Abstract
Encephalitozoon cuniculi is a microsporidium responsible for systemic illness in mammals. In the course of developing leads to new therapy for microsporidiosis, we found that a bis(phenylbenzyl)3-7-3 analog of spermine, 1,15-bis{N-[o-(phenyl)benzylamino}-4,12-diazapentadecane (BW-1), was a substrate for an E. cuniculi amine oxidase activity. The primary natural substrate for this oxidase activity was N′-acetylspermine, but BW-1 had activity comparable to that of the substrate. As the sole substrate, BW-1 gave linear reaction rates over 15 min and Km of 2 μM. In the presence of N′-acetylspermine, BW-1 acted as a competitive inhibitor of oxidase activity and may be a subversive substrate, resulting in increased peroxide production. By use of 13C-labeled BW-1 as a substrate and nuclear magnetic resonance analysis, two products were determined to be oxidative metabolites, a hydrated aldehyde or dicarboxylate and 2(phenyl)benzylamine. These products were detected after exposure of 13C-labeled BW-1 to E. cuniculi preemergent spore preparations and to uninfected host cells. In previous studies, BW-1 was curative in a rodent model of infection with E. cuniculi. The results in this study demonstrate competitive inhibition of oxidase activity by BW-1 and support further studies of this oxidase activity by the parasite and host.
Microsporidia are unicellular obligate intracellular parasites of many animals and insects. They lack typical mitochondria; instead, they possess a mitochondrial remnant organelle termed the mitosome (11). Members of the microsporidia are ubiquitous parasites, with >1,200 species infecting hosts representing many levels of biological complexity, from insects to humans (27). Present concerns about human microsporidial infections are exacerbated by the presence of organisms of several genera which target immunosuppressed patients, by findings that these are zoonotic pathogens, and by the lack of new, effective chemotherapeutic agents (26).
Metabolism and function of the polyamines spermidine and spermine have long been chemotherapeutic targets for neoplastic diseases as well as parasitic infections (5, 15). Polyamines are ubiquitous in all organisms, and their cell contents are carefully regulated, whether through synthesis, degradation, uptake, or export. Extensive studies have demonstrated their essentiality for cell proliferation and roles in cell signaling (18). Research over the past two decades has resulted in the synthesis of polyamine analogues which downregulate polyamine synthesis and uptake while increasing polyamine degradation and excretion (13, 16, 19, 21). Until recently, such analogs were straight chain (3-3-3 or 3-7-3) in configuration and symmetrically N-alkylated at the ends. Additional structural features have included nonsymmetrical alkylation (13) and the introduction of sterically hindered backbones (16), each of which has increased therapeutic potential.
In recent studies, we have examined such analogues for activity against Encephalitozoon cuniculi, a microsporidian parenteral parasite of humans that causes disseminated disease and has been found to be growth inhibitory in vitro and curative in laboratory model infections (1, 28). In particular, one such analogue, a bis-phenylbenzyl-substituted 3-7-3 compound (1,15-bis{N-[o-(phenyl)benzylamino}-4,12-diazapentadecane [BW-1]) (Fig. 1) was found to interfere with E. cuniculi polyamine oxidase (PAO) activity and ultimately to serve as a substrate for the parasite enzyme (4). In this study, we present evidence of the metabolism of this analog and the possibility of its selective cytotoxic action.
FIG. 1.
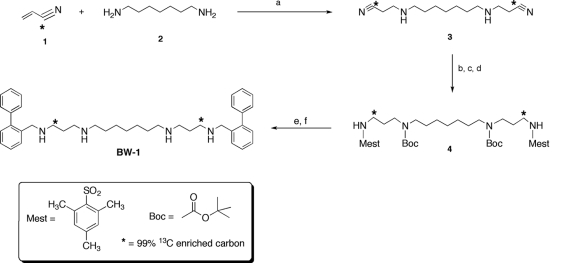
The synthesis of bis-α-99% 13C-enriched BW-1. Conditions were as follows. (a) Ethanol, reflux. (b) (Boc)2O, NaHCO3, CH2Cl2, H2O. (c) Raney Ni, NaOH, EtOH (50 lb/in2). (d) 2-Mesitylenesulfonyl chloride, 10% NaOH, CH2Cl2, H2O. (e) [2-(phenyl)]benzyl] chloride, NaH, dimethylformamide. (f) 30% HBr in acetic acid.
MATERIALS AND METHODS
Organism.
