Abstract
Phage therapy against Pseudomonas aeruginosa infections has received renewed attention owing to the increasing prevalence of antibiotic resistance in this bacterium. Here, we isolated and characterized two new potentially lytic bacteriophages (MPK1 and MPK6), which produced large and clear plaques on P. aeruginosa strain PAO1. Based on their morphology, MPK1 belongs to the Myoviridae, while MPK6 belongs to the Podoviridae. The group B polysaccharide of lipopolysaccharide was required for infection, suggesting that their host spectra are associated with the serotypes of P. aeruginosa strains. Intramuscular and intraperitoneal administration of MPK1 and, to a lesser extent, MPK6 significantly protected mice from mortality caused by PAO1-induced peritonitis-sepsis (P < 0.01). Mice treated with either phage also had lower bacterial burdens in their livers, lungs, and spleens. The antibacterial efficacy of MPK1 and MPK6 was also evaluated based on Drosophila melanogaster systemic infection caused by P. aeruginosa, for which phages were administered by feeding. Both phages significantly delayed the PAO1-induced killing of D. melanogaster (P < 0.001), although MPK1 persisted longer than MPK6 in uninfected D. melanogaster tissue samples. These results suggest that a mini-scale experiment using D. melanogaster infection is valid for evaluating the antibacterial efficacy of phage therapy against P. aeruginosa infections.
Pseudomonas aeruginosa is an opportunistic human pathogen that is ubiquitously found in various biotic and abiotic environments. It is frequently isolated from human patients afflicted with cystic fibrosis, otitis media, keratitis, and burn wound infections as an etiological agent of septicemia in immunocompromised individuals. This bacterium, generally from the environmental reservoir, can colonize large numbers of child patients before the age of 3 years (27) and adversely affect their pulmonary function (23). Furthermore, P. aeruginosa is also commonly found in peritonitis-sepsis cases secondary to ruptured appendices in otherwise healthy children (4). Peritonitis by P. aeruginosa is a serious threat also to the patients undergoing continuous ambulatory peritoneal dialysis (CAPD) (15), accounting for 10% of fatality cases associated with CAPD. The bacterial intoxication usually leads to high morbidity, CAPD failure, and late complications in those cases (14, 15, 17). Rodent models of P. aeruginosa peritonitis have been developed for understanding the pathophysiology implicated in peritonitis (32). The pathological consequences are generally bacteremia and infected livers, with interleukin-6 levels elevated within 6 h postinfection, and ultimately cause mortality within 48 h depending on P. aeruginosa strains and infection doses (5).
Although antibiotics are still widely used to control the bacterial infections, they are increasingly ineffective due to the emergence of antibiotic resistance. Selection and dissemination of intrinsic and acquired antibiotic resistance mechanisms increase the proclivity to resist the chemotherapy involving various antibiotics and promote the emergence of bacterial strains with multiple antibiotic resistances, which are associated with the mortality and morbidity in infected patients nowadays (22, 28). Hence, the development of new therapeutic and prophylactic strategies is compulsory for the control of bacterial infections.
An alternative and/or supplementary anti-infective modality for combating infections caused by antibiotic-resistant microorganisms which is currently being revisited in various countries is bacteriophages that are able to specifically target their host bacterial infections, and this modality is called phage therapy (29). Phage therapy is a method of harnessing phages as bioagents and was first introduced before the discovery of the first antibiotic, penicillin (30). Phages continue to be used in place of antibiotics for the treatment of bacterial infections in the former Soviet Union and Eastern Europe (25). Much more attention has recently been paid to phage therapy, as more and more bacteria have evolved antibiotic resistance. Thus, phage therapy may be a valuable alternative modality to antibiotics and has already been proven to be medically superior to antibiotics in certain cases (3, 19).
P. aeruginosa is a highly adaptable bacterium that could enhance its ecological fitness even in the presence of conventional antibiotic therapy. The rapid emergence of new P. aeruginosa strains as well as the persistence of the existing antibiotic-resistant clinical isolates has led to an urgent need to explore more sustainable alternative strategies such as phage therapy to manage P. aeruginosa-mediated infections. Recently, the therapeutic mixture of six lytic phages was shown to be effective in clinical ear infections caused by P. aeruginosa (26). The efficacy of phage therapy using a genetically modified filamentous phage (Pf3R) (10), lytic phage isolates, or phage cocktails has been also investigated against various experimental mouse infection models with P. aeruginosa that include burn wound infection (20) and gut-derived sepsis (31). Because the pathophysiology caused by P. aeruginosa infections is quite complicated, more and more relevant infection models need to be tested for the efficacy and relevancy of the antibacterial therapies.
