Abstract
Characterization of the time course and magnitude of enzyme induction due to multiple inducers is important for interpretation of clinical data from drug-drug interaction studies. A population interaction model was developed to quantify efavirenz autoinduction and further induction with concurrent carbamazepine coadministration. Efavirenz concentration data in the absence and presence of carbamazepine following single- and multiple-dose oral administrations in healthy subjects were used for model development. The proposed model was able to describe the time-dependent efavirenz autoinduction and the further induction with carbamazepine when the agents were combined. The estimated population averages of efavirenz oral clearance were 5.5, 9.4, 14.4, and 16.7 liters/h on days 1, 14, and 35 and at steady state for the interaction, respectively, for efavirenz monotherapy for 2 weeks followed by the coadministration of carbamazepine for 3 weeks. The estimated times to 50% of the steady state for efavirenz autoinduction and for the induction resulting from the concurrent administration of efavirenz and carbamazepine were similar (around 10 to 12 days). With this model-based analysis, efavirenz exposures can be projected prior to and at the steady state of induction, allowing a better understanding of the time course and magnitude of enzyme induction.
Drug-drug interaction studies are an important aspect of clinical pharmacology research and can be a critical step to optimize the use of selected medicines. In the treatment of human immunodeficiency virus (HIV) infection and HIV complications, polypharmacy is compulsory, and this necessitates the conduct of numerous interaction studies (3). Interpretation of results from interaction studies may be challenging when multiple enzyme inducers or inhibitors are involved while study duration is restricted. In some cases, it may be difficult to know when steady state is achieved or only a “pseudo”-steady state may be achievable during a controlled study period.
Enzyme induction has important clinical implications when enhanced drug metabolism results in lower drug concentrations, which leads to a suboptimal efficacious response or, even worse, the development of drug resistance. Enzyme induction can be due to (i) a drug affecting its own metabolism (autoinduction), (ii) comedication(s) with induction capability, or (iii) both, such as the concomitant use of efavirenz and carbamazepine.
Efavirenz is a potent nonnucleoside reverse transcriptase inhibitor approved for the treatment of HIV-1 infection in combination with other antiretroviral agents (efavirenz [Sustiva] package insert for capsules and tablets; Bristol-Myers Squibb Co., Princeton, NJ) (1). It is metabolized mainly by cytochrome P450 2B6 (CYP2B6) and possibly CYP3A4 or other CYP isoforms to a less extent (efavirenz package insert for capsules and tablets; Bristol-Myers Squibb Co.) (1, 29). Thus, drugs that induce CYP2B6/3A4 (e.g., rifampin, carbamazepine, and phenobarbital) may increase the clearance of efavirenz, resulting in a time-dependent decrease in efavirenz exposure. Furthermore, efavirenz is an inducer of CYP2B6/3A4. It increases its own metabolism as well as the metabolism of comedications that are substrates of CYP2B6/3A4 (efavirenz package insert for capsules and tablets; Bristol-Myers Squibb Co.) (9, 10, 29, 31).
Carbamazepine is an anticonvulsant commonly used for the management of neurological symptoms associated with HIV infection (carbamazepine [Tegretol] package insert for tablets; Novartis Pharmaceutical Corporation, East Hanover, NJ). The development of neurological symptoms in HIV-infected patients is not uncommon and is related either primarily to HIV infection or secondarily to opportunistic infections (19). Since the frequency of new-onset seizures in HIV infection has been estimated to be 3% (22), there is a medical need to use anticonvulsants in the management of these patients. Similar to efavirenz, carbamazepine exhibits an autoinduction property and increases the metabolism of concomitantly administered drugs. It is a substrate of CYP3A4, CYP2C8, and CYP1A2 predominantly and is a potent inducer of CYP2B6/3A4 (carbamazepine package insert for tablets; Novartis Pharmaceutical Corporation) (12, 13, 21). Therefore, an understanding of the time course and magnitude of enzyme induction when efavirenz and carbamazepine are coadministered is of clinical importance.
Clinical evaluation of enzyme induction may require unfeasibly prolonged study periods, especially in the presence of both autoinduction and induction due to a concomitantly administered drug. Evaluation of the efavirenz-carbamazepine interaction at steady state under well-controlled study conditions is therefore challenging, and to the best our knowledge, time to steady state and magnitude of induction at steady state of the interaction have not been adequately characterized.
