Abstract
MDCK cells transfected with the human β-galactoside α-2,6-sialyltransferase 1 gene (AX-4 cells) were used to determine the drug susceptibility and pharmacodynamically linked variable of oseltamivir for influenza virus. For dose-ranging studies, five hollow-fiber units were charged with 102 A/Sydney/5/97 (H3N2) influenza virus-infected AX-4 cells and 108 uninfected AX-4 cells. Each unit was treated continuously with different oseltamivir carboxylate concentrations in virus growth medium for 6 days. For dose fractionation studies, one hollow-fiber unit received no drug, one unit received a 1× 50% effective concentration (EC50) exposure to oseltamivir by continuous infusion, one unit received the same AUC0-24 (area under the concentration-time curve from 0 to 24 h) by 1-h infusion every 24 h, one unit received the same total exposure in two equal fractions every 12 h, and one unit received the same total exposure in three equal fractions every 8 h. Each infusion dose was followed by a no-drug washout, producing the appropriate half-life for this drug. The effect of the drug on virus replication was determined by sampling the units daily, measuring the amount of released virus by plaque assay, and performing a hemagglutination assay. The drug concentration in the hollow-fiber infection model systems was determined at various times by liquid chromatography-tandem mass spectrometry. The dose-ranging study showed that the EC50s for oseltamivir carboxylate for the A/Sydney/5/97 strain of influenza virus was about 1.0 ng/ml. The dose fractionation study showed that all treatment arms suppressed virus replication to the same extent, indicating that the pharmacodynamically linked variable was the AUC0-24/EC50 ratio. This implies that it may be possible to treat influenza virus infection once daily with a dose of 150 mg/day.
Influenza type A viruses H3N2 and H1N1 and influenza type B virus cause infections in people that lead to considerable morbidity and, among the very young and the very old, mortality on an annual basis (33). These yearly epidemics of influenza are caused by changes in the amino acid composition of the two glycoproteins, hemagglutinin (HA) and neuraminidase, found on the surface of the virus particle. This process is called antigenic drift (5). Occasionally, major changes occur in the antigenic makeup of the virus due to the acquisition of one or more genes when more than one influenza A virus replicates in the same cell. Replacement of the genes encoding the HA and/or neuraminidase proteins of one of the viruses with those of the other virus leads to the creation of viruses with completely new antigenic properties, a process called antigenic shift (5). These major changes in the antigenic properties of influenza A viruses have the potential to cause influenza pandemics if the viruses have the ability to spread easily from person to person. Such pandemics occurred in 1918, 1957, and 1968, killing 40 to 50 million people in 1918 and many thousands of people throughout the world in the latter two pandemics (25).
There are several licensed antiviral compounds for the prevention and treatment of influenza virus infections. The adamantanes, amantadine and rimantadine, are effective for the prevention and treatment of influenza caused by type A influenza viruses that are susceptible to these drugs (6). Unfortunately, the majority of strains of influenza A virus that circulate in the world today are resistant to these two relatively inexpensive drugs (9, 12, 19). The neuraminidase inhibitors, oseltamivir carboxylate and zanamivir, are effective in the prevention and treatment of influenza A and B virus infections (21, 29, 34) and have been approved by the Food and Drug Administration for the prevention and therapy of uncomplicated influenza virus infections. Wide-spread resistance to oseltamivir carboxylate has recently been observed (30). Two experimental neuraminidase inhibitors, peramivir and A-315675, are under development for the prevention and treatment of influenza virus infections (2, 3, 4, 10, 23, 24, 28). Thus, there are potentially effective therapies for the prevention and treatment of epidemic and pandemic influenza. Now the question that remains is how much drug to give and how often to give that much drug to maintain sufficient drug levels to cure an infected patient while decreasing the opportunity for the emergence of drug-resistant viruses during therapy.
Drusano and colleagues developed the in vitro pharmacodynamic hollow-fiber infection model (HFIM) system to determine the correct dose and schedule of administration of antiviral drugs against human immunodeficiency virus (HIV) (7, 8, 14-16, 31), and the results of these studies have been validated in clinical trials of these antiviral drugs (7, 14-16, 31). Clear recommendations were also generated from the HFIM for cidofovir against vaccinia virus (26).
Given that we have successfully examined dose and schedule for other antiviral compounds, we wished to examine whether oseltamivir could be used on a daily schedule. The currently recommended dose and schedule of oseltamivir for adults suffering from uncomplicated influenza is 75 mg given twice a day (34). Since daily therapy is better than twice-daily therapy because of adherence issues, we wished to use the HFIM to examine whether there was any change in anti-influenzal effect if oseltamivir was administered once versus twice daily.
MATERIALS AND METHODS
Cells.
MDCK cells (ATCC CCL-34) were obtained from the American Type Culture Collection. AX-4 cells, an MDCK cell line overexpressing the human β-galactoside α-2,6-sialyl transferase (ST6Gal 1) gene, was obtained from Y. Kawaoka of the University of Wisconsin (18). MDCK cells were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum, 1% sodium pyruvate, 1% MEM nonessential amino acids, 1% penicillin-streptomycin, and 1% glutamine. AX-4 cells were maintained in MEM supplemented with 5% fetal bovine serum, 1% penicillin-streptomycin, and 375 μl of puromycin (10 mg/ml) per 500 ml of medium to give a final concentration of 7.5 μg/ml. The cells were grown as monolayers in 75-cm2 or 25-cm2 cell culture flasks (Corning, Inc., Corning, NY) or in six-well tissue culture plates (Corning, Inc., Corning, NY) at 37°C and 5% CO2.