Encephalitozoon cuniculi, a parenteral parasite of mammals, including humans, was obtained from Ann Cali of Rutgers University and cultivated in minimal Eagle's medium plus 7% newborn calf serum, using RK-13 or HEK monolayers as host cells (24). Host monolayers were grown to 80% confluence in Falcon T25 or T75 flasks and then infected with 5 × 105 spores. After 1 week of culture, up to 50% infectivity was obtained. Alternatively, infected host cultures were split and allowed to multiply until confluence was obtained (3).
Enzyme preparations.
Mature spores were given off into the culture overlay and collected from culture flasks twice weekly, when fresh medium was added. Spores were pooled and stored aseptically at 0 to 4°C. Crude enzyme preparations were made from concentrated spores which had been washed in 150 mM phosphate-buffered 0.9% NaCl (PBS; pH 6.8) and resuspended in PBS containing 1% sodium dodecyl sulfate for 5 min. Resuspended spores were then concentrated (5,000 × g for 10 min), and a heavy suspension was made in 1 to 2 ml of PBS. Spores were disrupted from 4 to 8 min in microcentrifuge tubes containing 425- to 600-nm glass beads using a Disruptor Genie (Scientific Industries, Bohemia, NY). Homogenates were examined for >80% breakage (microscopically) and clarified by centrifuging at 2,200 × g for 10 min (2). These preparations were used for enzyme studies.
PAO assays.
PAO from the preparations described above was measured according to the method of Childs and Bardsley (14). Reaction mixtures (37°C) contained 0.1 M glycine buffer (pH 7.0), 10 U of horseradish peroxidase (Sigma Type VI A; 1 U oxidizes 1 μmol of 2,2′ azino-bis 3-ethylbenzthiazline-6-sulfonic acid [ABTS] per min at 25°C, pH 5.0), 5 mM ABTS, and 22 to 220 μg E. cuniculi protein in a volume of 1 ml. N1-Acetylspermine (1 mM) or another substrate, e.g., BW-1, was added to start the reaction. The extinction coefficient of ABTS at 420 nm is 3.6 × 104 M−1 cm−1 (14). Reactions were followed spectrophotometrically for 15 or 30 min, and the initial linear rate minus the control (no substrate) was taken for data presentation.
BW-1 kinetics.
The effect of BW-1 on the activity of PAO was determined by measuring the amount of peroxide produced using 10 μg of horseradish peroxidase, 5 μM ABTS in 0.1 M glycine buffer, pH 7.0. N-Acetylspermine was added to the cuvette at concentrations from 0.1 mM to 2.0 mM in the presence and absence of 0, 5, 50, and 100 μM BW-1. The reaction was started by the addition of 67 μg of E. cuniculi spore extract, and the change in absorbance was measured at 420 nm for 15 min. The results were analyzed using Hanes-Woolf plots with various amounts of [S] against [S]/V with each concentration of BW-1.
Isolation of intracellular forms of E. cuniculi.
Preemergent spores were isolated from infected RK-13 cells (∼50 to 75% infection) in T75 flasks. Infected monolayers were trypsinized, scraped, and homogenized in a glass vessel for 6 to 8 min at 0 to 4°C. The breakage medium consisted of PBS plus a protease inhibitor cocktail containing 2.7 mM Nα-p-tosyl-l-lysine chloromethyl ketone HCl (TLCK), 0.15 mM aprotinin, 2.2 mM leupeptin, and 0.5 mM dithiothreitol in 50 mM KCl-0.15 M NaPO4, pH 7.4. Cell homogenates were passed through 12-μm and 5-μm filters. Preemergent spores were harvested on a 60% (vol/vol) Percoll gradient using the large-scale gradient procedure described by Bacchi et al. (3). The heavy gradient fraction consisting largely of preemergent spores (specific gravity, 1.102 to 1.1190 g ml−1) was collected and washed in Weidner-Trager medium (25) containing 3 mM ATP, 1 mM GTP, 0.5 mM NAD+, 2 mM glucose-6-phosphate, 0.5 mM acetyl-coenzyme A, 1 mM Na pyruvate, 2 mM glucose, 138 mM Na3PO4, pH 6.8. The resulting pellet was resuspended in Weidner-Trager medium and used for incubation with 13C-labeled BW-1.
Synthesis of 13C-labeled BW-1.