In this study, we evaluated the therapeutic efficacy of newly isolated Caudovirales phage strains, MPK1 and MPK6, against mouse peritonitis-sepsis caused by intraperitoneal (i.p.) infection with P. aeruginosa strain PAO1 that may resemble the clinical pathophysiology of P. aeruginosa-induced peritonitis in humans. Based on the fact that P. aeruginosa is able to infect hosts of multiple phylogenetic backgrounds, such as plants, worms, and insects, and the fact that a set of virulence factors identified from a Drosophila melanogaster systemic infection-based screen are also required for mouse peritonitis (16), we here established a D. melanogaster model to evaluate phage therapy and verified the antibacterial efficacy of both MPK1 and MPK6, using this nonmammalian infection model.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Pseudomonas aeruginosa strain PAO1 was used as described elsewhere (11). The lipopolysaccharide (LPS) mutants (rmd, wbpM, and rmlC mutants) are gifts from Joe Lam (University of Guelph, Canada). Bacterial cells were grown in Luria-Bertani (LB) broth (1% tryptone, 0.5% yeast extract, and 1% NaCl) with aeration or 2% Bacto agar (Difco) or cetrimide agar (Pseudomonas isolation agar; Fluka) plates at 37°C.
Preparation of phage lysates.
Phage strains MPK1 and MPK6 are enriched by the plate lysate method, using P. aeruginosa strain PAO1 as the host, as described elsewhere (12). The culture suspension was centrifuged at 8,000 × g for 10 min at 4°C to remove the cell debris, and phage particles were precipitated from culture supernatant in the presence of 10% polyethylene glycol (average molecular weight, 8,000) and 1 M NaCl and then dissolved in 5 ml phage buffer (10 mM MgSO4, 10 mM Tris [pH 7.6], and 1 mM EDTA). Phage particles were concentrated by ultracentrifugation at 110,000 × g for 3 h at 4°C and resuspended in phage buffer. The phage suspension was placed on top of a discontinuous CsCl gradient (1.45, 1.50, 1.70 g/ml) and centrifuged at 87,000 × g for 2 h at 4°C. The phage band was collected and dialyzed.
TEM.
The virion morphology of phages MPK1 and MPK6 was determined by transmission electron microscopy (TEM) as described previously (12). Briefly, formvar-coated TEM grids were subjected to hydrophilic treatment (10 min) and floated with 1:100 diluted CsCl-purified phage samples (20 μl), and negative staining (5 s) using 20 μl of 2% uranyl acetate (pH 4.0) immediately followed. The grids were allowed to air dry for 30 min and examined under a transmission electron microscope (JEM 1010 EM; JEOL Ltd.) at ×120,000 to ×500,000 magnification.
Phage infection.
Phage infection is observed by either conventional plaque assay or spotting assay (11). For plaque assay, 10 μl of lysates that contain about 102 PFU phages was mixed with 107 CFU of P. aeruginosa cells grown to the late logarithmic growth phase (i.e., optical density at 600 nm [OD600] of 0.7) and resuspended in 100 μl of phage buffer. After 10 min of incubation, 3 ml of top agar was added, and the mixture was plated. Plaques were visualized after 16 to 24 h of incubation at 37°C.
Mouse experiments.
Mouse infection was carried out using female ICR mice (aged 4 weeks), following the protocol approved by the Animal Care and Use Committee at Sogang University. Bacterial cells were grown to the stationary growth phase (OD600 of 3.0), harvested, washed twice with phosphate-buffered saline (PBS) buffer (2.7 mM KCl, 137 mM NaCl, 10 mM Na2HPO4, and 2 mM KH2PO4 [pH 7.0]), and then resuspended in PBS buffer at 2 × 107 CFU/ml. To induce peritonitis, mice were infected i.p. with 100 μl of the bacterial suspension (i.e., 2 × 106 CFU). For phage therapy, after 6 to 12 h postinfection, phage solution containing either 2 × 106 or 2 × 107 PFU in PBS buffer (100 μl) was administered either i.p. or intramuscularly (i.m.). For the enumeration of bacterial burden from mouse tissues, infected mice were anesthetized by inhalation of ether at the designated time points (0.5, 12, 24, 36, and 48 h postinfection). Lung, liver, and spleen samples were obtained aseptically and homogenized with a tissue homogenizer (Yamato Scientific Co., Ltd., Tokyo, Japan) in PBS buffer (1 ml). Portions of blood and homogenized tissue samples were plated onto cetrimide agar, which were incubated for 24 h at 37°C. For phage pharmacokinetics, phages were administered either i.p. or i.m. into the uninfected mice, from which the blood, lung, and liver samples were obtained and homogenized at the designated time points (0.5, 12, 24, 36, and 48 h). The tissue homogenates were subjected to filtration, and the filtrates were used for PFU measurement by plaque assay.