Virus.
Influenza virus strain A/Sydney/5/97 R292 was obtained from Retroscreen, London, England. MDCK cells infected with this virus react with a monoclonal antibody specific for the influenza A virus nucleocapsid antigen and with a monoclonal antibody directed against the influenza virus H3 antigen, confirming that this isolate is an H3N2 subtype of type A influenza virus. Both fluorochrome-labeled monoclonal antibodies were obtained from Chemicon International, Inc., Temecula, CA.
Antiviral drug.
The d-tartrate salt of oseltamivir carboxylate was obtained from Angela Perrin of F. Hoffman-La Roche Ltd., Basel, Switzerland. Stocks of drug were prepared by suspending the powder in water to yield a final concentration of 13.6 mg/ml (equivalent to 10 mg/ml of oseltamivir carboxylate) and filter sterilized through a 0.2-μm filter, and the filtrate was stored at −80°C. Fresh stocks were prepared every 2 to 3 months.
EC50 determination.
The EC50 is the drug concentration that will reduce the number of PFU by 50% of the number obtained in the absence of drug. The procedure for determining EC50 has been described previously (27). In brief, to determine the EC50 for oseltamivir carboxylate for this influenza A virus isolate, AX-4 cell monolayers were prepared in 25-cm2 plastic tissue culture flasks. The following day, influenza A virus, diluted in MEM supplemented with 0.2% bovine serum albumin (Sigma Chemical Co., St. Louis, MO), 2 μg/ml of tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK)-trypsin (Sigma Chemical Co., St. Louis, MO), and 1% penicillin-streptomycin (virus growth medium [VGM]) to yield a multiplicity of infection (MOI) of 0.0001 PFU/cell, was added to monolayers of cells. After a 2-h incubation period at 36°C, the inoculum was removed and 5-ml amounts of VGM supplemented with various concentrations of the d-tartrate salt of oseltamivir carboxylate were added to the appropriate flasks. The infected monolayers were incubated at 36°C under an atmosphere of 5% CO2 for 48 to 72 h. The monolayers were observed daily for cytopathic effect. At 48 and 72 h postinfection, the medium containing released virus was collected and clarified by centrifugation at 800 × g for 5 min to remove floating cells and cell debris, and the clarified supernatant was divided into 1-ml samples and frozen at −80°C. The effects of different concentrations of oseltamivir carboxylate on the yield of influenza A virus were determined by plaque assay (20, 32). In brief, tenfold dilutions of samples of influenza A virus were made in VGM and 0.5 ml of each dilution was placed on a 1-day-old, confluent MDCK cell monolayer in six-well plates. After a 2-h adsorption period at 36°C under an atmosphere of 5% CO2, the inoculum was removed; a 0.5% agar overlay containing MEM, 0.2% bovine serum albumin, 2 μg/ml TPCK-trypsin, 0.5% DEAE- dextran, and 1% penicillin-streptomycin was added to each well; and the plates were incubated at 36°C and 5% CO2 for 48 to 72 h. The plaques were counted visually.
Hemagglutination assay.
To determine the amount of released virus produced in the hollow-fiber units, virus samples were diluted 1:10 in phosphate-buffered saline (PBS) in quadruplicate, followed by 11 twofold serial dilutions in PBS. Then, 50 μl of a 0.5% turkey red blood cell suspension was added to each well. After 30 to 45 min at room temperature, the wells were scored for hemagglutination. The hemagglutination titer was the row of wells at the lowest virus dilution with a positive hemagglutination response in half of the wells in the row.
HFIM system.
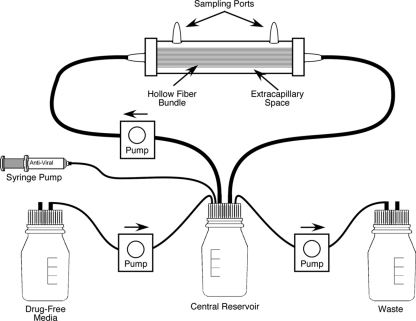
To determine the pharmacodynamically linked variable for antiviral compounds effective against viruses, we employed the HFIM system (Fig. 1). The use of the system with viruses has been previously described (26). For our studies with oseltamivir carboxylate and the A/Sydney/5/97 R292 strain of influenza virus, we used 4300-C2011 hollow-fiber cartridges (FiberCell Systems, Inc., Frederick, MD) containing high-molecular-cutoff (average pore size, 20 kDa) polysulfone fibers. Each hollow-fiber unit was treated with PBS for 2 days, followed by 1 day of treatment with VGM. Influenza A virus-infected cells were prepared by infecting an AX-4 cell monolayer in a 75-cm2 flask with A/Sydney/5/97 R292 virus at an MOI of 0.0001 PFU/cell. After 18 h of incubation, the cells were removed from the flask with 0.25% trypsin-EDTA solution and suspended in VGM. One aliquot was mixed with trypan blue, and the total number of viable cells was determined by counting the cells with a hemocytometer. Another aliquot was fixed with 10% formaldehyde, permeabilized, and treated with a fluorescein isothiocyanate-labeled monoclonal antibody to the influenza A nucleocapsid antigen (Chemicon International, Inc., Temecula, CA), and the percentage of antigen-positive cells was measured by flow cytometry. These two measurements determined the number of virus-infected AX-4 cells in the suspension of infected cells. One hundred influenza virus-infected AX-4 cells were mixed with 108 uninfected AX-4 cells in 25 ml of VGM and injected into the extracapillary space (ECS) of each hollow-fiber unit. For dose-ranging experiments, the units containing this mixture of uninfected and virus-infected cells were treated with various concentrations of oseltamivir carboxylate by continuous infusion for 6 to 7 days. To maintain the proper drug concentration, the medium in the central reservoirs was changed daily. For dose fractionation experiments, a dose of drug was administered as a continuous infusion or the total dose of drug was injected into the central reservoir over a 1-h period on a q24h (whole dose every 24 h), q12h (one-half dose every 12 h), or q8h (one-third dose every 8 h) schedule, followed by a no-drug washout with the appropriate half-life. All treated units received the same AUC0-24 (area under the concentration-time curve from 0 to 24 h) of oseltamivir carboxylate. In all cases, virus replication was monitored daily by sampling the medium in the ECS to determine the amount of cell-free virus by plaque assay and hemagglutination assay.