The synthesis of bis-α-99% 13C-enriched BW-1 was accomplished as shown in Fig. 1, using our previously described synthetic route (7). Thus, 1,7-diaminoheptane (Fig. 1, structure 2) was bis-cyanoethylated in the presence of [α-99% 13C]acrylonitrile (ethanol [EtOH], reflux) (Fig. 1, structure 1) to afford the corresponding bis-α-99% 13C-enriched dinitrile (Fig. 1, structure 3). The interior nitrogens were then N-Boc protected (di-tert-butyl dicarbonate, CH2Cl2, H2O, NaHCO3), the nitrile moieties were reduced (Raney Ni, NaOH, EtOH [50 lb/in2]), and the resulting primary amines were N-mesityl protected (2-mesitylenesulfonyl chloride, CH2Cl2, H2O, 10% NaOH), producing compound 4 (Fig. 1, structure 4). Bis-alkylation of intermediate 4 ([2-(phenyl)]benzyl] chloride, NaH, dimethylformamide) and deprotection (30% HBr in acetic acid), followed by crystallization of the product from ethanol, afforded the desired 1,15-bis-99% 13C-labeled BW-1 as the tetrabromide salt (Fig. 1, structure 4).
RESULTS
PAO activity from E. cuniculi uses N1-acetylspermine as the primary substrate and does not utilize spermine, as do mammalian PAOs (4). While examining the effects of polyamine analogues on the uptake and metabolism of polyamines in E. cuniculi, we found that a bis(phenylbenzyl) 3-7-3 analog (BW-1) (Fig. 1) was a substrate for PAO. The activity of BW-1 as a substrate was compared to that of N1-acetylspermine (Table 1). With 0.1 mM BW-1 as the substrate, the E. cuniculi PAO exhibited activity equivalent to that with 0.1 mM N1-acetylspermine. Concentrations of BW-1 of >0.1 mM resulted in precipitate formation in the reaction mixture, which interfered with the assay. A related bis-benzyl-3-7-3 analog, MDL 27695 [N,N1-bis{3-[(phenyl-methyl)amino]propyl}1,7-diaminoheptane], was also examined for activity as an oxidase substrate, since it had been found to be a substrate for mammalian PAO (8). This compound at 0.1 mM also had about the same substrate activity as N1-acetylspermine (Table 1).
TABLE 1.
E. cuniculi PAO activityc
a Activity compared to 0.1 mM N′-acetylspermine.
b Activity compared to 1 mM N′-acetylspermine.
Assay performed as described in Materials and Methods.
13C-labeled NMR studies.
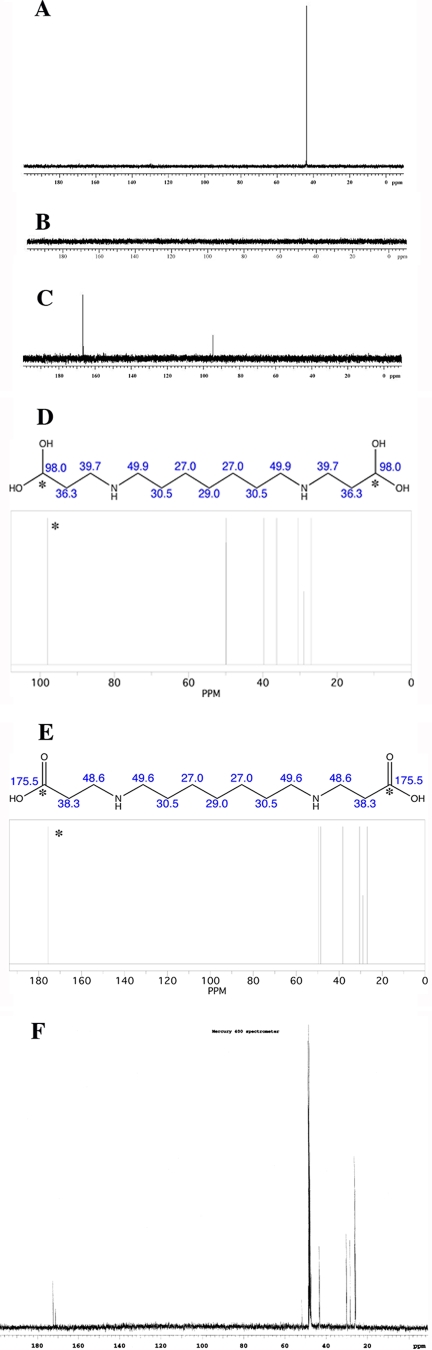
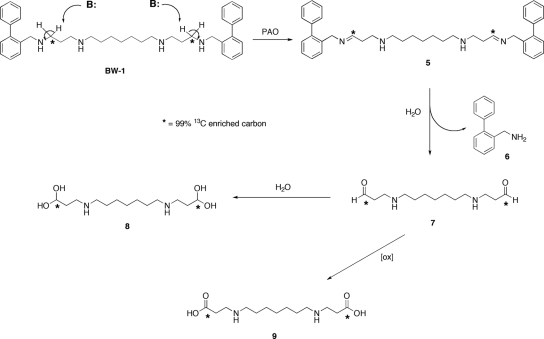
To determine how BW-1 was metabolized by E. cuniculi PAO, we studied uptake and metabolism of 13C-labeled BW-1 by intact preemergent spores. Freshly isolated preemergent spore preparations (200 μg) were incubated for 1 and 2 h with 99% 13C-enriched BW-1 (25 μM). All 13C-labeled nuclear magnetic resonance (NMR) analyses were conducted using a Varian Mercury 400 mHz NMR spectrometer equipped with an ATB tunable broadband probe. All spectra were collected in D2O at 25°C and referenced to the solvent deuterium lock signal as an internal standard. Representative spectra are shown in Fig. 2. After only 10 acquisitions, pure 13C-enriched BW-1 exhibited a strong resonance at 43.9 mHz, corresponding to the expected frequency for the α-labeled carbons (Fig. 2A). This observation is identical to the frequency previously assigned for this carbon in the 13C spectrum of unlabeled