Drosophila melanogaster experiments.
Drosophila melanogaster Oregon R was grown at 25°C using the corn meal-dextrose medium (0.93% agar, 6.24% dry yeast, 4.08% corn meal, 8.62% dextrose, 0.1% methyl paraben, and 0.45% [vol/vol] propionic acid) as described elsewhere (18), with slight modification. Briefly, infection of flies was performed by pricking 5-day-old adult flies in the dorsal thorax with a 10-μm needle (Ernest F. Fullam, Inc.). The needle was dipped halfway into PBS-diluted bacterial suspension containing 107 CFU/ml from the stationary-growth-phase (OD600 of 3.0) cultures. At this dilution, we introduced 50 to 200 bacteria/animal. Infected flies were transferred to a new medium overlaid with 100 μl phage solution containing 5 × 107 PFU. Fly mortality was monitored for up to 48 h postinfection. Flies that died within 15 h under this condition (less than 5%) were not included in mortality determination. Mortality studies were repeated at least five times. For the enumeration of bacterial burdens, infected flies were anesthetized with CO2 and ground in LB medium at the designated time points. Homogenates of individual infected flies were plated onto cetrimide agar, which were incubated at 37°C for 24 h to measure the number of CFU per fly. For phage pharmacokinetics in D. melanogaster, flies were fed with phages for 12 h and then transferred to a new medium without phages. Flies were homogenized at the designated time points (0.5, 12, 24, 36, and 48 h); the tissue homogenates were filtered, and the filtrates were used for PFU measurement by plaque assay.
Statistical analysis.
Kaplan-Meier log rank statistics was used to determine the statistical significance of the differences between the control and the treatment groups in mortality rates. The statistical significance in the numbers of viable bacteria recovered from blood and organs was verified by the Mann-Whitney U test.
RESULTS AND DISCUSSION
Identification of the new Caudovirales phage strains specific for P. aeruginosa.
Phages specific to P. aeruginosa strains were initially isolated from sewage samples, which had been obtained several times from Seoul and the suburban districts. Once isolated, phages were screened for their lytic activity on the basis of large and distinguishable plaque formation, a characteristic of lytic phages. We selected six potentially lytic phages for P. aeruginosa strain PAO1 (named MPK1 to MPK6), based on the plaque size (>3-mm diameter after 18 h incubation at 37°C) (data not shown) and clarity (see Fig. 2). We determined the morphology of those six phages by using a transmission electron microscope. Based on their virion structure, with an icosahedral head of ∼70 nm in diameter (data not shown) and contractile tails of ∼110 nm in length with fibers (Fig. 1A), phages MPK1 to MPK5 belong to the Myoviridae family (order Caudovirales). In contrast, MPK6 has a head similar to that of MPK1 but has a stubby tail of <10 nm in length (Fig. 1B) and, thus, belongs to morphotype C, subdivision C1 of the Podoviridae family (order Caudovirales) (1, 2, 8). Conclusively, we have isolated six Caudovirales phages, which are most likely not temperate for P. aeruginosa. We selected MPK1 from the Myoviridae phages and MPK6 for the next experiments.
FIG. 2.
Plaque formation by MPK1 and MPK6 on various LPS mutants. MPK1 and MPK6 phage lysates containing about 3 × 102 PFU were spotted onto the bacterial lawns of PAO1 (WT) and its congenic LPS mutants, the rmd (deficient for group A polysaccharide), wbpM (deficient for group B polysaccharide), and rmlC (deficient for core, group A, and group B polysaccharides) mutants (see text for details).
FIG. 1.
Transmission electron micrographs of MPK1 (A) and MPK6 (B) negatively stained with uranyl acetate, revealing their virion structure. Bar, 100 nm.
Receptor of phages MPK1 and MPK6.
