Abstract
A remarkable prevalence of qnrB (54%) and, at a lower level, of qnrS (14%) was discovered in pools of commensal enterobacteria from 310 healthy children living in Peru and Bolivia, using a metagenomic approach. Analysis of randomly selected enterobacterial pools revealed that qnrB was mainly carried by Escherichia coli and qnrS by Klebsiella pneumoniae. Investigation of 11 qnrB-positive isolates and 9 qnrS-positive isolates revealed the presence of plasmid-borne qnrB19 (n = 8), qnrB2 (n = 2), qnrB10 (n = 1), and qnrS1 (n = 9) genes.
Several plasmid-mediated quinolone resistance (PMQR) mechanisms have been discovered during the past decade, including Qnr proteins, QepA transporters, and the acetyltransferase AAC(6′)-Ib-cr (11, 13). Qnr proteins, the first discovered PMQR mechanism, are small pentapeptide repeat proteins that bind and protect type II DNA topoisomerases from inhibition by fluoroquinolones (17-19). Different lineages of Qnr proteins have been described (QnrA, QnrB, QnrS, and, more recently, QnrC and QnrD), with several allelic variants known for some of them (3, 9, 11, 20). qnr-like genes have also been detected on chromosomes from both gram-negative and gram-positive bacteria (9), and, recently, as class 1 integron gene cassettes in the chromosome of Vibrio cholerae (7). Although Qnr proteins only determine a moderate reduction of quinolone susceptibility, this may favor the selection of additional resistance mechanisms leading to higher-level quinolone resistance of clinical significance, and the dissemination of qnr genes and other PMQR determinants is believed to be an important promoter for evolution of quinolone resistance (11-13).
qnr genes have been reported worldwide, especially in enterobacteria. However, they have mostly been investigated in clinical isolates with specific resistance traits (e.g., quinolone resistance or extended-spectrum β-lactamase phenotype) (11 and references therein), while their prevalence in commensal bacteria remains largely unknown. In this study, we investigated the prevalence of qnr genes in commensal enterobacteria from healthy children by a PCR-based metagenomic approach. We also tested a simplified dot blot DNA hybridization method as a less-labor-intensive and expensive tool to perform the metagenomic analysis.
The analysis was carried out on commensal enterobacterial pools from 310 healthy children, ages 6 to 72 months, living in four urban areas of Latin America: two in Peru (Moyobamba, San Martin Department; and Yurimaguas, Loreto Department) and two in Bolivia (Camiri, Santa Cruz Department; and Villa Montes, Tarija Department). The enterobacterial pools, consisting of the bacterial growth obtained by plating fecal samples (one sample per child) onto MacConkey agar (MCA) plates (Oxoid, Milan, Italy), were randomly selected among samples (n = 3,193) obtained during a survey performed in 2005 in the same areas (1) and stored at −70°C. A loopful of each pool was plated on an MCA plate supplemented with 0.12 μg/ml ciprofloxacin (MCA-CIP). This ciprofloxacin concentration was used for screening purposes since (i) it was lower than MICs usually exhibited by enterobacterial strains harboring qnr genes as the sole quinolone resistance mechanism (reference 11 and references therein), while being higher than the wild-type MIC distribution for Escherichia coli and Klebsiella pneumoniae (http://www.escmid.org/research_projects/eucast/); (ii) a similar ciprofloxacin MIC threshold has previously been used for screening of qnr-positive bacteria (4, 8, 14, 15, 21). In case of growth onto MCA-CIP, a loopful of bacteria was directly used for total DNA extraction (10), and about 100 ng of metagenomic DNA was used as template in PCRs (50 μl) to detect the presence of qnr genes. Primers and conditions for PCR amplification of qnr genes were described previously in references 14 (qnrA and qnrS) and 2 (qnrB). Controls for qnr genes were kindly provided by Patrice Nordmann and Laurent Poirel (Université Paris-Sud, K.-Bicêtre, France). The specificity of the PCR products was confirmed by partial sequence analysis of randomly selected amplicons (Macrogen, Seoul, Korea).