BW-1 (data not shown). Because of the low number of acquisitions, no other resonances appeared in the spectra of labeled BW-1. Following incubation with E. cuniculi, the organism was collected and separated from the supernatant by centrifugation, and each fraction was subjected to analysis by 13C-labeled NMR. As shown in Fig. 2B, there was no detectable NMR signal in the supernatant fraction, an indication that 13C-labeled BW-1 had been transported into the organism and that all subsequent metabolism had occurred intracellularly. In contrast, there were significant 13C-labeled NMR signals observed in the pelleted material following centrifugation of the culture and extraction with 10% trichloroacetic acid (Fig. 2C). The resonance at 43.9 mHz corresponding to BW-1 was completely absent, and two new resonances at 94.7 and 166.7 mHz were observed. Calculations derived from the NMR chemical shift estimator function of ChemBio-Draw Ultra 11.0 (CambridgeSoft, Cambridge, MA) and the spectra of the pure dicarboxylate (Fig. 2D and E) support the conclusions that these peaks could correspond to a hydrated aldehyde (estimated chemical shift, 98.0 ppm) (Fig. 2D) and a dicarboxylate formed through oxidation of BW-1 by PAO in E. cuniculi (estimated chemical shift, 175.5 ppm) (Fig. 2E), generated as shown in Fig. 3. Enzyme-catalyzed abstraction of an α-hydrogen in BW-1 would produce the corresponding bis-imino intermediate 5 (Fig. 3, structure 5) which, following hydrolysis and elimination of 2-(phenyl)benzylamine (Fig. 3, structure 6), would afford the corresponding bis-aldehyde (Fig. 3, structure 7). This intermediate would become partially hydrated in aqueous solution to afford intermediate 8 (Fig. 3, structure 8), thus accounting for the appearance of a new peak at 94.7 mHz. A portion of the aldehyde (Fig. 3, structure 7) could also spontaneously oxidize to form the dicarboxylate (Fig. 3, structure 9), thus accounting for the second new peak at 166.7 mHz. In order to support this hypothesis, an authentic sample of the dicarboxylate derivative was synthesized (22) and a 13C-labeled NMR spectrum was collected (CD3OD) for this compound (Fig. 2F). The chemical shift observed for the carboxylate carbon was 173 ppm, which agrees with both the estimated value of 175.5 ppm and the observed value of 166.7 ppm. In addition, the aliphatic peaks observed for the authentic dicarboxylate correlate with those in the estimated spectrum shown in Fig. 2E. The minor differences in carboxylate resonance can be explained by variations in the solvent (D2O) for the biological samples against CD3OD for the synthesized dicarboxylate, concentration, and pH of the respective solutions, and thus the spectra in Fig. 2F support the mechanism proposed in Fig. 3. Such a mechanism is consistent with that described for other amine oxidases, such as monoamine oxidase, and is similar to the oxidation of the naturally occurring polyamine spermine in the presence of hydroxyl radical, as previously described by our laboratories (17). Additional studies are required to determine whether the oxidation of BW-1 in E. cuniculi proceeds by this or another related mechanism.