Application of modeling and simulation to the characterization of enzyme induction have emerged over the years (2, 3, 23, 24, 33) as effective means to improve our understanding of the temporal pattern and magnitude of induction. With this approach, enzyme induction can be quantified with data collected prior to steady state. The approach allows the projection of induction status under untested conditions by using model-based simulation and helps to better assess the clinical significance of induction in drug treatment.
We employed a model-based approach in this analysis to aim at developing a population drug-drug interaction model for describing enzyme induction in order to (i) characterize the time course and magnitude of efavirenz autoinduction and the further induction associated with concurrent carbamazepine treatment and (ii) project efavirenz exposures when chronically coadministered with carbamazepine.
MATERIALS AND METHODS
This section provides brief descriptions of the data used for model development, model development steps, and the method for model-based simulation. A detailed description of the model-based analysis is provided in the Appendix.
Clinical data for model development.
To better understand the change in efavirenz exposure over time due to enzyme induction, clinical data from two phase I studies were employed in this analysis, namely, an efavirenz single-dose study for characterizing efavirenz clearance in the absence of codrug-caused induction and an efavirenz-carbamazepine multiple-dose interaction study for characterizing the change in efavirenz clearance over time in the absence and presence of carbamazepine.
(i) Efavirenz single-dose study.
The efavirenz single-dose study was a phase I, open-label, three-period crossover bioavailability study with healthy adult subjects. Only data from subjects who received a 600-mg efavirenz tablet (i.e., label-recommended dose and formulation) were used for model development. Plasma concentrations were measured for efavirenz over 504 h following a single dose. The data were utilized to estimate baseline efavirenz clearance in the absence of enzyme induction.
(ii) Efavirenz-carbamazepine interaction study.
The efavirenz-carbamazepine interaction study was designed to evaluate the effect of carbamazepine on the pharmacokinetics (PK) of efavirenz in healthy adult subjects. Details of the study design and subject demographics were reported previously (16). In brief, subjects received 600-mg efavirenz tablets once daily (QD) for 14 days, followed by coadministration of carbamazepine with titrated doses from 200 mg QD, to 200 mg twice daily, to 400 mg QD between days 15 and 35. Plasma concentrations were measured over 24 h on day 14 for efavirenz and day 35 for efavirenz and carbamazepine. Trough concentrations of efavirenz and carbamazepine were measured every other day between day 2 and day 35. Methods for measurement of efavirenz and carbamazepine plasma levels were reported previously (16).
In both studies, efavirenz plasma concentrations were measured with a validated liquid chromatography method (16). The lower limits of quantitation for efavirenz were 0.05 and 0.1 μg/ml in the single- and multiple-dose studies, respectively.
Model development steps.
Nonlinear mixed-effects modeling was employed in this analysis with the NONMEM computer program (version VI, level 1.1), compiled with GNU FORTRAN, version 77 (4). The installation of NONMEM has been validated, and all relevant patches and upgrades were installed. Diagnostic graphics, exploratory analyses, and postprocessing of NONMEM output were performed with S-Plus software (version 7.0 for Linux; Insightful, Seattle, WA). Model development was comprised of three steps: (i) the development of a base model for the characterization of efavirenz PK in which efavirenz clearance was assumed to be constant, (ii) the development of an autoinduction model in which efavirenz clearance was described as a function of time, and (iii) the development of drug-drug interaction models in which the effect of carbamazepine-associated induction on efavirenz clearance was described either as a function of time (model A) or as a function of carbamazepine exposures (model B). Furthermore, the effect of subject demographics on efavirenz clearance was examined. Detail descriptions of these models are provided in the Appendix.
Simulation to project efavirenz exposure.
Model-based simulation was performed with the time-dependent interaction model (model A). One thousand subjects were simulated to examine changes in efavirenz exposure when the drug was dosed alone at 600 mg QD for 14 days followed by the coadministration of 400 mg carbamazepine OD for 3 and 11 weeks (i.e., at 86% and 95% of the steady state of interaction, respectively). Efavirenz exposure levels were computed.