A number of surface receptors on P. aeruginosa cells have been implicated as bacteriophage receptors. Especially, the type IV pili (TFP) have been shown to function as the primary receptors for various P. aeruginosa phages, including filamentous phage Pf (6), single-stranded RNA phage PP7 (8), Bradley B type phage PO4 (7), and temperate transducing phages such as MP22 (12). We first determined whether TFP is required for the MPK1 and MPK6 infection and found that a pilA mutant of PAO1 was nonetheless sensitive to MPK1 and MPK6, which was further substantiated by the phage susceptibility of the twitching motility-defective clinical isolates in our culture collection (data not shown). The next candidate is LPS, although detailed molecular components in the LPS as the phage receptors remain elusive. Only the LPS core region is known to act as the phage receptor for phi PLS27 (13) and phi CTX (33). To determine whether LPS might act as the receptor for MPK1 and MPK6, we used a series of LPS mutants derived from strain PAO1: the rmd (deficient for group A polysaccharide), wbpM (deficient for group B polysaccharide), and rmlC (deficient for core polysaccharide and, hence, for group A and B polysaccharides as well) mutants. As shown in Fig. 2, while both phages could form clear plaques on the wild-type and rmd bacteria, no plaques were formed on rmlC bacteria. Interestingly, while MPK6 formed no plaque on wbpM mutant bacteria, MPK1 displayed tiny plaques on wbpM mutant bacteria, suggesting that the normal receptor might be affected by the group B polysaccharide mutation with alternative receptor(s) still present for MPK1 in wbpM mutant bacteria. Therefore, the LPS group B polysaccharide is most likely the primary receptor for both phage MPK1 and MPK6 entry into the PAO1 cells, although the molecular determinants for phage-receptor interactions seem to differ for both phages.
Lytic activity of phages MPK1 and MPK6 in vitro.
The lytic activity of both MPK1 and MPK6 phages was examined based on the multistep bacterial killing curve in the phage life cycle. P. aeruginosa PAO1 cells (∼107 CFU) were incubated in the medium containing 2 × 106 PFU of either MPK1 or MPK6 in 100 μl PBS. The number of viable bacteria gradually decreased up to ∼102 CFU for MPK1 after 30 min of incubation versus ∼103 CFU for MPK6 after 120 min (Fig. 3), demonstrating that both phages have potent lytic activity against P. aeruginosa PAO1 and that MPK1 is the more potent than is MPK6 for rapidly killing P. aeruginosa, at least under this growth condition. The capability of growth inhibition in vitro by these phages led us to further evaluate the antibacterial efficacy against experimental infections (in vivo) caused by P. aeruginosa.
FIG. 3.
Lytic activity of MPK1 and MPK6 in vitro. The PAO1 culture suspension was mixed with the phage lysate of 2 × 106 PFU of either MPK1 (•) or MPK6 (▪) in LB medium and then incubated further. The number of viable bacteria (CFU) was measured at the appropriate dilutions to count about 102 CFU.
Pharmacokinetics of phages MPK1 and MPK6 in mice.
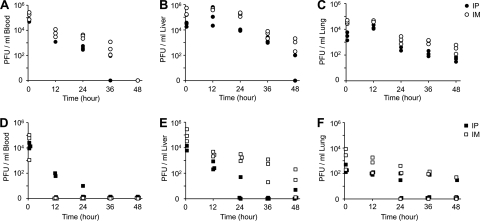
Prior to evaluating the therapeutic efficacy, we examined the pharmacokinetics of both phages to determine which way is better to deliver phages in mice—the i.p. or i.m. route. Phages (5 × 107 PFU) were introduced via the i.p. or i.m. route into uninfected mice. Three mice from each group receiving phage samples i.p. or i.m. were euthanized at 0.5, 12, 24, 36, and 48 h postinfection. The number of phages was enumerated from organ (liver and lung) and blood samples (per milliliter) (Fig. 4). In each tissue sample examined, a consistent pattern of the relative PFU levels after administration of both phages by the different routes was observed: PFU levels after i.m. administration were higher than those after i.p. administration (i.m. > i.p.), which is consistent with the previous study using a phage cocktail containing 108 PFU against burn wound infections by P. aeruginosa (20). Under all conditions, MPK1 displayed better persistence than does MPK6, although similar numbers were observed right after (i.e., 30 min) phage administration. MPK6 was hardly recovered from blood, especially when MPK6 was delivered by the i.m. route (Fig. 4D). These results suggest that both P. aeruginosa phages have similar pharmacokinetics in mice, but MPK1 displayed better persistence than did MPK6 in animal tissues.