FIG. 2.
NMR analysis of purified 13C-labeled BW-1 and subsequent metabolic studies. (A) 13C-labeled NMR spectrum for pure 13C-labeled BW-1. (B) 13C-labeled NMR spectrum of supernatant fraction following incubation of BW-1 in E. cuniculi and centrifugation. (C) 13C-labeled NMR spectrum of pellet following incubation of BW-1 in E. cuniculi and extraction with 10% trichloroacetic acid. (D) Calculated estimate of chemical shift values for the putative hydrated aldehyde derived from BW-1. (E) Calculated estimate of chemical shift values for the putative dicarboxylate derived from BW-1. (F) 13C-labeled NMR spectrum for an authentic sample of the unlabeled dicarboxylate (compound 9). The resonance at 173 corresponds to the carboxylate carbon, while the small peak at 171 corresponds to a small amount of the monocarboxylate generated during the synthesis.
FIG. 3.
Proposed oxidative metabolism of BW-1.
In order to determine whether BW-1 was also metabolized by the mammalian cells in the feeder layer, RK-13 rabbit kidney cells (400 μg) were exposed to bis-α-99% 13C-enriched BW-1 at a concentration of 10 μM for 3 h. The cells were then collected by centrifugation, and 13C-labeled NMR spectra were collected for the supernatant, a 10% extract of the pellet material, and a sample of sterile medium (control). No peaks corresponding to 99% 13C-enriched BW-1 or any putative metabolite were observed in the supernatant following 15,000 acquisitions (i.e., the spectrum was identical to that shown in Fig. 2B), indicating that all of the 99% 13C-enriched BW-1 had been removed from the medium, presumably by transport into the RK-13 cells. The spectrum obtained for the supernatant fraction was identical to that of sterile medium that had not been exposed to 99% 13C-enriched BW-1. In contrast, 13C-labeled NMR analysis of the pellet material (15,000 acquisitions) revealed two strong peaks at 94.7 and 166.7 ppm that were identical to those observed for metabolism of BW-1 by E. cuniculi (i.e., the spectrum was identical to that shown in Fig. 2C). These data suggest that BW-1 is readily taken up by RK-13 rabbit kidney cells and that BW-1 is metabolized in these cells by a route that is analogous to the E. cuniculi pathway.
Kinetics of BW-1 inhibition.
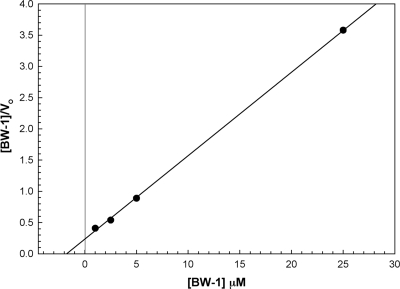
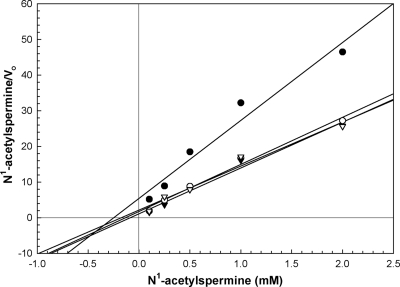
Used as sole substrate, BW-1 gave linear reaction rates over 15 min, a Km of 2 μM, and a Vmax of 7.6 μM/min (Fig. 4) in a substrate range of 1 to 30 μM. The effect of BW-1 on E. cuniculi PAO was analyzed by using kinetic plots with N-acetylspermine as the substrate (Fig. 5). The Km value for N-acetylspermine alone is 30 μM, whereas in the presence of BW-1 the apparent Km value for N-acetylspermine is 15 μM (Table 2). The ratio of Vmax/Km for the reaction increases 3.5-fold from 0.33 to 1.17 in the presence of BW-1, which is indicative of the additional peroxide formed in the presence of BW-1. From the Hanes-Woolf plot of BW-1 versus that of N-acetylspermine, it can be determined that the Ki for BW-1 is 40 μM. These results are indicative that BW-1 acts as a competitive inhibitor of the enzyme and may be a subversive substrate, resulting in additional peroxide production.
FIG. 4.
Hanes-Woolf plot of E. cuniculi PAO activity with BW-1 as substrate. BW-1 concentration was varied from 1 to 30 μM using the assay conditions described in Materials and Methods. The assay contained 50 to 200 μg E. cuniculi protein in a volume of 1 ml, and the absorbance change of ABTS was recorded at 420 nm. The Km for BW-1 was calculated to be 2 μM and the Vmax 7.57 μM/min.