RESULTS
This section describes a summary of data used for modeling, results of the base model, autoinduction, and drug-drug interaction models for efavirenz and model evaluation results.
Data summary.
A total of 336 efavirenz plasma concentrations from 21 subjects who participated in the single-dose study and 584 efavirenz plasma concentrations from 16 subjects who participated in the multiple-dose interaction study were pooled for model development. The demographic characteristics for the subjects in this combined data set were a median weight of 78 kg (range, 47 to 102 kg) and a median age of 28 years (range, 18 to 45 years), with 29 males and 8 females. Data for one subject who participated in the interaction study were not included in the analysis because the plasma concentrations for this subject were severalfold higher than those in others (outlier) (Fig. 1). Possible reasons for this are provided in Discussion.
FIG. 1.
Efavirenz plasma concentration-time profiles after dosing with efavirenz alone and with coadministration of carbamazepine. Subjects received 600 mg efavirenz (EFV) QD for 14 days, followed by coadministration with carbamazepine (CBZ) for 21 days. Plasma samples were collected during day 14 and day 35 and at predose between days 1 and 14 and days 15 and 35.
Base model for efavirenz.
Data from the single-dose study and the multiple-dose study when efavirenz was administered alone were pooled for developing this model, with an assumption that there was no time-dependent change in efavirenz oral clearance (CL/F). The parameter estimates generated from this model are presented in Table 1 (base model). The estimated population average of the efavirenz CL/F (7 liters/h) with this model was found to be higher than those reported previously for single-dose studies (4 to 5 liters/h) (2, 8). Since the efavirenz CL/F was assumed to be constant over time, the estimated CL/F was, in fact, an average value over the 14-day dosing period. It was therefore hypothesized in the subsequent autoinduction model that the efavirenz CL/F could change over time following multiple-dose administration.
TABLE 1.
Population model parameter estimates for efavirenz
| Parameter | Valuea
|
|||||
|---|---|---|---|---|---|---|
| Base model
|
Autoinduction model
|
Coinduction model A
|
||||
| Avg (RSE) | IIV (%) (RSE) | Avg (RSE) | IIV (%) (RSE) | Avg (RSE) | IIV (%) (RSE) | |
| Ka (h−1) | 0.428 (14) | 49 (38) | 0.420 (12) | 49 (34) | 0.431 (19) | 48 (32) |
| V2/F (liters) | 183 (15) | 44 (25) | 173 (11) | 46 (30) | 170 (10) | 46 (19) |
| V3/F (liters) | 317 (10) | 335 (9) | 349 (9) | |||
| Q (liters/h) | 16.4 (23) | 15.9 (16) | 15.1 (12) | |||
| CLi (liters/h) | 8.16 (12) | |||||
| CLiday1 (liters/h) | 6.52 (7) | 6.14 (7) | ||||
| AImax (liters/h) | 8.29 (30) | |||||
| AIday14 (liters/h) | 10.2 (32) | |||||
| DDImax (liters/h) | 13.5 (21) | |||||
| T50 (h) | 245 (67) | 309 (56) | ||||
| T50′ (h) | 275 (42) | |||||
| CL/F (liters/h) | 7.01 | 31 (49) | ||||
| CL/Fday1 (liters/h) | 5.76 | 5.47 | ||||
| CL/Fday14 (liters/h) | 9.22 | 9.39 | ||||
| CL/Fday35 (liters/h) | 14.4 | |||||
| CL/FssAI (liters/h) | 11.4 | 26 (36) | ||||
| CL/FssDDI (liters/h) | 16.7 | 31 (32) | ||||
| RR_sd (%) | 21 (11) | 21 (12) | ||||
| RR_md (%) | 33 (17) | 27 (9) | ||||
Abbreviations: DDImax, maximal change of CLi at steady state of coinduction since carbamazepine was coadministered; T50′, time to 50% of DDImax; RSE, relative standard error, represented as percentage of the population average; IIV, interindividual variability; RR, residual error, sd, single-dose study; md, multiple-dose study. CL/F values at the specified time were calculated from the CLi according to equations 1, 2, and 4 in the Appendix.
Autoinduction model for efavirenz.
