS.
This work was supported by the Belgian Fonds de la Recherche Scientifique Médicale (grant no. 3.4.597.06); the Belgian program of Interuniversity Poles of Attraction initiated by the Federal Office for Scientific Technical and Cultural Affairs (research projects IAP5/33 and IAP6/19); the European Commission within the EUR-INTAFAR network European project (LSHM-CT-2004-512138); and a grant in aid from Johnson & Johnson Pharmaceutical Research and Development, Raritan, NJ.
Footnotes
Published ahead of print on 16 March 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Alexander, E. H., and M. C. Hudson. 2001. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl. Microbiol. Biotechnol. 56:361-366. [DOI] [PubMed] [Google Scholar]
- 2.Amsler, K. M., T. A. Davies, W. Shang, M. R. Jacobs, and K. Bush. 2008. In vitro activity of ceftobiprole against pathogens from two phase 3 clinical trials of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 52:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, S. D., and J. G. Gums. 2008. Ceftobiprole: an extended-spectrum anti-methicillin-resistant Staphylococcus aureus cephalosporin. Ann. Pharmacother. 42:806-816. [DOI] [PubMed] [Google Scholar]
- 4.Angehrn, P., P. Hebeisen, I. Heinze-Krauss, M. Page, and V. Runtz. June 1998. Preparation of vinylpyrrolidine derivatives of cephalosporins with basic substituents, p. 1-54. European Union patent no. EP0849269.
- 5.Bamberger, D. M. 2007. Bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: the potential role of daptomycin. Ther. Clin. Risk Manag. 3:675-684. [PMC free article] [PubMed] [Google Scholar]
- 6.Barcia-Macay, M., S. Lemaire, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Evaluation of the extracellular and intracellular activities (human THP-1 macrophages) of telavancin versus vancomycin against methicillin-susceptible, methicillin-resistant, vancomycin-intermediate and vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:1177-1184. [DOI] [PubMed] [Google Scholar]
- 7.Barcia-Macay, M., C. Seral, M. P. Mingeot-Leclercq, P. M. Tulkens, and F. Van Bambeke. 2006. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob. Agents Chemother. 50:841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dancer, S. J. 2008. The effect of antibiotics on methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 61:246-253. [DOI] [PubMed] [Google Scholar]
- 9.de Lencastre, H., D. Oliveira, and A. Tomasz. 2007. Antibiotic resistant Staphylococcus aureus: a paradigm of adaptive power. Curr. Opin. Microbiol. 10:428-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deresinski, S. C. 2008. The efficacy and safety of ceftobiprole in the treatment of complicated skin and skin structure infections: evidence from 2 clinical trials. Diagn. Microbiol. Infect. Dis. 61:103-109. [DOI] [PubMed] [Google Scholar]
- 11.Fuda, C., D. Hesek, M. Lee, W. Heilmayer, R. Novak, S. B. Vakulenko, and S. Mobashery. 2006. Mechanistic basis for the action of new cephalosporin antibiotics effective against methicillin- and vancomycin-resistant Staphylococcus aureus. J. Biol. Chem. 281:10035-10041. [DOI] [PubMed] [Google Scholar]
- 12.Gaze, W., C. O'Neill, E. Wellington, and P. Hawkey. 2008. Antibiotic resistance in the environment, with particular reference to MRSA. Adv. Appl. Microbiol. 63:249-280. [DOI] [PubMed] [Google Scholar]
- 13.Gee, K. R., H. C. Kang, T. I. Meier, G. Zhao, and L. C. Blaszcak. 2001. Fluorescent Bocillins: synthesis and application in the detection of penicillin-binding proteins. Electrophoresis 22:960-965. [DOI] [PubMed] [Google Scholar]
- 14.Graves-Woodward, K., and R. F. Pratt. 1998. Reaction of soluble penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus with beta-lactams and acyclic substrates: kinetics in homogeneous solution. Biochem. J. 332(Pt 3):755-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluytmans-Vandenbergh, M. F., and J. A. Kluytmans. 2006. Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clin. Microbiol. Infect. 12(Suppl. 1):9-15. [DOI] [PubMed] [Google Scholar]
- 17.Lemaire, S., C. Fuda, F. Van Bambeke, P. M. Tulkens, and S. Mobashery. 2008. Restoration of susceptibility of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics by acidic pH: role of penicillin-binding protein PBP 2a. J. Biol. Chem. 283:12769-12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemaire, S., A. Olivier, F. Van Bambeke, P. M. Tulkens, P. C. Appelbaum, and Y. Glupczynski. 2008. Restoration of susceptibility of intracellular methicillin-resistant Staphylococcus aureus to beta-lactams: comparison of strains, cells, and antibiotics. Antimicrob. Agents Chemother. 52:2797-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, Y. Glupczynski, and P. M. Tulkens. 2007. Role of acidic pH in the susceptibility of intraphagocytic methicillin-resistant Staphylococcus aureus strains to meropenem and cloxacillin. Antimicrob. Agents Chemother. 51:1627-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaire, S., F. Van Bambeke, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2005. Activity of three β-lactams (ertapenem, meropenem and ampicillin) against intraphagocytic Listeria monocytogenes and Staphylococcus aureus. J. Antimicrob. Chemother. 55:897-904. [DOI] [PubMed] [Google Scholar]
- 21.Leonard, F. C., and B. K. Markey. 2008. Meticillin-resistant Staphylococcus aureus in animals: a review. Vet. J. 175:27-36. [DOI] [PubMed] [Google Scholar]
- 22.Lovering, A., F. Danel, M. G. P. Page, and N. J. Strynadka. 2006. Mechanism of action of ceftobiprole: structural basis for the anti-MRSA activity, abstr. 1506. Program Abstr. 16th Eur. Conf. Clin. Microbiol. Infect.