FIG. 5.
Hanes-Woolf plot showing the comparative inhibition kinetics obtained with BW-1 on E. cuniculi PAO. The activity of PAO was measured in the presence of 100, 250, 500, 1,000, and 2,000 μM N1-acetylspermine either in the absence of BW-1 (•) or presence of 5 μM (○), 50 μM (▾), or 100 μM (▿) BW-1. Incubations contained 0.1 M glycine buffer (pH 7.0), 10 U of horseradish peroxidase and 22 to 220 μg E. cuniculi protein in a volume of 1 ml. The change in absorption of ABTS was measured at 420 nm.
TABLE 2.
Substrate kinetics of E. cuniculi PAOa
| Substrate | Vmax (μM min−1) | Km (μM) |
|---|---|---|
| N1-acetylspermine | 10.0 | 30 |
| BW-1 | 7.6 | 2 |
| N1-acetylspermine + BW-1 | 17.5 | 15 |
Kinetic constants were obtained from Hanes-Woolf plots of various substrate concentrations for N1-acetylspermine (100 to 2,000 μM) and BW-1 (1 to 25 μM). The values for the N1-acetylspermine plus BW-1 mixture were determined from Hanes-Woolf plots of various N1-acetylspermine (100 to 2,000 μM) concentrations with fixed increasing concentrations of BW-1 (5 to 100 μM).
DISCUSSION
Early studies of polyamine content and function in eukaryotic cells also focused on inhibition of their synthesis as a potential major mechanism for inhibition of cell growth. With the realization that polyamine interconversion and excretion were also important facets of polyamine metabolism, research activity then turned to development of antipolyamine agents which would be taken up through polyamine transporters, accelerate polyamine breakdown, and downregulate their synthesis (19). One of the first agents developed in this family of polyamine analogues was MDL 27695, which had a 3-7-3 amine backbone and terminal benzyl groups (Table 1) (10). Initial studies demonstrated that this compound was debenzylated by a mammalian oxidase to H2O2, benzylaldehyde, and the 3-7-3 amine backbone. The last was capable of downregulating polyamine synthesis, increasing polyamine interconversion and excretion, and lowering polyamine levels in the cell (8). The potential utility of MDL 27695 has been demonstrated as an inhibitor of mammalian tumor cell growth and as an antimalarial and antileishmanial agent (6, 9, 23). Recent studies with MDL 27695 against Pneumocystis carinii indicate that MDL 27695 was highly effective in a rat model of infection and interfered with the polyamine uptake and metabolism of the parasite (20).
The present study strongly indicates that the parasite E. cuniculi is capable of assimilating the related bis(phenylbenzyl) analogue BW-1 and rapidly oxidizing it to 2-phenyl-benzylamine and a diaminoheptane diethyaldehyde or diethylcarboxylate. Future work will focus on the products of oxidation of bis-α-99% 13C-enriched BW-1 by purified human PAO and spermine oxidase in vitro. The proposed oxidation products of BW-1 differ from those metabolites of bis-benzylated 3-7-3 analogue MDL 27695 produced by mammalian cells through the mammalian PAO; in that reaction, benzylaldehyde and the free 3-7-3 bis-amine derivative were the products. In a model of Plasmodium berghei infection, the oxidation of the bis-benzyl polyamine analogue (MDL 27695) to the free amine took place in mouse erythrocytes. The bis-benzyl form was necessary for accumulation in the erythrocyte, and metabolism to the free amine took place therein (10). The free bis-3-7-3 amine derivative did not penetrate the erythrocyte, and mice treated with an amine oxidase inhibitor were not cured of the infection, indicating that the PAO oxidation of the parent compound was essential for antimalarial activity. In contrast, in P. carinii, MDL 27695 was not metabolized to the 3-7-3 derivative, and the debenzylated product (3-7-3 amine) was not growth inhibitory, indicating that the intact molecule was responsible for the antipneumocystis effects (20).