The same data set for developing the base model was used to develop the autoinduction model but with an assumption that the efavirenz CL/F was time dependent. In this model, the intrinsic efavirenz clearance (CLi) was described as a nonlinear function of time (see equation 5 in the Appendix). The parameter estimates generated are provided in Table 1 (autoinduction model). According to the model estimation, the population averages of CL/F on day 1, on day 14, and at the steady state of efavirenz autoinduction were 5.76, 9.22, and 11.4 liters/h, respectively, suggesting that the efavirenz CL/F increased over time, and the steady-state CL/F was approximately twofold higher than the baseline value. The estimated average time to achieve 50% of the maximal change in intrinsic clearance from baseline due to autoinduction (AImax) was approximately 10 days (245 h) (Table 1). Following an efavirenz dosing of 600 mg QD for 2 weeks, 81% of the steady-state CL/F was attained by day 14. It appears that 3 months of dosing may be needed in order to attain a 95% steady state due to the nonlinear time course of enzyme induction.
By incorporating a time factor into the model, the interindividual variability for efavirenz CL/F was reduced from 31% to 26% compared to that estimated by the base model (Table 1), and the objective function value was reduced by 35 points (P < 0.001), indicating that time is a meaningful covariate in estimating efavirenz CL/F during the course of autoinduction.
The efavirenz AImax was further tested as a function of baseline CLi at day 1 (CLiday1) (see equation 6 in the Appendix). Results revealed that the parameters generated from this test model were similar to those generated from the reference model (see equation 5 in the Appendix). The objective function values of the two models were the same, indicating that the AImax can be either estimated as an independent parameter or reparameterized as “A”-fold of CLiday1. Since the estimated proportional constant “A” was 1.27, AImax can be expressed as 1.27-fold of CLiday1.
An observation noted from the individual efavirenz concentration-time profiles was that subjects who showed higher efavirenz concentrations at the beginning of treatment appeared to be able to maintain higher concentrations during the autoinduction at the dose level tested (Fig. 1). The finding implied that the magnitude of autoinduction was not driven primarily by the efavirenz concentrations and that the higher concentrations were a consequence of less extensive autoinduction that rooted from the lower baseline clearance (Fig. 2). Therefore, the individual intrinsic capacity of induction appeared to be the primary factor to determine the extent of autoinduction for each subject.
FIG. 2.

Efavirenz oral clearance (CL/F) in the absence and in the presence of carbamazepine according to model-based simulation. Subjects received 600 mg efavirenz (EFV) QD for 14 days, followed by coadministration with carbamazepine (CBZ) for 21 days.
The main population PK parameter estimates at the steady state of autoinduction derived from this model using data from healthy subjects are consistent with literature model parameters for HIV-infected patients (2, 24), suggesting that the enzyme induction process does not appear to be affected by HIV infection. The similarity between the parameters in healthy and patient populations lends credence to the appropriateness of applying the model to analyze data from HIV-infected patients. Demographics (age, body weight, and gender) were tested as covariates on the efavirenz CL/F; no effect was detected with the given data set.
Interaction model for efavirenz (model A).
All data from the single- and multiple-dose studies were used for developing the interaction model for efavirenz. The efavirenz CL/F was described as a function of time in the absence and in the presence of carbamazepine. The parameter estimates are provided in Table 1 (coinduction model A). The population estimates of efavirenz CL/F right before carbamazepine dosing (i.e., day 14), on the last day of carbamazepine dosing (day 35), and at the steady state of carbamazepine interaction were 9.39, 14.4, and 16.7 liters/h, respectively. The CL/F increased approximately threefold at steady state for coinduction compared to the efavirenz baseline CL/F.
The temporal profiles of individual efavirenz CL/F in the absence and in the presence of carbamazepine are depicted in Fig. 2. Notably, subjects who showed a lesser effect of autoinduction (i.e., lower CL/F) on day 14 also had less response to the carbamazepine-caused induction, indicating that the two agents appeared to induce CYP isoforms via similar mechanisms, in agreement with in vitro findings that both agents induced CYP2B6 and CYP3A4 preferentially through the same receptor (hCAR), and the two CYP isoforms appear to be induced simultaneously (13).