- 23.Lowy, F. D. 2000. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8:341-343. [DOI] [PubMed] [Google Scholar]
- 24.Mempel, M., C. Schnopp, M. Hojka, H. Fesq, S. Weidinger, M. Schaller, H. C. Korting, J. Ring, and D. Abeck. 2002. Invasion of human keratinocytes by Staphylococcus aureus and intracellular bacterial persistence represent haemolysin-independent virulence mechanisms that are followed by features of necrotic and apoptotic keratinocyte cell death. Br. J. Dermatol. 146:943-951. [DOI] [PubMed] [Google Scholar]
- 25.Mouton, J. W., A. Schmitt-Hoffmann, S. Shapiro, N. Nashed, and N. C. Punt. 2004. Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141. Antimicrob. Agents Chemother. 48:1713-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noel, G. J., K. Bush, P. Bagchi, J. Ianus, and R. S. Strauss. 2008. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin. Infect. Dis. 46:647-655. [DOI] [PubMed] [Google Scholar]
- 27.Page, M. G. 2007. Emerging cephalosporins. Expert Opin. Emerg. Drugs 12:511-524. [DOI] [PubMed] [Google Scholar]
- 28.Pantosti, A., A. Sanchini, and M. Monaco. 2007. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2:323-334. [DOI] [PubMed] [Google Scholar]
- 29.Preston, D. A., C. Y. Wu, L. C. Blaszczak, D. E. Seitz, and N. G. Halligan. 1990. Biological characterization of a new radioactive labeling reagent for bacterial penicillin-binding proteins. Antimicrob. Agents Chemother. 34:718-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabath, L. D., S. J. Wallace, and D. A. Gerstein. 1972. Suppression of intrinsic resistance to methicillin and other penicillins in Staphylococcus aureus. Antimicrob. Agents Chemother. 2:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakoulas, G., and R. C. Moellering, Jr. 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin. Infect. Dis. 46(Suppl. 5):S360-S367. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg, A., J. H. R. Hessler, R. L. Skov, J. Blom, and N. Frimødt-Moller. 2009. Intracellular activity of antibiotics against Staphylococcus aureus in a mouse peritonitis model. Antimicrob. Agents Chemother. 53:1874-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seral, C., F. Van Bambeke, and P. M. Tulkens. 2003. Quantitative analysis of gentamicin, azithromycin, telithromycin, ciprofloxacin, moxifloxacin, and oritavancin (LY333328) activities against intracellular Staphylococcus aureus in mouse J774 macrophages. Antimicrob. Agents Chemother. 47:2283-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha, B., and M. Herrmann. 2005. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb. Haemost. 94:266-277. [DOI] [PubMed] [Google Scholar]
- 35.Stryjewski, M. E., and H. F. Chambers. 2008. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl. 5):S368-S377. [DOI] [PubMed] [Google Scholar]
- 36.Tulkens, P. M. 1991. Intracellular distribution and activity of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 10:100-106. [DOI] [PubMed] [Google Scholar]
- 37.Utsui, Y., and T. Yokota. 1985. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 28:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Bambeke, F., M. Barcia-Macay, S. Lemaire, and P. M. Tulkens. 2006. Cellular pharmacodynamics and pharmacokinetics of antibiotics: current views and perspectives. Curr. Opin. Drug Discov. Dev. 9:218-230. [PubMed] [Google Scholar]
- 39.Villegas-Estrada, A., M. Lee, D. Hesek, S. B. Vakulenko, and S. Mobashery. 2008. Co-opting the cell wall in fighting methicillin-resistant Staphylococcus aureus: potent inhibition of PBP 2a by two anti-MRSA beta-lactam antibiotics. J. Am. Chem. Soc. 130:9212-9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vonk, A. G., and C. M. Vandenbroucke-Grauls. 2007. Methicillin-resistant Staphylococcus aureus (MRSA) in the community. Ned. Tijdschr. Geneeskd. 151:401-407. (In Dutch.) [PubMed] [Google Scholar]
- 41.Wijaya, L., L. Y. Hsu, and A. Kurup. 2006. Community-associated methicillin-resistant Staphylococcus aureus: overview and local situation. Ann. Acad. Med. Singapore 35:479-486. [PubMed] [Google Scholar]
- 42.Zbinden, R., V. Punter, and A. von Graevenitz. 2002. In vitro activities of BAL9141, a novel broad-spectrum pyrrolidinone cephalosporin, against gram-negative nonfermenters. Antimicrob. Agents Chemother. 46:871-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhanel, G. G., A. Lam, F. Schweizer, K. Thomson, A. Walkty, E. Rubinstein, A. S. Gin, D. J. Hoban, A. M. Noreddin, and J. A. Karlowsky. 2008. Ceftobiprole: a review of a broad-spectrum and anti-MRSA cephalosporin. Am. J. Clin. Dermatol. 9:245-254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.