FIG. 1.
HFIM system. Each hollow-fiber cartridge contains semipermeable hollow fibers which allow gases, low-molecular-weight nutrients, and low-molecular-weight antiviral compounds to pass through the membranes while keeping cells and viruses outside the membranes. Uninfected and virus-infected cells are added to the cartridge through one of the sampling ports on the top of the cartridge. Medium from the reservoir is pumped through the hollow fibers to nourish the cells that grow on the outside surface of the hollow fibers. The contents of the ECS of the hollow-fiber units are sampled for cells, cell-free virus, and drug through the ports on the top of each unit, and the concentration of drug entering the hollow-fiber unit can be determined by sampling the medium as it enters the hollow-fiber unit from the reservoirs.
Drug assay.
To determine the actual concentration of oseltamivir carboxylate in the medium entering each hollow-fiber unit at each time point, a sample was removed daily from the medium leaving the reservoir and entering the hollow-fiber units, and the concentration of drug was determined by high-pressure liquid chromatography (HPLC)-tandem mass spectrometry (LC-MS-MS) (Applied Biosystems, Inc.). Samples (0.050 ml) in VGM were diluted with HPLC water (0.050-ml sample into 0.050 ml water) and were analyzed by LC-MS-MS for oseltamivir carboxylate concentrations. The LC-MS-MS system was comprised of a Shimadzu Prominence HPLC system and an Applied Biosystems/MDS Sciex API5000 LC-MS-MS system. Chromatographic separation was performed by using a 100- by 3.0-mm Phenomenex Onyx Monolithic C18 column with a mobile phase consisting of 80% 5 mM ammonium acetate, pH 3.5, and 20% methanol at a flow rate of 1.0 ml/min. Oseltamivir carboxylate concentrations were obtained by using LC-MS-MS, monitoring the MS-MS transition m/z 285 → m/z 138. The run time of the analysis was 5.0 min. The assay was linear over a range of 0.25 to 10.0 ng/ml (r2 > 0.994). The interday coefficients of variation for the quality control samples, analyzed in replicates of three at three concentrations on each analysis day (0.500, 1.00, and 5.00 ng/ml), ranged from 4.95 to 12.4%, with accuracies (percent recovery) ranging between 91.5% and 112%.
Statistical analysis.
An inhibitory sigmoid-Emax (maximal effect) model of the form {effect = control effect − [Emax × exposureH/(exposureH + EC50H)]} was fit to the data. Control effect is the measured viral output in the absence of drug, Emax is the greatest reduction in viral output produced by drug exposure, and H is Hill's constant. The model was fit to the data by nonlinear regression analysis, performed within the ADAPT II package of programs of D'Argenio and Schumitzky (11).
RESULTS
EC50s of oseltamivir carboxylate for A/Sydney/5/97 R292 strain of influenza virus grown in AX-4 cells.
To use the HFIM system to determine the pharmacodynamically linked variable for an antiviral drug for a particular virus, one must know the EC50 of that drug for the virus under study in the particular cell line used for the host. To that end, we determined the effect of freshly prepared d-tartrate salt of oseltamivir carboxylate on the replication of the A/Sydney/5/97 strain of influenza virus in AX-4 cell monolayers in six-well plates. The results showed that the average EC50 for the A/Sydney/5/97 R292 strain of influenza virus grown in AX-4 cells is 10.23 ± 8.66 ng/ml (mean ± standard deviation; range of 2 to 29 ng/ml) of oseltamivir carboxylate.
Growth of the A/Sydney/5/97 R292 strain of influenza virus in AX-4 cells in hollow-fiber units.
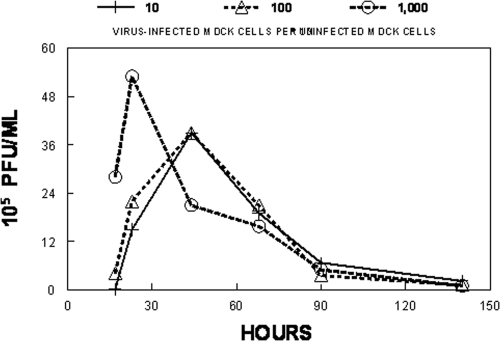
To determine the best conditions for the replication of the A/Sydney/5/97 R292 strain of influenza virus in AX-4 cells when the cells are growing in the hollow-fiber units, 101, 102, or 103 A/Sydney/5/97 R292 influenza virus-infected AX-4 cells were mixed with 108 uninfected AX-4 cells and placed in three hollow-fiber units. Each unit was continuously infused with VGM for 6 days. At various times postinfection, the ECS was sampled and the amount of infectious virus released into the medium was determined by plaque assay. The results are illustrated in Fig. 2. The hollow-fiber units initiated with 101 or 102 virus-infected cells produced around 105 PFU/ml at 17 h postinfection, with virus production peaking at 39 × 105 PFU/ml at 44 h postinfection, followed by a slow decline in the amount of infectious virus over the 140-h time course of the experiment. This decline in virus infectivity is most likely due to the lack of fresh target cells to keep the infection going. The hollow-fiber unit initiated with 103 virus-infected cells produced more infectious virus at 17 h postinfection, but the infection peaked at 23 h postinfection, followed by a rapid decline in the amount of infectious virus produced in the hollow-fiber units under conditions of a higher MOI. The data clearly demonstrated that under these low-MOI conditions, the A/Sydney/5/97 R292 strain of influenza virus can replicate in AX-4 cells in the hollow-fiber system.
