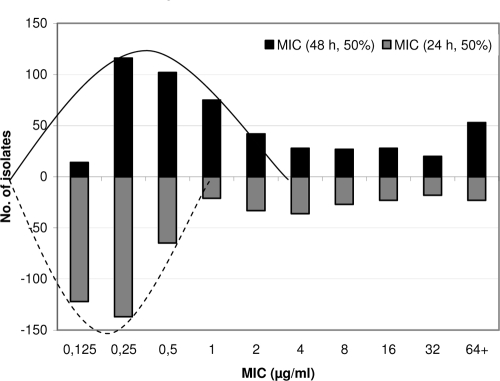
Recent modifications to the CLSI susceptibility testing standard M27-A on fluconazole MIC determination highlight the time of incubation (48 or 24 h) and the end point criteria (80% growth inhibition for macrodilution and 50% for microdilution) (2, 4). The use of the 50% inhibition end point after 24 h resulted in one- to twofold-lower MICs than were found after 48 h, as exemplified in Fig. 1. The 24- and 48-h MIC limits for the two recommended quality control strains are lowered correspondingly: for Candida parapsilosis ATCC 22019, 0.5 to 4 mg/liter versus 1 to 4 mg/liter, and for Candida krusei ATCC 6258, 8 to 64 mg/liter versus 16 to 128 mg/liter (3). Yet, the authors conclude that this does not give rise to a need for lowering fluconazole breakpoints.
FIG. 1.
MICs obtained by the CLSI method, using 48 h of incubation and 50% inhibition or using 24 h of incubation and 50% inhibition.
A one- to twofold-lower MIC will not alter the classification of susceptibility for the majority of C. albicans isolates, as the MICs are far below the breakpoint for susceptibility (MIC ≤ 8 mg/liter). However, one- to twofold-lower MICs will have a significant impact on the susceptibility of isolates belonging to the naturally more resistant species Candida glabrata and Candida krusei, with typical MICs of 4 to 64 mg/liter, as well as for isolates with acquired resistance. For instance, 3 of the 10 isolates (30%) that Baddley et al. defined as resistant (MIC ≥ 64 mg/liter) using the 48-h and 80% or 50% inhibition criteria would be reclassified as susceptible in a dose-dependent manner (SDD) if the early reading and 50% end point definition were used, while this was the case for 30/53 isolates (56.6%) in Ostrosky-Zeichner's study (2, 4). In addition, the percentage of clinical response for SDD strains would decrease from 86% to 55% when the MICs were obtained at 48 h versus 24 h (4).
Both authors conclude that even with an earlier reading and a 50% growth inhibition end point, the MIC/pharmacodynamic outcome relationship supports the use of the original breakpoints. However, we have the following concerns. Both studies pool infections caused by different species, thus assuming that the virulence levels of the yeasts and the host groups are identical. This may not be the case (1). Furthermore, at least for the Baddley study, there were fewer than 10 cases involving isolates with MICs of >1 mg/liter in most groups, making percentage calculations inappropriate (2). Classification and regression tree (CART) analysis (CART 6.0; Salford Systems, CA) produces receiver operating characteristic (ROC) area curves and relative errors which are of insufficient quality for any conclusions to be drawn (Table 1). If such analysis is performed anyway, the lowest relative error and highest ROC area for predicting mortality are obtained with a 48-h incubation, an 80% inhibition end point, and a log2 MIC of 2.5 mg/liter as the threshold (Table 1), which suggests a breakpoint for resistance of >4 mg/liter or ≥8 mg/liter (Table 2).
TABLE 1.
CART analysis of the relationship of MIC versus mortality in patients with candidemia (data taken from reference 2)a
| Incubation period and growth inhibition end point | MIC split (mg/liter) | Relative errorb | ROC curve areac |
|---|---|---|---|
| 24 h | |||
| 50% | ≤32/>32 | 1.033 | 0.468 |
| 80% | ≤4/>4 | 0.942 | 0.524 |
| 48 h | |||
| 50% | ≤4/>4 | 0.883 | 0.560 |
| 80% | ≤4/>4 | 0.714 | 0.646 |
CART analysis (CART 6.0; Salford Systems, CA) was performed with the following methodological conditions: Gini method, minimum cost tree regardless of the size for selecting the best tree, 10 v-fold-cross-validation, equal priors, no costs, and no penalties.
A relative error of 0 means no error, or a perfect fit, and 1 represents the performance of random guessing.
A ROC curve area of 1 means a perfect prediction (100% sensitivity and 0% false positives), and 0.5 represents a random guess.
TABLE 2.
Relationship of MIC to mortality in patients with candidemia (data taken from reference 2)
| Susceptibility classification | No. of nonsurvivors/total no. in group (%) for indicated breakpoint criteria, incubation period, and growth inhibition end point |
|||||||
|---|---|---|---|---|---|---|---|---|
| Alternativea |
CLSIb |
|||||||
| 24 h |
48 h |
24 h |
48 h |
|||||
| 50% | 80% | 50% | 80% | 50% | 80% | 50% | 80% | |
| Susceptible | 17/65 (26.1) | 16/58 (27.6) | 12/55 (21.8) | 12/52 (23) | 20/74 (27) | 20/74 (27) | 18/71 (25) | 17/67 (25.4) |
| Intermediate/SDD | 3/8 (37.5) | 1/12 (8.3) | 3/11 (27.3) | 1/9 (11.1) | 0/3 (0) | 0/2 (0) | 0/3 (0) | 1/5 (20) |
| Resistant | 4/10 (40) | 7/14 (50) | 9/18 (50) | 11/21 (52.3) | 4/7 (57.1) | 4/8 (50) | 6/10 (60) | 6/10 (60) |
Susceptibility, MIC of <2 mg/liter; resistance, MIC of >4 mg/liter.
Susceptibility, MIC of ≤8 mg/liter; resistance, MIC of ≥64 mg/liter.
If data mining tools for ascertaining the statistical power of in vitro correlation with outcome do not produce results that reach statistical significance, we suggest that changes in a susceptibility test leading to one- to twofold-lower MICs should be accompanied with a similar decrease in breakpoints.
REFERENCES
- 1.Arendrup, M., T. Horn, and N. Frimodt-Møller. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30:286-291. [DOI] [PubMed] [Google Scholar]
- 2.Baddley, J. W., M. Patel, S. M. Bhavnani, S. A. Moser, and D. R. Andes. 2008. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob. Agents Chemother. 52:3022-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 4.Ostrosky-Zeichner, L., J. H. Rex, M. A. Pfaller, D. J. Diekema, B. D. Alexander, D. Andes, S. D. Brown, V. Chaturvedi, M. A. Ghannoum, C. C. Knapp, D. J. Sheehan, and T. J. Walsh. 2008. Rationale for reading fluconazole MICs at 24 hours rather than 48 hours when testing Candida spp. by the CLSI M27-A2 standard method. Antimicrob. Agents Chemother. 52:4175-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]