FIG. 4.
Pharmacokinetics of MPK1 and MPK6 in mice. Phage samples (5 × 107 PFU in PBS) of either MPK1 (○ and •) (A to C) or MPK6 (□ and ▪) (D to F) were administered i.p. (closed symbols) or i.m. (open symbols) to uninfected mice. Groups of mice (n = 3) were sacrificed at 0.5, 12, 24, 36, and 48 h postinfection; blood (A and D), liver (B and E), and lung (C and F) were extracted, and their homogenates were used to measure the PFU per unit volume (ml) as described in Materials and Methods. The PFU/ml is shown on a log scale.
Therapeutic efficacy of phages MPK1 and MPK6 against mouse peritoneal infection.
Phages MPK1 and MPK6 were injected by either the i.m. or i.p. route with different doses (2 × 106 PFU and 2 × 107 PFU in 100 μl PBS) of P. aeruginosa strain PAO1 6 h postinfection. The 2 × 107 PFU dose of both phages significantly protected the infected mice compared to the untreated mice (Fig. 5A and B). No significant protection was observed with the 2 × 106 PFU dose of MPK6 administered by the i.m. route (Fig. 5A) (P = 0.316). Despite the better pharmacokinetics of the i.m. administration, as shown in Fig. 4, the i.p. administration displayed better efficacy than the i.m. administration for both MPK1 and MPK6. It is most likely that the i.p. administration can deliver the phages more directly or effectively to the infection site, since we used the peritonitis model.
FIG. 5.
Protective effect of MPK1 and MPK6 in mice. (A and B) Mortality of PAO1-infected mice with MPK1 (○ and •) and MPK6 (□ and ▪) doses of 2 × 107 PFU per 100 μl PBS (○ and □) or 2 × 106 PFU per 100 μl PBS (• and ▪), which were administered by the i.m. (A) or i.p. (B) route at 6 h postinfection. PAO1-infected mice without phage treatment (⋄) were 100% moribund within 48 h. The statistical significance based on a log rank test is indicated as follows: **, P < 0.01; ***, P < 0.001. (C) Bacterial burdens in lung, spleen, and liver of mice treated with or without i.p. phage treatment (2 × 107 PFU in 100 μl PBS) as in panel B. The numbers of viable bacteria (CFU) were measured from the organs of live mice at 24 h postinfection. The CFU per unit volume (ml) were measured as described in Materials and Methods and are shown on a log scale.
To verify whether the administration of phages could inhibit the bacterial proliferation, we determined the bacterial loads in mouse organs, including spleen, lung, and liver, since peritonitis is concomitant with bacterial infiltration at the liver of infected animals (5). The organ samples were obtained from live animals at 24 h postinfection. As shown in Fig. 5C, treatment with both phages could significantly reduce the bacterial loads; MPK1 treatment could reduce the bacterial burdens by about 2 to 3 logs in lung, spleen, and liver of live mice at 24 h postinfection, whereas slightly less reduction (by about 2 logs) was observed in the MPK6-treated live mice. These results suggest that both phages are highly effective in controlling P. aeruginosa-induced peritonitis by inhibiting bacterial proliferation in vivo, for which MPK1 displays better efficacy than MPK6 does.
Pharmacokinetics and therapeutic efficacy of phages MPK1 and MPK6 in D. melanogaster.
The D. melanogaster systemic infection model is well established for studying the bacterial virulence mechanisms and needs a very small number of P. aeruginosa cells (50 to 200 CFU) to infect, compared to the murine peritonitis model (107 CFU). It causes death within 48 h as a result of systemic infection by bacterial proliferation of up to ∼107 CFU under our optimized experimental conditions (16, 18). To establish the D. melanogaster model to evaluate phage therapy against P. aeruginosa, we exploited phage feeding on a group of flies (n = 50) in a fly vial by overlaying the phage samples containing 5 × 107 PFU onto a fly vial containing 2.5 ml of corn meal medium.