FIG. 2.
Growth of influenza virus in the HFIM system—effect of MOI on virus yield. For growth of the R292 strain of A/Sydney/5/97 H3N2 influenza virus in AX-4 cells in the HFIM system, various amounts of virus-infected AX-4 cells were mixed with 108 uninfected AX-4 cells and the cell mixtures were inoculated into hollow-fiber units. VGM was continuously circulated through each hollow-fiber unit at 36°C and 5% CO2 for 6 days. At various times postinfection, virus-infected cells and cell-free virus were removed from the ECS through ports on the top of the hollow-fiber cartridges. The cells were removed by low-speed centrifugation, and the amount of infectious virus in the supernatant was determined by plaque assay. The data show that at a high MOI (103 virus-infected cells per 108 uninfected cells), virus replicated rapidly in the HFIM system, followed by a decline in infectious virus. At lower MOIs (101 and 102 virus-infected cells per 108 uninfected cells), virus grew well, reaching a peak of infectious virus at 48 h postinfection. Since initiating infections at 102 infected cells per 108 uninfected cells gave a peak of virus replication at 48 h postinfection (mimicking natural infection in humans), all of the studies reported in this paper used that MOI.
Production of infectious virus in nasal secretions in experimental human influenza virus infections peaks at 48 h postinfection (13). Since it is important to attempt to model the human infection in the HFIM system, we chose to initiate the infection in the hollow-fiber units with 102 virus-infected cells and 108 uninfected AX-4 cells, an experimental condition that allowed virus replication to peak at 48 h postinfection, followed by a slow decline in virus infectivity. All experiments reported in this paper were performed by initiating the HFIM systems with 102 virus-infected AX-4 cells and 108 uninfected AX-4 cells.
Dose-ranging study of oseltamivir carboxylate for the A/Sydney/5/97 R292 strain of influenza virus.
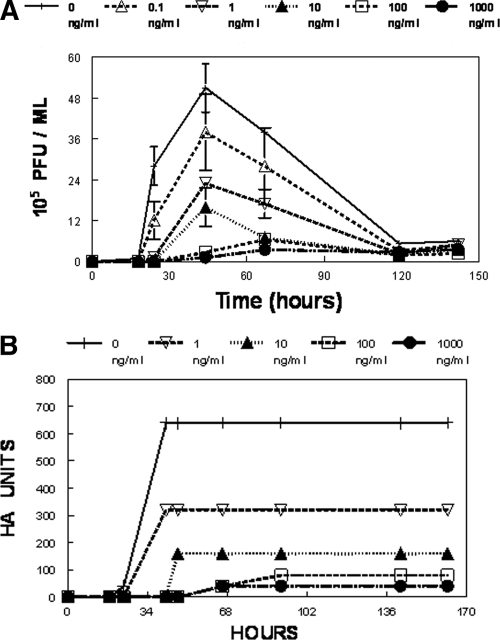
The EC50 for oseltamivir carboxylate for the R292 isolate of A/Sydney/5/97 influenza virus in AX-4 cells grown in flasks is about 10 ± 8 ng/ml. To determine the EC50 of oseltamivir carboxylate for the A/Sydney/5/97 R292 strain of influenza virus in AX-4 cells in the HFIM system, five hollow-fiber units were set up with 102 virus-infected AX-4 cells and 108 uninfected AX-4 cells. Each hollow-fiber unit was continuously infused with a different concentration of oseltamivir carboxylate for 6 days. Figure 3A and B show the effects of different concentrations of oseltamivir carboxylate on the production of A/Sydney/5/97 influenza virus. In the absence of drug, the virus grew well, with a peak titer of 51 × 105 PFU/ml or 640 hemagglutination units at 44 h postinfection. At later times after infection, the amount of infectious virus declined due to the heat sensitivity of the virus and to lack of new cells to infect. The decrease was not evident in the hemagglutination assay, most likely because the hemagglutination antigen was more heat stable than virus infectivity. In the hollow-fiber units, at exposures of a continuous concentration of 1 ng/ml, oseltamivir carboxylate inhibited the production of infectious virus or hemagglutination units by approximately 50% of the control value (sigmoid-Emax modeling provided the following estimates: Emax, 49.85 PFU/ml; EC50, 0.726 ng/ml; H, 0.624; Econ (control effect), 50.5 PFU/ml; and r2, 0.978). At higher concentrations, the drug inhibited the production of infectious virus and the number of HA units to a greater extent. At 100 and 1,000 ng/ml of oseltamivir carboxylate, virus production was almost completely suppressed. This information was used to perform a dose fractionation experiment to determine the pharmacodynamically linked variable for this compound for this virus.