Based on model predictions, the administration of efavirenz alone for 14 days reached 81% of steady-state autoinduction levels, and coadministration with carbamazepine for 21 days reached 86% of steady-state efavirenz-carbamazepine coinduction levels. The model-estimated mean percent reduction in the efavirenz area under the concentration-time curve (AUC) between day 35 and day 14 was 35%, which was in agreement with the 36% reduction estimated by using noncompartmental analysis (16). In addition, the model was able to estimate that the mean difference in the efavirenz AUC between the steady state of autoinduction and the steady state of efavirenz-carbamazepine coinduction was 32%. The estimated average time to achieve 50% of the maximal change in CLi due to coinduction is approximately 11 days, or 275 h counting from the coadministration of carbamazepine.
Simulation based on model A indicated that the mean efavirenz AUC was 65 μg·h/ml when dosed alone at 600 mg QD for 14 days and that the mean AUC values were 42 and 38 μg·h/ml, respectively, with the coadministration of 400 mg carbamazepine QD for 3 and 11 weeks. A substantial reduction in the efavirenz AUC was achieved with a 3-week course of carbamazepine coadministration, and a further reduction in AUC should not be substantial following the long-term coadministration of carbamazepine (Fig. 3).
FIG. 3.
Model-projected daily exposures to 600 mg efavirenz in the absence (day 14) and in the presence (day 35 and day 90) of 400 mg/day carbamazepine. Horizontal lines represent the 5th and 95th percentiles of the simulated day 90 AUC values for monotherapy with 600 mg efavirenz per day. The line in the middle of the box represents the 50th percentile, and the margins of box represent the 25th and 75th percentiles of the simulated AUC. The vertical bars represent the whiskers. The data for day 14 represent the efavirenz AUC after 14 doses of monotherapy. The data for day 35 and day 90 represent the efavirenz AUC following coadministration with 400 mg/day carbamazepine for 3 and 11 weeks, respectively.
Interaction model for efavirenz (model B).
The effect of carbamazepine concentrations on the efavirenz CL/F was evaluated with model B. The estimated population mean CL/F values on days 1, 14, and 35 were 5.5, 9.1, and 13 liters/h, respectively. The estimates were comparable to those generated with model A (Table 1). The mean carbamazepine trough concentration on day 35 required to achieve 50% of the maximal change in CLi during coinduction (DDImax) (see the Appendix) was estimated to be 0.84 μg/ml. The similarity in the parameter estimation with the two different approaches (i.e., models A and B) suggests that either time or carbamazepine concentrations at near the steady state of interaction can be used as a covariate to describe the enzyme induction-related change in the efavirenz CL/F. However, a limitation of model B is that although the magnitude of induction associated with the concomitant administration of efavirenz and carbamazepine can be estimated, the time to the steady state of the interaction cannot be projected (see equation 8 in the Appendix).
Model evaluation.
Model diagnostic plots were used for the evaluation of the goodness of fit of tested models. It revealed that there was no systematic bias in goodness of fit in the autoinduction and efavirenz-carbamazepine coinduction models, and the models well described the observed data. The efavirenz exposure parameters generated from the model-based simulation were comparable with the parameters estimated by the model-independent noncompartmental analysis.
A visual predictive check was performed for the time-dependent interaction model (model A). There were more than 90% of observed concentrations within the 90% prediction interval (Fig. 4), indicating that the interaction model was able to reasonably project efavirenz plasma concentrations over time in the absence and in the presence of carbamazepine.
FIG. 4.
Predictive check plot representing model-predicted and actual measured efavirenz plasma concentrations. Subjects received 600 mg efavirenz (EFV) QD for 14 days, followed by coadministration with carbamazepine (CBZ) for 21 days. The solid line represents the model-predicted median (50th percentile) efavirenz concentration, and the gray area represents the 5th and 95th percentiles of model-predicted efavirenz concentrations. The circles represent observed efavirenz concentrations.