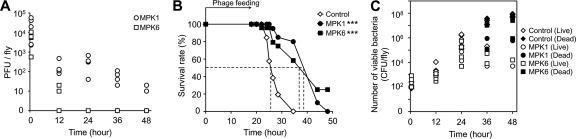
We first examined the pharmacokinetics of both phages which could be orally administered to D. melanogaster, in order to assess their potential toxicity as well as their persistence in fly tissues. Uninfected flies were fed with either phage (5 × 107 PFU) and transferred to a new medium without phage feeding. As shown in Fig. 6A, phages were recovered from fed flies, indicating that phages might be successfully administered by the overlay on top of a medium. About 104 PFU of both phages were transferred by only 12 h of feeding. Also, the MPK1 level was maintained for up to 48 h after transfer to a new medium, although the PFU gradually decreased by about 3 logs at 48 h. Interestingly, MPK6 was poorly recovered and disappeared before 12 h without bacterial infection after transfer to a new medium. Furthermore, both phages were not toxic at all by 12 h of feeding. No fly died even with phage feeding for more than 72 h (data not shown). These results suggest that phage feeding can be exploited to administer phages for phage therapy for D. melanogaster infection.
FIG. 6.
Bacteriophage therapy against D. melanogaster systemic infection. (A) Pharmacokinetics of MPK1 and MPK6. Phage samples (5 × 107 PFU in PBS) of either MPK1 (A) (○ and •) or MPK6 (B) (□ and ▪) were overlaid on the surface of the fly media. Groups of flies (n = 5) were collected at 0.5, 12, 24, 36, and 48 h, and their homogenates were removed to measure the PFU per fly, which is shown on a log scale. (B) Mortality of PAO1-infected flies fed with phages. Infected flies were transferred to a new medium overlaid with no phages (⋄) or with phage samples (5 × 107 PFU in PBS) of either MPK1 (•) or MPK6 (▪). The dotted lines represent the time required to reach 50% mortality. The statistical significance based on a log rank test is indicated as follows: ***, P < 0.001. (C) Bacterial burdens in fly homogenates fed with phages as described for panel A. The number of viable bacteria (CFU) was measured from the homogenates of an individual live (open symbols) and dead (solid symbols) fly at 0.5 h, 12 h, 24 h, or 48 h postinfection. The CFU per fly are shown on a log scale. ⋄ and ♦, no phage treatment; ○ and •, MPK1; □ and ▪, MPK6.
Next, the antibacterial efficacy of both phages was evaluated, based on their protective effects from PAO1-induced mortality and proliferation in the D. melanogaster infection model. Phages MPK1 and MPK6 were administered by overlaying 5 × 107 PFU on a fly vial to accommodate 50 PAO1-infected flies (i.e., feeding 106 phage per fly). Both phages significantly protected the infected flies, compared to no phage feeding (Fig. 6B). Similar levels of efficacy were observed for both phages (P = 0.637), although a better pharmacokinetics of MPK1 was observed in fly tissues.
We examined the protective effect on the bacterial proliferation in fly tissues by phage feeding. As shown in Fig. 6C, MPK6 and, to the larger extent, MPK1 inhibited the bacterial proliferation under this infection condition. Since P. aeruginosa levels reach 107 CFU (i.e., by 5 log increase) when the infected flies died (18), >2 log inhibition could be sufficient to resist PAO1-induced killing when flies started to die. These results suggest that both phages are highly effective in controlling P. aeruginosa-induced D. melanogaster killing by inhibiting bacterial proliferation in vivo.
Conclusion.
In this study, we have isolated two new P. aeruginosa phages (MPK1 and MPK6), whose primary receptor is the group B polysaccharide of P. aeruginosa LPS, and evaluated their antibacterial efficacy based on mouse and Drosophila melanogaster infection models. Several cases of phage therapy against the experimental infections by P. aeruginosa in mice were reported, which include gut-derived sepsis and burn wound infections (20, 31). A recent study of pyocin therapy was based on a mouse peritonitis model (24). All those experimental infection models appear relevant, considering that the complicated pathophysiology was caused by P. aeruginosa infections. More importantly, P. aeruginosa is a multihost pathogen that can intoxicate phylogenetically diverse hosts, including mammals, insects, worms, and plants, involving a distinct set of virulence factors. Thus, various infection models should be exploited to evaluate and confirm the antibacterial efficacy of antimicrobial agents.