FIG. 3.
(A and B) Dose range experiment for A/Sydney/5/97 R292 influenza virus and oseltamivir carboxylate in the HFIM system. To determine the dose of oseltamivir carboxylate that will inhibit the replication of A/Sydney/5/97 R292 in AX-4 cells in the HFIM system, 102 virus-infected cells and 108 uninfected AX-4 cells were loaded into hollow-fiber units and continuously infused with various concentrations of oseltamivir carboxylate for 6 days. Each hollow-fiber unit was sampled daily, and the amount of virus produced was measured by plaque assay (A) and by hemagglutination assay (B). For panel A, the relationship between drug exposure and viral inhibition given is as follows: PFU/ml × 105 at 44 h = 50.5 × 105 − {49.9 × 105[Oselt conc0.624]/(Oselt conc0.624 + 0.7260.624)}; r2 = 0.978. Error bars show standard deviations.
Dose fractionation study.
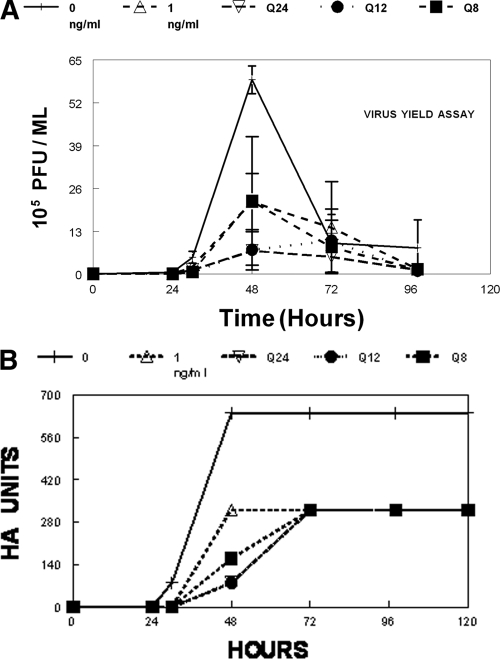
To determine the pharmacodynamically linked variable for oseltamivir carboxylate for the A/Sydney5/97 R292 strain of influenza virus, the EC50 concentration of oseltamivir carboxylate for this isolate was administered as a continuous infusion (AUC0-24 = 1 ng/ml × 24 h = 24 ng/ml/h) or the same 24-h AUC was administered as fractionated doses over a 1-h period as q24h, q12h, and q8h doses. All fractionated doses were followed by a no-drug wash out. The results shown in Fig. 4A and B show that in the absence of drug, the virus grew well in the hollow-fiber unit, with a peak in the production of infectious virus at 48 h postinfection, followed by a decline in the production of infectious virus. In the arm that received a dose of 1 ng/ml of drug by continuous infusion, the production of infectious virus at 48 h was substantially reduced. A similar result was obtained when the total dose was delivered q24h, q12h, or q8h, followed by a no-drug washout. We analyzed the data in a sigmoid-Emax effect model and found that the model estimates and their standard deviations for the fractionated regimens overlapped, with 95% confidence intervals that were not different. The observed PFU/ml values and their 95% confidence intervals also overlap. We concluded that the pharmacodynamically linked variable for this drug for influenza A virus is the AUC/EC50 ratio. The data in Fig. 4B show the results of a hemagglutination assay for the same samples used to measure infectivity in the virus yield assay (Fig. 4A). The results are essentially the same. In the absence of drug, the virus grew well, producing many HA units at 48 h postinfection. Continuous infusion of drug at 1 ng/ml reduced the amount of HA units by half at 72 h postinfection. Delivering the same total dose fractionated into q24h, q12h, and q8h doses gave the same amount of virus suppression, suggesting that the pharmacodynamically linked variable is the AUC/EC50 ratio.
FIG. 4.
(A and B) Dose fractionation study for A/Sydney/5/97 R292 and oseltamivir carboxylate in the HFIM system. To determine the pharmacodynamically linked variable for oseltamivir carboxylate for the A/Sydney5/97 R292 strain of influenza virus, six hollow-fiber units were set up as described in the legend of Fig. 2. One hollow-fiber unit was continuously fed with medium with no drug (“0” in key). One hollow-fiber unit received a continuous infusion of oseltamivir carboxylate at 1 ng/ml (“1” in key). One unit received a total dose equivalent to continuous infusion of 1 ng/ml for 24 h with the dose delivered q24h followed by a no-drug washout with an 8-h half-life. One unit received a total dose equivalent to 1 ng/ml for 24 h with the dose given as q12h followed by a no-drug washout with an 8-h half-life. One unit received a dose equivalent to 1 ng/ml for 24 h with the dose given as q8h followed by a no-drug washout with an 8-h half-life. The units receiving continuous infusions had their medium changed daily to maintain the proper drug concentration. Error bars show standard deviations.
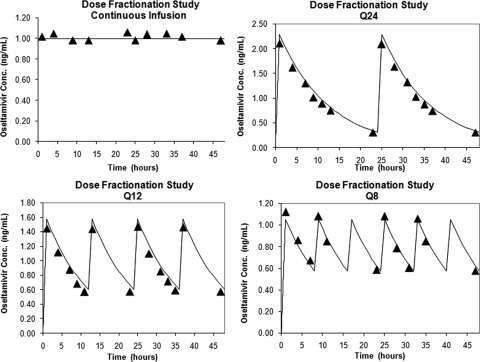
To confirm that the correct doses were delivered at the correct times and that the intended drug concentration was attained, each hollow-fiber unit was sampled at various times throughout the dose fractionation study and the amount of oseltamivir carboxylate present was determined by LC-MS-MS. The data in Fig. 5 show these results. The intended drug concentrations in the continuous arm (1 ng/ml) were attained in the hollow-fiber units. For the dose-fractionated arms, the data show that the intended concentration-time profile was achieved.