The presence of qnr genes in the enterobacterial pools was also investigated by dot blot DNA hybridization using a rapid method, essentially as described by Srinivasan et al. (16). Briefly, a loopful of the bacterial growth on MCA-CIP was transferred to 150 μl of lysis solution (0.4 N NaOH, 10 mM EDTA) and incubated at 70°C for 2 h. The bacterial lysate (100 μl) was directly blotted onto Hybond-N+ nylon membranes (Amersham Bioscience, Buckinghamshire, United Kingdom) using a BIO-dot microfiltration apparatus (Bio-Rad Laboratories, Milan, Italy). Nylon membranes were washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), fixed by UV light, and hybridized with digoxigenin-dUTP (DIG)-labeled qnr probes (generated by PCR using the positive controls, as described above) using the DIG system according to the manufacturer's instructions (Roche Diagnostics SpA, Milan, Italy).
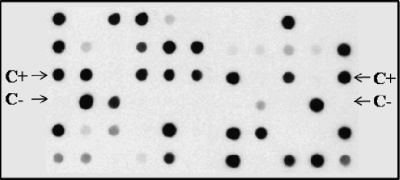
Growth of enterobacterial pools on MCA-CIP was observed with 275 of 310 samples (89%). Analysis of the metagenomes prepared from this bacterial growth by PCR revealed overall positivities of 54% for qnrB and 14% for qnrS, while qnrA was not detected (Table 1). Dot blotting showed signals of variable intensity (Fig. 1) and an overall lower detection sensitivity, but results were fully consistent with those of PCR (i.e., all positive samples in the dot blot, including weak signals, were also positive by PCR) (Table 1). This evidently reflected a variable copy number of target genes in the metagenomic DNA from various samples, with a number of cases in which the amount of target genes could only be detected by the more sensitive gene amplification approach.
TABLE 1.
Prevalence of qnr genes in commensal enterobacteria from 310 healthy children living in Peru and Boliviaa
| Study area | No. of samples | No. (%) of samples grown on MCA-CIPb | No. of samples (% [95% CI]) forc:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Any qnr
|
qnrB
|
qnrS
|
||||||
| PCR | Dot blot | PCR | Dot blot | PCR | Dot blot | |||
| Peru | 164 | 154 (94) | 113 (69 [61-76]) | 93 (57 [49-64]) | 107 (65 [57-72]) | 87 (53 [45-61]) | 37 (23 [16-30]) | 11 (7 [3-12]) |
| Bolivia | 146 | 121 (83) | 63 (43 [35-52]) | 51 (35 [27-43]) | 60 (41 [33-50]) | 49 (34 [26-42]) | 7 (5 [2-10]) | 2 (1 [0-5]) |
| Total | 310 | 275 (89) | 176 (57 [51-62]) | 144 (46 [40-52]) | 167 (54 [48-60]) | 136 (44 [38-50]) | 44 (14 [11-19]) | 13 (4 [2-7]) |
Statistical differences were determined by the chi-square test with the EpiInfo software package, version 6 (Centers for Disease Control and Prevention, Atlanta, GA). The binomial exact 95% confidence interval (95% CI) was calculated by Stata Software release 8.0 (2003; StataCorp LP, College Station, TX).
Rates of growth on MCA-CIP plates were not significantly different (P > 0.5).
The percentage of samples was calculated for the total samples. qnr genes were significantly more prevalent in Peru than in Bolivia: P = 0.01 for any qnr and qnrB, and P < 0.001 for qnrS. All positive samples in the dot blot were also positive in the PCR.
FIG. 1.
Nylon membrane prepared with bacterial lysates, hybridized with DIG-labeled qnrB probe. All positive signals, including the weakest ones, were positive in PCR experiments. Positive and negative controls (C+ and C−, respectively) are indicated by arrows.
Concerning geographical differences, even though the rates of growth of enterobacterial pools on MCA-CIP were not significantly different, qnr genes were significantly more prevalent in Peru than in Bolivia (Table 1). Interestingly, these differences mirrored the higher quinolone resistance rates found in the commensal E. coli microbiota of the same population of children from Peru (62% for nalidixic acid and 39% for ciprofloxacin) as compared to Bolivia (51% for nalidixic acid and 26% for ciprofloxacin) (1), which supports an association between dissemination of PMQR determinants and quinolone resistance.