DISCUSSION
The common approach to evaluate enzyme induction is to conduct a controlled clinical trial within a specified time period in order to attain a “steady state” of induction evidenced by negligible changes in drug trough concentrations over several consecutive days. Although this approach is effective in terms of capturing rapid changes in exposure during the earlier phase of induction and minimizing the time and resource for conducting the trial, a question remains whether the steady state for induction was attained, especially when coadministered drugs exhibited both autoinduction and potentially further induction when combined, such as efavirenz and carbamazepine. In comparison to a conventional data analysis approach (e.g., noncompartmental analysis) for enzyme induction, the model-based assessment exhibits advantages in assessing time-dependent events, such as autoinduction and codrug-related induction, and in better handling data collected prior to steady state for induction.
An understanding of the temporal profile of efavirenz induction in the absence and in the presence of other enzyme inducers is of clinical and scientific interest. Although several population PK models were developed for efavirenz (2, 8, 24), this is the first attempt to fully characterize the time course and magnitude of efavirenz-related enzyme induction. The data used for this analysis were unique for assessing the time effect because (i) the data were obtained from well-controlled clinical studies with concentrations measured over time, (ii) the data were from a homogeneous study population during both autoinduction and efavirenz-carbamazepine interaction phases with minimal interference with disease-related factors, and (iii) no interference of other comedications was allowed. These attributes enable us to develop a model that can better understand the enzyme induction process and that can properly interpret clinical data collected at different stages of induction and allow us to project efavirenz exposures beyond the period of clinical trial.
An interaction between efavirenz and carbamazepine is anticipated due to the involvement of common metabolic enzymes (CYP2B6 and CYP3A4) (31) (carbamazepine package insert for tablets [Novartis Pharmaceutical Corporation] and efavirenz package insert for capsules and tablets [Bristol-Myers Squibb Co.]). Both drugs induce CYP2B6 and CYP3A4 preferentially through a constitutive androstane receptor (hCAR), which regulates CYP2B6 and CYP3A4 gene expression (12, 13, 15, 21). Efavirenz-related enzyme induction has been reported to be time and concentration dependent (13, 20). Since only one dose level (i.e., 600 mg) of efavirenz was evaluated, the time dependency of induction was investigated using model A. Nevertheless, the 600-mg dose is the label-recommended efavirenz dose for the treatment of HIV in combination with other antiretroviral agents (efavirenz package insert for capsules and tablets; Bristol-Myers Squibb Co.), which underscores the clinical relevance of the current investigation.
Results from in vitro experiments suggest that efavirenz induction is nonlinear with respect to concentration. At an efavirenz concentration of 10 μM, which is equivalent to the average in vivo concentration at 600 mg efavirenz administered QD for humans, CYP2B6 induction in human hepatocytes was comparable to that for rifampin at 10 μM and approached near-maximal induction (12, 13). This in vitro finding may explain why a relationship between the efavirenz concentration and the extent of induction was not observed at the 600-mg efavirenz dose, presumably due to the fact that maximal induction was likely achieved. Furthermore, as mentioned above, subjects who had higher efavirenz concentrations at baseline were able to maintain higher concentrations during autoinduction and the efavirenz-carbamazepine interaction (Fig. 1), suggesting that higher concentrations did not necessarily lead to a more substantial induction (or lower exposure). Therefore, the magnitude of induction at the 600-mg dose appears to be associated mainly with the individual intrinsic capacity of CYP2B6/3A4 induction. Moreover, literature data suggest that the magnitude of induction and intersubject variability in the induction may be associated with a genetic polymorphism of CYP2B6 (1, 7, 11, 18, 26, 30).
This model-based assessment suggests that the magnitude of induction at steady state appears to be associated with baseline efavirenz clearance. The estimated efavirenz CL/F values at steady state for autoinduction and following the coadministration of efavirenz and carbamazepine were two- and threefold of the baseline value, respectively. As a result, the contribution of efavirenz autoinduction to the total induction with carbamazepine was computed to be two-thirds. Consistent with our findings, the efavirenz CL/F was reported to be increased by 2.6-fold after the coadministration of 600 mg/day rifampin for 10 days compared to its baseline value, and the magnitude of change for the individual CL/F on day 10 was strongly associated with the individual baseline CL/F (10), implying that the baseline efavirenz CL/F may be an indicator for the projection of the magnitude of such induction.