FIG. 5.
Analysis of the simulated pharmacokinetics of the dose fractionation experiment with an 8-h half-life. The medium entering each hollow-fiber unit was sampled at the indicated times, and the amount of oseltamivir carboxylate was determined by LC-MS-MS. The data show that the hollow-fiber unit receiving 1 ng/ml actually received that amount of drug for the first 48 h of the experiment. The hollow-fiber units receiving the three fractionated doses achieved the correct peak concentrations at the appropriate times, and the drug was washed out according to the correct schedule.
DISCUSSION
Oseltamivir has become an important component of our defense against influenza virus. This is particularly true for a pandemic strain, where mortality and morbidity may be great. The production of vaccines varies from year to year, and their protective ability is not wholly predictable from currently available data. Consequently, antiviral chemotherapy assumes a major importance. Oseltamivir carboxylate has become, de facto, the only reliable anti-influenza agent, as resistance to amantadine and rimantadine has risen to more than 90% for H3N2 viruses and about 10% for H1N1 influenza viruses (9, 12, 19). Further, while resistance has emerged to oseltamivir, it is nowhere near the rate at which resistance to the adamantanes is emerging (9, 30). This is probably because of the number of mutations in the M2 open reading frame that result in complete resistance, while the mutant viruses maintain good biofitness and the ability to spread from human to human. Therefore, it is important to explore the pharmacodynamics of oseltamivir carboxylate and identify the pharmacodynamically linked variable.
To that end, we used our in vitro pharmacodynamic HFIM system to determine the optimal dose and administration schedule for oseltamivir carboxylate for the A/Sydney/5/97 H3N2 R292 isolate of influenza virus in AX-4 cells. Our results show that continuous infusion of oseltamivir carboxylate at 1 ng/ml inhibited virus replication in AX-4 cells in the HFIM system by 50% and that continuous infusion of oseltamivir carboxylate at 100 and 1,000 ng/ml completely suppressed virus replication. Dose fractionation studies showed that when a dose equivalent to the EC50 dose in the HFIM system (1 ng/ml) was given by continuous infusion or the same total dose was administered q24h, q12h, or q8h, followed by a no drug-wash out at the appropriate half-life, the suppression of virus replication was essentially the same in all cases. The results of the dose fractionation study demonstrate that the pharmacodynamically linked variable for oseltamivir carboxylate for influenza virus is the AUC/EC50 ratio. Therefore, oseltamivir carboxylate could be administered once a day at twice the dose relative to the currently recommended therapy to effectively treat influenza virus infections. This point requires clinical validation.
We set up the HFIM to mimic the course of influenza illness seen in humans, where viral titers peak at around 48 to 72 h (13). The basic reproductive number, R0, is the average number of second-generation infections produced by a single infected cell placed in a population of entirely susceptible cells. Baccam et al. (1) calculated that influenza, under the assumptions of their model, would have an R0 of approximately 11.0, where 1.0 is the breakpoint for having an infection that would be unable to propagate and would die out. Cell-to-cell spread would be expected to be rapid. Therefore, it is not surprising that the output from the system tended to decline after 48 to 72 h, as target cells would be destroyed by the infection. Nonetheless, the time course of the model mimics that of human disease.
The system allowed clear delineation of an exposure response when the drug was administered as a continuous infusion. Concentrations of 1 ng/ml demonstrated viral suppression around the EC50, irrespective of whether one examined a hemagglutination assay (Fig. 3B) or a plaque assay (Fig. 3A). At an oseltamivir concentration of 5 ng/ml, an effect approximating an EC75 was achieved when both endpoints were examined. Higher concentrations (100 and 1,000 ng/ml) achieved near-maximal viral inhibition. Of interest, in contrast to the exposure response curve seen in HIV therapeutics (7, 14-16, 31), the exposure response curve here rises rather slowly, requiring 25 times the approximate EC50 to achieve an EC95 (EC50 to EC95 in HIV agents usually encompasses a four- to fivefold drug concentration range).
We chose to examine a constant concentration of 1 ng/ml (approximate EC50) for the delineation of the dynamically linked variable in the dose fractionation experiment because this was near the middle of the exposure response curve and thus would be most sensitive to factors changing the response, such as schedule of administration. The outcome was quite clear. At 48 to 72 h, the inhibition seen was consistent with AUC/EC50 (or AUC/EC95, if preferred) as the pharmacodynamic driver. For the plaque assay endpoint at 48 h, the least inhibition was generated by continuous infusion and by q8h administration schedules. The other modes of administration showed more inhibition but were not statistically significantly different. For the HA endpoint, at 48 h, the continuous mode of administration generated the least effect and the greatest effect was generated by q24h administration. However, at 72 and 96 h, all modes of administration were exactly equivalent. Overall, the inference is that the AUC/EC50 ratio is the pharmacodynamic driver.
It should be noted that these relationships were developed in an in vitro system. The concentration-time profiles constructed were limited to those that the preliminary experiments suggested. In humans, the number of concentration-time profiles would be very large due to between-patient variance. For this and other reasons, clinical validation for these findings should be sought. It should be noted, however, that the pharmacodynamically linked variable in an animal model for the neuraminidase inhibitor peramivir was also the AUC/EC50 ratio (17).