To investigate the nature of the bacterial hosts of qnr genes in the commensal microbiota, enterobacterial pools yielding metagenomes positive for qnrB (n = 42) or qnrS (n = 22), selected at random, were plated on MCA-CIP to yield isolated colonies. All colonies with a different morphological appearance were collected and subjected to molecular analysis to investigate the presence of qnrB and qnrS genes by PCR (up to four colonies were analyzed per sample). All qnr-positive isolates were identified by the API 20E system (BioMérieux, Marcy l'Etoile, France). When two or more isolates of the same species and carrying the same qnr gene were observed in a sample, only one isolate was included in the data analysis.
Isolates carrying qnrB or qnrS were detected in 36 of 42 (86%) and 14 of 22 (64%) of the selected qnrB- and qnrS-positive enterobacterial pools, respectively (Table 2). The lack of recovery of qnr-positive isolates from some samples was likely due to a lower frequency of such isolates in those enterobacterial pools. In fact, the success in isolating qnr-positive isolates from the enterobacterial pools was consistently higher when the corresponding metagenome had been found qnr positive also by dot blotting (89% versus 47%) (Table 2), in agreement with the hypothesis that, in those cases, qnr genes were carried by a dominant bacterial population.
TABLE 2.
Detection and identification of qnr-carrying bacterial hosts from enterobacterial pools yielding qnr-positive metagenomes
| Enterobacterial pool (no. of samples) | No. (%) of samples in which qnr hosts were identified | No (%) of qnr bacterial hosts
|
||
|---|---|---|---|---|
| E. coli | K. pneumoniae | Othersa | ||
| qnrB positive | ||||
| PCR and dot blot positive (35) | 32 (91) | 22 (69) | 5 (16) | 5 (16) |
| PCR positive only (7) | 4 (57) | 4 (100) | ||
| Total (42) | 36 (86) | 26 (72) | 5 (14) | 5 (14) |
| qnrS-positive | ||||
| PCR and dot blot positive (12) | 10 (83) | 3 (30) | 7 (70) | |
| PCR positive only (10) | 4 (40) | 1 (25) | 1 (25) | 2 (50) |
| Total (22) | 14 (64) | 4 (29) | 8 (57) | 2 (14) |
qnrB was detected in E. cloacae (n = 2), K. oxytoca (n = 1), C. freundii (n = 1), and E. hermannii (n = 1). qnrS was detected in K. oxytoca (n = 2).
Identification of the qnr-positive isolates showed that qnrB was mainly carried by E. coli and, more rarely, by K. pneumoniae or other enterobacterial species, including Enterobacter cloacae, Klebsiella oxytoca, Citrobacter freundii, and Escherichia hermannii (Table 2). On the other hand, qnrS was mainly found in K. pneumoniae and, more rarely, in E. coli and K. oxytoca (Table 2). In two cases, both qnr genes were found to be carried by the same E. coli isolate.
MICs of nalidixic acid, ciprofloxacin, and levofloxacin were determined by agar dilution and interpreted according to CLSI (5, 6) for the 48 qnr-harboring isolates. Resistance to nalidixic acid was common (77%), while 32% and 17% of isolates were nonsusceptible to ciprofloxacin and levofloxacin, respectively (analytical data are reported in Table S1 in the supplemental material).
The location and nature of qnr genes were investigated in 20 selected isolates representative of different species (11 qnrB and 9 qnrS) by Southern blotting on nylon membranes, as described for dot blot hybridization, and by sequencing of PCR amplicons generated with primers designed on flanking sequences (EU624315 and EU715254 for qnrB and EU939771 for qnrS). qnrB genes were located on either low- or high-molecular-weight plasmids and included mostly qnrB19 but also qnrB2 and a new allele of qnrB10 showing 7 nucleotide differences compared to qnrB10 DQ631414. All qnrS genes were qnrS1 and were located on high-molecular-weight plasmids (Table 3; and data not shown).