One of the meaningful aspects of the present study is that we established a new therapeutic animal model to evaluate the antibacterial activity in vivo using a nonmammalian model host, D. melanogaster. We are currently investigating how phages are administered and where they are specifically localized within fly tissues to optimize this model for further studies. In recent studies, a nonmammalian live-animal infection model using a nematode (Caenorhabditis elegans) infection model has been successfully exploited for high-throughput screens to isolate new antimicrobial compounds against Enterococcus faecalis or Candida albicans (9, 21). A major advantage of using nonmammalian model hosts is that the research costs (mammalian animals and space, etc.) and, more importantly, the experimental scale can be significantly reduced, since the small size of the nonmammalian model animals requires far less space and smaller amounts of pathogens and antimicrobials for the experimental settings, which in turn enables the high-throughput analysis from the chemical libraries as well. Another great advantage of such live-animal infection models is that new antimicrobial compounds can be isolated, which do not inhibit the growth of the target microorganisms but do attenuate the virulence pathways and/or enhance the host immune response. Therefore, a further optimization of the D. melanogaster model to evaluate the antibacterial efficacy of various phages against P. aeruginosa infection will enhance our insights into antimicrobial therapy and provide a platform to specifically modulate the interface between bacterial virulence and host immunity.
Acknowledgments
This work was supported by grants from the 21C Frontier Microbial Genomics and Applications Center and the Seoul R&BD Program. Y.-J.H., Y.-R.L., and H.-H.J. were recipients of the BK21 Fellowship.
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Ackermann, H. W. 2001. Frequency of morphological phage descriptions in the year 2000. Brief review. Arch. Virol. 146:843-857. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann, H. W. 1998. Tailed bacteriophages: the order Caudovirales. Adv. Virus Res. 51:135-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alisky, J., K. Iczkowski, A. Rapoport, and N. Troitsky. 1998. Bacteriophages show promise as antimicrobial agents. J. Infect. 36:5-15. [DOI] [PubMed] [Google Scholar]
- 4.Aronoff, S. C., M. M. Olson, M. W. Gauderer, M. R. Jacobs, J. L. Blumer, and R. J. Izant, Jr. 1987. Pseudomonas aeruginosa as a primary pathogen in children with bacterial peritonitis. J. Pediatr. Surg. 22:861-864. [DOI] [PubMed] [Google Scholar]
- 5.Barekzi, N. A., K. A. Poelstra, A. G. Felts, I. A. Rojas, J. B. Slunt, and D. W. Grainger. 1999. Efficacy of locally delivered polyclonal immunoglobulin against Pseudomonas aeruginosa peritonitis in a murine model. Antimicrob. Agents Chemother. 43:1609-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley, D. E. 1973. The length of the filamentous Pseudomonas aeruginosa bacteriophage Pf. J. Gen. Virol. 20:249-252. [DOI] [PubMed] [Google Scholar]
- 7.Bradley, D. E. 1973. A pilus-dependent Pseudomonas aeruginosa bacteriophage with a long noncontractile tail. Virology 51:489-492. [DOI] [PubMed] [Google Scholar]
- 8.Bradley, D. E. 1967. Ultrastructure of bacteriophage and bacteriocins. Bacteriol. Rev. 31:230-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breger, J., B. B. Fuchs, G. Aperis, T. I. Moy, F. M. Ausubel, and E. Mylonakis. 2007. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 3:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagens, S., A. Habel, U. von Ahsen, A. von Gabain, and U. Blasi. 2004. Therapy of experimental Pseudomonas infections with a nonreplicating genetically modified phage. Antimicrob. Agents Chemother. 48:3817-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo, Y.-J., I.-Y. Chung, K. B. Choi, and Y.-H. Cho. 2007. R-type pyocin is required for competitive growth advantage between Pseudomonas aeruginosa strains. J. Microbiol. Biotechnol. 17:180-185. [PubMed] [Google Scholar]
- 12.Heo, Y.-J., I.-Y. Chung, K. B. Choi, G. W. Lau, and Y.-H. Cho. 2007. Genome sequence comparison and superinfection between two related Pseudomonas aeruginosa phages, D3112 and MP22. Microbiology 153:2885-2895. [DOI] [PubMed] [Google Scholar]