This has important implications. First, it is highly likely that oseltamivir carboxylate will be equivalently efficacious when administered as 75 mg q12h or as 150 mg daily if the higher single dose is well tolerated. Obviously, the ability to administer the agent daily will optimize treatment adherence relative to that seen with twice-daily administration.
Because of issues of availability of oseltamivir carboxylate in a pandemic situation, Holodny et al. (22) examined every-other-day dosing of oseltamivir supplemented with q6h dosing of probenecid and daily dosing supplemented with q12h dosing of probenecid. There are no data on a minimum target value of AUC0-24 for oseltamivir carboxylate to predict a high likelihood of a good clinical outcome. Nonetheless, the finding that AUC/EC50 is the pharmacodynamic driver for oseltamivir should give pause to the idea of employing dosing regimens that fall outside the “80-125 rule.” In order to put the findings of the Holodny study (22) into perspective, it would be important to generate an AUC0-24/EC50 target for outcome and to employ Monte Carlo simulation to ascertain how frequently the alternative regimens (including probenecid) attain such a target. Given the noted point estimates of the means and standard deviations, it is highly likely that such alternative regimens would fall short of an acceptable target attainment for the population. It is important to investigate the pharmacodynamics of anti-influenza drugs to identify regimens highly likely to be efficacious during a pandemic situation.
Acknowledgments
This work was supported by grant R01-AI079729-01 from NIAID to the Emerging Infections and Pharmacodynamics Laboratory and by grants from Roche Pharmaceuticals, Inc., Palo Alto, CA, and the Charitable Leadership Foundation, Clifton Park, NY.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
The authors have no conflicts to disclose.
Footnotes
Published ahead of print on 13 April 2009.
REFERENCES
- 1.Baccam, P., C. Beauchemin, C. A. Macken, F. G. Hayden, and A. S. Perelson. 2006. Kinetics of influenza A virus infection. J. Virol. 80:7590-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantia, S., C. S. Arnold, C. D. Parker, R. Upshaw, and P. Chand. 2006. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antivir. Res. 69:39-45. [DOI] [PubMed] [Google Scholar]
- 3.Bantia, S., C. D. Parker, S. L. Ananth, L. L. Horn, K. Andries, P. Chand, P. L. Kotian, A. Dehghani, Y. El-Kattan, T. Lin, T. L. Hutchison, J. A. Montgomery, D. L. Kellog, and Y. S. Babu. 2001. Comparison of the antiinfluenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baz, M., Y. Abed, B. Nehme, and G. Boivin. 2009. Activity of the oral neuraminidase inhibitor A-322278 against the oseltamivir-resistant H274Y (A/H1N1) influenza virus mutant in mice. Antimicrob. Agent Chemother. 53:791-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe, R. B. 2005. The origins of pandemic influenza: lessons from the 1918 virus. N. Engl. J. Med. 353:2209-2211. [DOI] [PubMed] [Google Scholar]
- 6.Belshe, R. B., M. H. Smith, C. B. Hall, R. Betts, and A. J. Hay. 1988. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J. Virol. 62:1508-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilello, J. A., G. Bauer, M. N. Dudley, G. A. Cole, and G. L. Drusano. 1994. Effect of 2′,3′-didehydro-3′-deoxythymidine in an in vitro hollow fiber pharmacodynamic model system correlates with results of dose ranging clinical studies. Antimicrob. Agent Chemother. 38:1386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilello, J. A., P. A. Bilello, J. J. Kort, M. N. Dudley, J. Leonard, and G. L. Drusano. 1995. Efficacy of constant infusion of A77003, an inhibitor of the HIV protease, in limiting acute HIV-1 infection in vitro. Antimicrob. Agents Chemother. 39:2523-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bright, R. A., D. K. Shay, B. Shu, N. J. Cox, and A. I. Klimov. 2006. Adamantane resistance among influenza A viruses isolated early during 2005-2006 influenza season in the United States. JAMA 295:891-894. [DOI] [PubMed] [Google Scholar]
- 10.Chand, P., S. Banita, P. L. Kotian, Y. El-Kattan, T. H. Lin, and Y. S. Babu. 2005. Comparison of the anti-influenza virus activity of cyclopentane derivatives with oseltamivir and zanamivir in vivo. Bioorg. Med. Chem. 13:4071-4077. [DOI] [PubMed] [Google Scholar]
- 11.D'Argenio, D. Z., and A. Schumitzky. 1997. ADAPT II. A program for simulation, identification, and optimal experimental design. User manual. Biomedical Simulations Resource, University of Southern California, Los Angeles, CA.
- 12.Deyde, V. M., T. Nguyen, R. A. Bright, A. Balish, B. Shu, S. Lindstrom, A. I. Klimov, and L. V. Gubareva. 2009. Detection of molecular markers of antiviral resistance in influenza A (H5N1) viruses using pyrosequencing method. Antimicrobial. Agents Chemother. 53:1039-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas, W. R. 1975. Influenza in man, p. 397-446. In E. D. Kilbourne (ed.), Influenza viruses and influenza. Academic Press, New York, NY.