TABLE 3.
qnr genes in commensal enterobacteria from healthy children in Peru and Bolivia
| Isolatea | Origin | qnr gene | MIC (μg/ml)b
|
||
|---|---|---|---|---|---|
| NAL | CIP | LEV | |||
| E. coli Y1 | Yurimaguas, Peru | qnrB19 | 32 | 0.5 | 1 |
| E. coli M1 | Moyobamba, Peru | qnrB19 | 16 | 0.25 | 0.5 |
| E. coli C1 | Camiri, Bolivia | qnrB19 | 32 | 0.5 | 1 |
| E. coli V1 | Villa Montes, Bolivia | qnrB19 | 32 | 0.25 | 1 |
| K. pneumoniae Y1 | Yurimaguas, Peru | qnrB19 | 64 | 2 | 1 |
| K. pneumoniae M1 | Moyobamba, Peru | qnrB19 | 32 | 2 | 2 |
| K. pneumoniae V1 | Villa Montes, Bolivia | qnrB2 | 32 | 1 | 1 |
| E. hermannii C1 | Camiri, Bolivia | qnrB19 | 32 | 0.25 | 0.25 |
| E. cloacae V1 | Villa Montes, Bolivia | qnrB2 | 32 | 2 | 2 |
| K. oxytoca M1 | Moyobamba, Peru | qnrB19 | 16 | 0.25 | 0.5 |
| C. freundii V1 | Villa Montes, Bolivia | qnrB10 | 16 | 0.25 | 0.5 |
| E. coli Y2 | Yurimaguas, Peru | qnrS1 | 16 | 0.5 | 0.5 |
| E. coli Y3 | Yurimaguas, Peru | qnrS1 | 16 | 0.5 | 0.5 |
| E. coli M2 | Moyobamba, Peru | qnrS1 | 16 | 0.5 | 0.5 |
| E. coli M3 | Moyobamba, Peru | qnrS1 | 32 | 0.5 | 2 |
| K. pneumoniae Y2 | Yurimaguas, Peru | qnrS1 | 32 | 1 | 2 |
| K. pneumoniae M2 | Moyobamba, Peru | qnrS1 | 32 | 4 | 1 |
| K. pneumoniae C2 | Camiri, Bolivia | qnrS1 | 32 | 2 | 2 |
| K. pneumoniae V2 | Villa Montes, Bolivia | qnrS1 | 16 | 1 | 2 |
| K. oxytoca M2 | Moyobamba, Peru | qnrS1 | >128 | 4 | 4 |
The 11 qnrB-harboring isolates were selected as follows: four E. coli isolates, one from each study area; three K. pneumoniae isolates, each from different study areas (no qnrB-harboring K. pneumoniae isolate detected in Camiri, Bolivia); and four isolates representative of species other than E. coli or K. pneumoniae. The nine qnrS-harboring isolates were selected as follows: all of the qnrS-harboring E. coli isolates (n = 4); four K. pneumoniae isolates, one from each study area; and one of the two qnrS-harboring K. oxytoca isolates.
NAL, nalidixic acid; CIP, ciprofloxacin; LEV, levofloxacin.
Concluding remarks.
This study demonstrated a remarkable dissemination of qnrB and, at a lower level, qnrS determinants in commensal enterobacteria from healthy children living in urban areas of Peru and Bolivia. The few studies that have investigated the prevalence of qnr genes in clinical isolates from South American countries showed that alleles belonging to the qnrB are the most frequently reported (11 and references therein), in accordance with the high prevalence of qnrB observed in this study. To our best knowledge, this is the first study on the prevalence of qnr genes in human commensal bacteria, and present findings suggest that the commensal enterobacterial microbiota could be an important reservoir of similar genes. It will thus be interesting to investigate if a similar situation is also found in the adult population and in other geographical settings.
Since data collected about household use of antibiotics excluded previous use of fluoroquinolones in the children included in this study (1), selection of qnr genes could be related to linkage with other resistance genes carried on the same plasmids. Further studies are under way to investigate this issue.
Nucleotide sequence accession number.
The nucleotide sequence of the new allele of qnrB10 has been submitted to GenBank and assigned accession no. FJ769283.
Supplementary Material
Acknowledgments
This study was carried out as a follow-up to research activities of the ANTRES Project (a project on antibiotic use and resistance in Latin America, supported by the European Commission within the INCO-DEV Program of the FP5).