In the present study, BW-1 was taken up and rapidly oxidized to apparently identical products by E. cuniculi and the uninfected RK-13 feeder cells. Control studies using sterile complete medium showed there was no metabolism of 13C-labeled BW-1. The newborn calf serum used with culture medium for the RK-13 feeder layer had no oxidase activity using BW-1, N1-acetylspermine, or benzylamine as the substrate. The identity of the enzyme(s) responsible for oxidation of BW-1 in E. cuniculi is not clear. The parasite enzyme with N1-acetylspermine as a substrate is not inhibited by the PAO inhibitor MDL 72527, but activity is significantly affected by pargyline, a Cu-amine monoamine oxidase inhibitor, and aminoguanidine, which targets monoamine oxidase (4). While the optimum catalytic pH for monoamine oxidase and polyamine oxidases is about 10, the E. cuniculi oxidase activity is between pH 7 and 8 (4). It is not known whether the oxidation of BW-1 is essential for activity against microsporidia. The compound is a potent inhibitor of E. cuniculi growth in an in vitro culture system (50% inhibitory concentration, 0.47 μM), without toxicity to the feeder layer, and cured an E. cuniculi mouse model of infection (1 or 5 mg/kg of body weight/day for 10 days) (4, 28) without toxicity to the host. In vitro, BW-1 at micromolar levels blocked spermine uptake and interconversion by E. cuniculi preemergent spores (4). However, in mammalian cell lines, the related compound MDL 27695 did not compete with polyamine transport and appeared to enter cells by a separate transport system (12). It remains to be seen whether intact BW-1 or its amine products are active as growth inhibitors against E. cuniculi, whether oxidation of BW-1 occurs in host cells prior to entry into the parasite, and whether the 3-7-3 oxidation product can enter the parasite.
Acknowledgments
This work was supported by grants from the National Institutes of Health (no. RO1-CA85509 to P. M. Woster and RO1-041398 to M. Wittner).
MDL 27695 was a gift from Marion Merrell Dow (Cincinnati, OH).
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Bacchi, C. J., S. Lane, B. Frydman, V. Valasinas, V. Reddy, J. S. Sun, L. J. Marton, L. M. Weiss, I. Khan, N. Yarlett, and M. Wittner. 2002. Novel synthetic polyamines are effective in treatment of experimental microsporidiosis, an opportunistic, AIDS-associated infection. Antimicrob. Agents Chemother. 46:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacchi, C. J., S. S. Lane, J. Rhoden, N. Yarlett, M. Wittner, and L. M. Weiss. 1999. Polyamine synthesis in Encephalitozoon cuniculi. J. Eukaryot. Microbiol. 46:31S. [PubMed] [Google Scholar]
- 3.Bacchi, C. J., S. Lane, L. M. Weiss, N. Yarlett, P. Takvorian, A. Cali, and M. Wittner. 2001. Polyamine synthesis and interconversion by the microsporidian Encephalitozoon cuniculi. J. Eukaryot. Microbiol. 48:374-381. [DOI] [PubMed] [Google Scholar]
- 4.Bacchi, C. J., D. Rattendi, E. Faciane, N. Yarlett, L. M. Weiss, B. Frydman, P. Woster, B. Wei, L. J. Marton, and M. Wittner. 2004. Polyamine metabolism in a member of the phylum Microspora (Encephalitozoon cuniculi): effects of polyamine analogues. Microbiology 150:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacchi, C. J., and N. Yarlett. 2002. Polyamine metabolism as chemotherapeutic target in protozoan parasites. Mini Rev. Med. Chem. 2:553-563. [DOI] [PubMed] [Google Scholar]
- 6.Baumann, R. J., W. L. Hanson, P. P. McCann, A. Sjoerdsma, and A. J. Bitonti. 1990. Suppression of both antimony-susceptible and antimony-resistant Leishmania donovani by a bis(benzyl)polyamine analog. Antimicrob. Agents Chemother. 34:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellevue, F. H., M. L. Boahbedason, R. H. Wu, R. A. Casero, Jr., D. Rattendi, S. Lane, C. J. Bacchi, and P. M. Woster. 1996. Structural comparison of alkylpolyamine analogues with potent in vitro antitumor or antiparasitic activity. Bioorg. Med. Chem. Lett. 6:2765-2770. [Google Scholar]
- 8.Bitonti, A. J., T. L. Bush, and P. P. McCann. 1989. Regulation of polyamine biosynthesis in rat hepatoma (HTC) cells by a bisbenzyl polyamine analogue. Biochem. J. 257:769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitonti, A. J., J. A. Dumont, T. L. Bush, M. L. Edwards, D. M. Stemerick, P. P. McCann, and A. Sjoerdsma. 1989. Bis(benzyl) polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with α-diflouromethylornithine cure murine malaria. Proc. Natl. Acad. Sci. USA 86:651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bitonti, A. J., J. A. Dumont, T. L. Bush, D. M. Stemerick, M. L. Edwards, and P. P. McCann. 1990. Bis(benzyl) polyamine analogs as novel substrates for polyamine oxidase. J. Biol. Chem. 265:382-388. [PubMed] [Google Scholar]
- 11.Burri, L., B. A. P. Williams, D. Bursac, T. Lithgow, and P. J. Keeling. 2006. Microsporidian mitosomes retain elements of the general mitochondrial targeting systems. Proc. Natl. Acad. Sci. USA 103:15916-15920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byers, T. L., A. J. Bitonti, and P. P. McCann. 1990. Bis(benzyl) polyamine analogues are substrates for a mammalian cell-transport system which is distinct from the polyamine-transport system. Biochem. J. 269:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casero, R. A., and P. M. Woster. 2001. Terminally alkylated polyamine analogues as chemotherapeutic agents. J. Med. Chem. 44:1-26. [DOI] [PubMed] [Google Scholar]
- 14.Childs, R. E., and W. G. Bardsley. 1975. The steady-state kinetics of peroxidase with 2,2"-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. J. 145:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen, S. S. 1998. A guide to the polyamines, p. 69-93. Oxford University Press, New York, NY.