Carbamazepine-mediated enzyme induction has been reported to be time and concentration dependent (carbamazepine package insert for tablets; Novartis Pharmaceutical Corporation) (5, 13). Therefore, the effect of carbamazepine concentrations, represented as trough concentrations on day 35, on the efavirenz CL/F was examined with model B. Simulation based on this model projected that the increase in efavirenz CL/F was marginal in the target therapeutic carbamazepine concentration range (4 to 12 μg/ml) (6, 25) (carbamazepine package insert for tablets; Novartis Pharmaceutical Corporation), because ∼90% of the steady-state efavirenz CL/F was achieved at a carbamazepine trough concentration of 5 μg/ml. An increase in the carbamazepine trough concentration from 5 to 10 μg/ml would result in an increase in the efavirenz CL/F of only <5% (from 10.9 to 11.4 liters/h) and a marginal reduction in the efavirenz daily AUC of only <5%. This simulation indicates that although the carbamazepine dose was tested up to 400 mg in the efavirenz-carbamazepine interaction study, an increased carbamazepine dose to >400 mg in clinical practice would not be expected to have a substantially different enzyme induction effect as that at the 400-mg dose.
Since CYP2B6 accounts for approximately 90% of efavirenz metabolism along with other CYP isoforms (14, 17, 30) and is coregulated with CYP3A4 (18), significant drug interactions between efavirenz and a number of CYP2B6/3A4 inducers or inhibitors have been found (efavirenz package insert for capsules and tablets; Bristol-Myers Squibb Co.). Given the magnitude of the interaction (i.e., reduction in efavirenz AUC by one-third), the clinical implication of the decrease in efavirenz exposure on long-term efficacy outcomes and on the virus-resistant profile to efavirenz should be further evaluated.
Despite the fact that induction occurred in most subjects, the absence of induction appeared in one subject in the interaction study (Fig. 1). The concentration increased continuously throughout the 35-day treatment period, regardless of dosing with efavirenz alone or concurrent dosing with carbamazepine. The demographic characteristics and adverse-event profiles of this subject were comparable to those of other subjects in the study.
Assuming a lack of efavirenz- and carbamazepine-mediated enzyme induction, the PK profile of this subject was simulated with model A, where the baseline clearance was included but where values of AIday14 and DDImax were set to zero. The simulated concentration-time profile corresponded with the observed profile as depicted in Fig. 5. This finding not only implies that there is no apparent induction in this subject but also suggests that model A can be used to describe efavirenz exposures in the absence and in the presence of carbamazepine from noninducible to fully inducible subjects. It has been hypothesized that the higher efavirenz concentration in this subject might be due to the genetic polymorphism of CYP2B6 (17, 26, 30). In addition, since carbamazepine also induces CPY3A4 and since efavirenz is a substrate of CYP3A4, it was expected that the carbamazepine induction may decrease efavirenz exposure in subjects with low CYP2B6 enzyme activity and normal CYP3A4 activity. However, this simulation suggests a lack of induction in this subject, implying that either CYP3A4 may not be inducible in subjects with low CYP2B6 activity or there is a strong coregulation of CYP3A4 and CYP2B6 or a minor contribution of CYP3A4 to efavirenz metabolism.
FIG. 5.
Observed and simulated efavirenz plasma concentration-time profiles in a subject with a lack of enzyme induction (outlier). The subject received 600 mg efavirenz QD for 14 days, followed by coadministration with carbamazepine for 21 days.
The model developed in this analysis may have several clinical applications, as discussed below.
(i) Better understanding of relationships between efavirenz exposure and clinical outcome.
The autoinduction model has been successfully used to estimate efavirenz exposures for a phase IV patient trial in which concomitantly administered drugs do not show an apparent interaction with efavirenz. Relationships among individual efavirenz exposures, viral loads, adverse events, and tolerability data at a given time were assessed and provided a better understanding of treatment-related clinical outcomes. In addition, applying this autoinduction model to a large patient population verified that the parameter estimates generated from healthy subjects in the current assessment were comparable to those obtained from a larger-scale (n = 200 subjects) patient trial with longer durations of treatment (24 weeks), which served as an external validation of the efavirenz autoinduction model to a certain extent. The efavirenz PK in subjects who showed much higher efavirenz concentrations was handled successfully with a mixture model.
(ii) Evaluation of efavirenz dose adjustments.