Footnotes
Published ahead of print on 13 April 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Bartoloni, A., L. Pallecchi, C. Fiorelli, T. Di Maggio, C. Fernandez, A. L. Villagran, A. Mantella, F. Bartalesi, M. Strohmeyer, A. Bechini, H. Gamboa, H. Rodriguez, C. Kristiansson, G. Kronvall, E. Gotuzzo, F. Paradisi, and G. M. Rossolini. 2008. Increasing resistance in commensal Escherichia coli, Bolivia and Peru. Emerg. Infect. Dis. 14:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cattoir, V., L. Poirel, V. Rotimi, C. J. Soussy, and P. Nordmann. 2007. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 60:394-397. [DOI] [PubMed] [Google Scholar]
- 3.Cavaco, L. M., H. Hasman, S. Xia, and F. M. Aarestrup. 2009. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovars Kentucky and Bovismorbificans of human origin. Antimicrob. Agents Chemother. 53:603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavaco, L. M., N. Frimodt-Møller, H. Hasman, L. Guardabassi, L. Nielsen, and F. M. Aarestrup. 2008. Prevalence of quinolone resistance mechanisms and associations to minimum inhibitory concentrations in quinolone-resistant Escherichia coli isolated from humans and swine in Denmark. Microb. Drug Resist. 14:163-169. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing. Supplement M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Fonseca, E. L., F. Dos Santos Freitas, V. V. Vieira, and A. C. Vicente. 2008. New qnr gene cassettes associated with superintegron repeats in Vibrio cholerae O1. Emerg. Infect. Dis. 14:1129-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins, K. L., M. Day, and E. J. Threlfall. 2008. Plasmid-mediated quinolone resistance in Salmonella enterica, United Kingdom. Emerg. Infect. Dis. 14:340-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacoby, G., V. Cattoir, D. Hooper, L. Martínez-Martínez, P. Nordmann, A. Pascual, L. Poirel, and M. Wang. 2008. qnr gene nomenclature. Antimicrob. Agents Chemother. 52:2297-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, J. L. 1994. Similarity analysis of DNAs, p. 655-682. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. ASM Press, Washington, DC.
- 11.Martínez-Martínez, L., M. Eliecer Cano, J. Manuel Rodríguez-Martínez, J. Calvo, and A. Pascual. 2008. Plasmid-mediated quinolone resistance. Expert Rev. Anti Infect. Ther. 6:685-711. [DOI] [PubMed] [Google Scholar]
- 12.Poirel, L., V. Cattoir, and P. Nordmann. 2008. Is plasmid-mediated quinolone resistance a clinically significant problem? Clin. Microbiol. Infect. 14:295-297. [DOI] [PubMed] [Google Scholar]
- 13.Robicsek, A., G. A. Jacoby, and D. C. Hooper. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 6:629-640. [DOI] [PubMed] [Google Scholar]
- 14.Robicsek, A., J. Strahilevitz, D. F. Sahm, G. A. Jacoby, and D. C. Hooper. 2006. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 50:2872-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saga, T., T. Akasaka, H. Takase, M. Tanaka, K. Sato, and M. Kaku. 2007. First detection of the plasmid-mediated quinolone resistance determinant qnrA in Enterobacteriaceae clinical isolates in Japan. Int. J. Antimicrob. Agents 29:738-739. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan, U., L. Zhang, A. M. France, D. Ghosh, W. Shalaby, J. Xie, C. F. Marrs, and B. Foxman. 2007. Probe hybridization array typing: a binary typing method for Escherichia coli. J. Clin. Microbiol. 45:206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran, J. H., and G. A. Jacoby. 2002. Mechanism of plasmid-mediated quinolone resistance. Proc. Natl. Acad. Sci. USA 99:5638-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein QnrA with Escherichia coli topoisomerase IV. Antimicrob. Agents Chemother. 49:3050-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran, J. H., G. A. Jacoby, and D. C. Hooper. 2005. Interaction of the plasmid-encoded quinolone resistance protein Qnr with Escherichia coli DNA gyrase. Antimicrob. Agents Chemother. 49:118-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, M., Q. Guo, X. Xu, X. Wang, X. Ye, S. Wu, D. C. Hooper, and M. Wang. 2 March 2009. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. [Epub ahead of print.] doi: 10.1128/AAC.01400-08. [DOI] [PMC free article] [PubMed]
- 21.Yang, H., H. Chen, Q. Yang, M. Chen, and H. Wang. 2008. High prevalence of plasmid-mediated quinolone resistance genes qnr and aac(6′)-Ib-cr in clinical isolates of Enterobacteriaceae from nine teaching hospitals in China. Antimicrob. Agents Chemother. 52:4268-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.