- 16.Frydman, B., and A. Valasinas. 1999. Polyamine-based chemotherapy of cancer. Exp. Opin. Ther. Patents 9:1055-1068. [Google Scholar]
- 17.Ha, H. C., N. S. Sirisoma, P. Kuppusamy, J. L. Zweier, P. M. Woster, and R. A. Casero. 1998. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 95:11140-11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer, D. L., and E. W. Gerner. 2004. Therapeutic strategies targeting polyamines, p. 327-345. In G. J. Kellof, E. T. Hawk, and C. C. Sigman (ed.), Cancer chemoprevention: promising cancer chemoprevention agents, vol. 1. Humana Press, Inc., Totowa, NJ. [Google Scholar]
- 19.Marton, L. J., and A. E. Pegg. 1995. Polyamines as targets for therapeutic intervention. Annu. Rev. Pharmacol. Toxicol. 35:55-91. [DOI] [PubMed] [Google Scholar]
- 20.Merali, S., M. Saric, K. Chin, and A. B. Clarkson, Jr. 2000. Effect of a bis-benzyl polyamine analogue on Pneumocystis carinii. Antimicrob. Agents Chemother. 44:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter, C. W., R. J. Bernacki, J. Miller, and R. J. Bergeron. 1993. Antitumor activity of N1,N11-bis(ethyl)norspermine against human melanoma xenografts and possible biochemical correlates of drug action. Cancer Res. 53:581-586. [PubMed] [Google Scholar]
- 22.Tabor, H., and C. W. Tabor. 1983. Synthesis of putreanine and of spermic acid. Methods Enzymol. 94:418-420. [Google Scholar]
- 23.Thomas, T. J., N. Shah, C. A. Faaland, M. A. Gallo, E. Yurkow, P. G. Satyaswaroop, and T. T. Thomas. 1997. Antitumor effects of a bis(benzyl)spermine analog on MCF-7 breast cancer cells in culture and nude mice xenografts. Oncol. Rep. 4:5-13. [PubMed] [Google Scholar]
- 24.Visvesvara, G. S., G. J. Leitch, H. Moura, S. Wallace, R. Weber, and R. T. Bryan. 1991. Culture, electron microscopy and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J. Protozool. 38:105S-111S. [PubMed] [Google Scholar]
- 25.Weidner, E., and W. Trager. 1973. Adenosine triphosphate in the extracellular survival of an intracellular parasite (Nosema michaelis). J. Cell Biol. 57:586-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss, L. M. 2003. Microsporidia 2003: IWOP-8. J. Eukaryot. Microbiol. 50:566-568. [DOI] [PubMed] [Google Scholar]
- 27.Wittner, M., and L. M. Weiss. 1999. The microsporidia and microsporidiosis. American Society for Microbiology, Washington, DC.
- 28.Zou, Y., Z. Wu, N. Sirisoma, P. M. Woster, R. A. Casero, L. M. Weiss, D. Rattendi, S. Lane, and C. J. Bacchi. 2001. Novel alkylpolyamine analogues that possess both antiparasitic and antimicrosporidial activity. Bioorg. Med. Chem. Lett. 11:1613-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]