- 14.Drusano, G. L., P. A. Bilello, W. T. Symonds, D. S. Stein, J. McDowell, A. Bye, and J. A. Bilello. 2002. Pharmacodynamics of abacavir in an in vitro hollow fiber model system. Antimicrob. Agents Chemother. 46:464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drusano, G. L., J. A. Bilello, S. L. Preston, E. Omara, S. Kaul, S. Schnittman, and R. Echols. 2001. Hollow fiber unit evaluation of a new human immunodeficiency virus (HIV)-1 protease inhibitor, BMS232632, for determination of the linked pharmacodynamic variable. J. Infect. Dis. 183:1126-1129. [DOI] [PubMed] [Google Scholar]
- 16.Drusano, G. L., K. H. P. Moore, J. P. Kleim, W. Prince, and A. Bye. 2002. Rational dose selection for a nonnucleoside reverse transcriptase inhibitor through use of population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 46:913-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drusano, G. L., S. L. Preston, D. Smee, K. Bush, K. Bailey, and R. W. Sidwell. 2001. Pharmacodynamic evealuation of RWJ 270201, a novel neuraminidase inhibitor, in a lethal murine model of influenza, predicts efficacy for once-daily dosing. Antimicrob. Agents Chemother. 45:2115-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatakeyama, S., Y. Sakai-Tagawa, M. Kiso, H. Goto, C. Kwakami, K. Mitamura, N. Sugaya, Y. Suzuki, and Y. Kawaoka. 2005. Enhanced expression of an α2,6-linked sialic acid on MDCK cells improved isolation of human influenza viruses and evaluation of their sensitivity to a neuraminidase inhibitor. J. Clin. Microbiol. 43:4139-4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayden, F. G. 2006. Antiviral resistance in influenza viruses: implications for management and pandemic response. N. Engl. J. Med. 354:785-788. [DOI] [PubMed] [Google Scholar]
- 20.Hayden, F. G., K. M. Cote, and R. G. Douglas, Jr. 1980. Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrobial. Agents Chemother. 17:865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden, F. G., L. V. Gubareva, A. S. Monto, T. C. Klein, M. J. Elliott, J. M. Hammond, S. J. Sharp, and M. J. Ossi for the Zanamivir Family Study Group. 2000. Inhaled zanamivir for the prevention of influenza in families. N. Engl. J. Med. 343:1282-1289. [DOI] [PubMed] [Google Scholar]
- 22.Holodniy, M., S. R. Penzak, T. M. Straight, R. T. Davey, K. K. Lee, M. B. Goetz, D. W. Raisch, F. Cunningham, E. T. Lin, N. Olivo, and L. R. Deyton. 2008. Pharmacokinetics and tolerability of oseltamivir combined with probenecid. Antimicrob. Agent Chemother. 52:3013-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ison, M. G., V. P. Mishin, T. J. Braciale, F. G. Hayden, and L. V. Gubareva. 2006. Comparative activities of oseltamivir and A-322278 in immunocompetent and immunocompromised murine models of influenza virus infection. J. Infect. Dis. 193:765-772. [DOI] [PubMed] [Google Scholar]
- 24.Kati, W. M., D. Montgomery, R. Carrick, L. Gubareva, C. Maring, K. McDaniel, K. Steffy, A. Molla, F. Hayden, D. Kempf, and W. Kohlbrenner. 2002. In vitro characterization of A-315675, a highly potent inhibitor of A and B strain influenza virus neuraminidases and influenza virus replication. Antimicrob. Agent Chemother. 46:1014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilbourne, E. D. 2006. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 12:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McSharry, J. J., M. R. Deziel, K. Zager, Q. Weng, and G. L. Drusano. 2009. Pharmacodynamics of cidofovir for vaccinia virus infection in an in vitro hollow fiber infection model system. Antimicrob. Agents Chemother. 53:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McSharry, J. J., A. C. McDonough, B. A. Olson, and G. L. Drusano. 2004. Phenotypic drug susceptibility assay for influenza virus neuraminidase inhibitors. Clin. Diagn. Lab. Immunol. 11:21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molla, A., W. Kati, R. Carrick, K. Steffy, Y. Shi, D. Montgomery, N. Gusick, V. S. Stoll, K. D. Stewart, T. I. Ng, C. Maring, D. J. Kempf, and W. Kohlbrenner. 2002. In vitro selection and characterization of influenza A (A/N9) virus variants resistant to a novel neuraminidase inhibitor, A-315675. J. Virol. 76:5380-5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moscona, A. 2005. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 353:1363-1373. [DOI] [PubMed] [Google Scholar]
- 30.Moscona, A. 2009. Global transmission of oseltamivir-resistant influenza. N. Engl. J. Med. 360:953-956. [DOI] [PubMed] [Google Scholar]
- 31.Preston, S. L., P. J. Piliero, J. A. Bilello, D. S. Stein, W. T. Symonds, and G. L. Drusano. 2003. In vitro-in vivo model for evaluating the antiviral activity of amprenavir in combination with ritonavir administered at 600 and 100 milligrams, respectively, every 12 hours. Antimicrob. Agents Chemother. 47:3393-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sidwell, R. W., and D. F. Smee. 2000. In vitro and in vivo assay systems for study of influenza virus inhibitors. Antivir. Res. 48:1-16. [DOI] [PubMed] [Google Scholar]
- 33.Thompson, W. W., D. K. Shay, E. Weintraub, L. Brammer, N. Cox, L. J. Anderson, and K. Fukuda. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179-186. [DOI] [PubMed] [Google Scholar]
- 34.Treanor, J. J., F. G. Hayden, P. S. Vrooman, R. Barbarash, R. Bettis, D. Riff, S. Singh, N. Kinnersley, P. Ward, and R. G. Mills for the US Oral Neuraminidase Study Group. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized, controlled trial. JAMA 283:1016-1024. [DOI] [PubMed] [Google Scholar]