- 13.Jarrell, K. F., and A. M. Kropinski. 1981. Pseudomonas aeruginosa bacteriophage φPLS27-lipopolysaccharide interactions. J. Virol. 40:411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, C. C., J. Baldessarre, and M. E. Levison. 1997. Peritonitis: update on pathophysiology, clinical manifestations, and management. Clin. Infect. Dis. 24:1035-1045. (Quiz, 24:1046-1047.) [DOI] [PubMed] [Google Scholar]
- 15.Juergensen, P. H., F. O. Finkelstein, R. Brennan, S. Santacroce, and M. J. Ahern. 1988. Pseudomonas peritonitis associated with continuous ambulatory peritoneal dialysis: a six-year study. Am. J. Kidney Dis. 11:413-417. [DOI] [PubMed] [Google Scholar]
- 16.Kim, S.-H., S.-Y. Park, Y.-J. Heo, and Y.-H. Cho. 2008. Drosophila melanogaster-based screening for multihost virulence factors of Pseudomonas aeruginosa PA14 and identification of a virulence-attenuating factor, HudA. Infect. Immun. 76:4152-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krothapalli, R., W. B. Duffy, C. Lacke, W. Payne, H. Patel, V. Perez, and H. O. Senekjian. 1982. Pseudomonas peritonitis and continuous ambulatory peritoneal dialysis. Arch. Intern. Med. 142:1862-1863. [PubMed] [Google Scholar]
- 18.Lee, J.-S., Y.-J. Heo, J. K. Lee, and Y.-H. Cho. 2005. KatA, the major catalase, is critical for osmoprotection and virulence in Pseudomonas aeruginosa PA14. Infect. Immun. 73:4399-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuzaki, S., M. Rashel, J. Uchiyama, S. Sakurai, T. Ujihara, M. Kuroda, M. Ikeuchi, T. Tani, M. Fujieda, H. Wakiguchi, and S. Imai. 2005. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J. Infect. Chemother. 11:211-219. [DOI] [PubMed] [Google Scholar]
- 20.McVay, C. S., M. Velasquez, and J. A. Fralick. 2007. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob. Agents Chemother. 51:1934-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moy, T. I., A. R. Ball, Z. Anklesaria, G. Casadei, K. Lewis, and F. M. Ausubel. 2006. Identification of novel antimicrobials using a live-animal infection model. Proc. Natl. Acad. Sci. USA 103:10414-10419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pruitt, B. A., Jr., A. T. McManus, S. H. Kim, and C. W. Goodwin. 1998. Burn wound infections: current status. World J. Surg. 22:135-145. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld, M., B. W. Ramsey, and R. L. Gibson. 2003. Pseudomonas acquisition in young patients with cystic fibrosis: pathophysiology, diagnosis, and management. Curr. Opin. Pulm. Med. 9:492-497. [DOI] [PubMed] [Google Scholar]
- 24.Scholl, D., and D. W. Martin, Jr. 2008. Antibacterial efficacy of R-type pyocins towards Pseudomonas aeruginosa in a murine peritonitis model. Antimicrob. Agents Chemother. 52:1647-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slopek, S., B. Weber-Dabrowska, M. Dabrowski, and A. Kucharewicz-Krukowska. 1987. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch. Immunol. Ther. Exp. (Warsz.) 35:569-583. [PubMed] [Google Scholar]
- 26.Soothill, J., C. Hawkins, E. Anggard, and D. Harper. 2004. Therapeutic use of bacteriophages. Lancet Infect. Dis. 4:544-545. [DOI] [PubMed] [Google Scholar]
- 27.Speert, D. P., M. E. Campbell, D. A. Henry, R. Milner, F. Taha, A. Gravelle, A. G. Davidson, L. T. Wong, and E. Mahenthiralingam. 2002. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis in British Columbia, Canada. Am. J. Respir. Crit. Care Med. 166:988-993. [DOI] [PubMed] [Google Scholar]
- 28.Steinstraesser, L., Y. Oezdogan, S. C. Wang, and H. U. Steinau. 2004. Host defense peptides in burns. Burns 30:619-627. [DOI] [PubMed] [Google Scholar]
- 29.Summers, W. C. 2001. Bacteriophage therapy. Annu. Rev. Microbiol. 55:437-451. [DOI] [PubMed] [Google Scholar]
- 30.Summers, W. C. 1999. Felix d'Herelle and the origins of molecular biology. Yale University Press, New Haven, CT.
- 31.Watanabe, R., T. Matsumoto, G. Sano, Y. Ishii, K. Tateda, Y. Sumiyama, J. Uchiyama, S. Sakurai, S. Matsuzaki, S. Imai, and K. Yamaguchi. 2007. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 51:446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinstein, W. M., A. B. Onderdonk, J. G. Bartlett, and S. L. Gorbach. 1974. Experimental intra-abdominal abscesses in rats: development of an experimental model. Infect. Immun. 10:1250-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokota, S., T. Hayashi, and H. Matsumoto. 1994. Identification of the lipopolysaccharide core region as the receptor site for a cytotoxin-converting phage, φCTX, of Pseudomonas aeruginosa. J. Bacteriol. 176:5262-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]