The preservation of sufficient efavirenz anti-HIV activity when the exposure is altered by interacting drugs, especially during long-term treatment, is important. The proposed model can be used for trial simulation to evaluate efavirenz exposure if the dose is modified during the chronic coadministration of carbamazepine. Any potential dose adjustment needs to be further evaluated in conjunction of safety, efficacy, and genomic data.
(iii) Optimization of design of drug-drug interaction studies.
The model developed in this study provides valuable information on the time course and magnitude of efavirenz enzyme induction that can help design future efavirenz interaction studies. Safety margin, study period, number of subjects, and optimal PK sampling time can be better selected.
In summary, the proposed models characterize the time course of efavirenz autoinduction and concurrent induction with carbamazepine and quantify the magnitude of the change in efavirenz exposure at any given time of the interaction. A model-based analysis approach not only improves our understanding of such an interaction but, most importantly, also generates scientific insight on multi-inducer-mediated enzyme induction.
APPENDIX
The following content describes details of the model-based analysis. All models developed in this work consisted of two-component models: (i) a structural model to describe the disposition of efavirenz following oral administration and (ii) pharmacostatistical models to describe the interindividual variability and residual errors.
The structural model.
A two-compartment model with first-order absorption and first-order elimination was found to adequately describe the PK of efavirenz. This served as the structural model for the base model, autoinduction models, and drug-drug interaction models. The parameters estimated from the structural model were the absorption rate constant (Ka), the apparent volume of distribution of the drug in the central compartment (V2/F), the apparent volume of distribution of the drug in the peripheral compartment (V3/F), the CL/F, and the intercompartment clearance (Q). The PREDPP subroutines ADVAN4/TRANS4 provided in NONMEM were used to describe the disposition of efavirenz.
The interindividual variability model.
The interindividual variability model describes the unexplained random variability in structure model parameters among individual subjects. The individual value for a structural model parameter was assumed to be log-normally distributed. The interindividual variability for a structural model parameter, P, was given as follows:
 |
(1) |
where Pi is the value of parameter P for the ith individual, Pavg is the population average of the parameter, and ηP,i is a realization of a normally distributed random variable with a mean of zero and a variance of  , given as follows:
, given as follows:
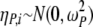 |
(2) |
The residual-error model.
The residual-error model describes the random variability between observed and predicted values within an individual subject due to measurement error as well as model misspecification error. An exponential residual error model for the jth observation in the ith individual was used:
 |
(3) |
where C(t)ij is the jth observed plasma concentration of individual i, C^(t)ij is the jth model predicted value (plasma concentration) for individual i, and ɛij is a normally distributed residual error for the jth measurement of individual i, with a mean of 0 and a variance of σ2. The residual errors (ɛ) for single- and multiple-dose studies were tested separately.
Base model for efavirenz.
The plasma concentration-time data when efavirenz was administered alone were used for model building (i.e., data from the single-dose study and over days 1 and 14 from the interaction study). A first-order absorption and two-compartment disposition model was employed as the structural model. The CL/F of efavirenz was estimated as a time-independent variable in this model. The interindividual variability of a structural model parameter (P) was assumed to be log-normally distributed. The residual errors for the two studies were estimated separately with log-normal residual-error models. The first-order conditional estimation with interaction was used as the minimization method, and the likelihood ratio test (28) and goodness-of-fit diagnostic plots were utilized for model discrimination.
As hepatic enzymes are involved in efavirenz metabolism (20) and renal excretion of efavirenz is negligible (efavirenz package insert for capsules and tablets; Bristol-Myers Squibb Co.), the CL of efavirenz was estimated as a function of the CLi based on a “well-stirred” model (24, 27):
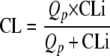 |
(4) |
where Qp is the hepatic plasma flow fixed to a physiological value of 50 liters/h (24). CL is computed from the estimated CLi from the model. F was fixed at 1 (24).
Autoinduction model for efavirenz.
The same data set used for developing the base model was used for developing the efavirenz autoinduction model. A first-order absorption and two-compartment disposition model was used as the structural model. To model the course of efavirenz autoinduction, CLi was expressed as a nonlinear function of time:
 |
(5) |
where AImax is the maximal change of CLi at the steady state of autoinduction from baseline (CLiday1) and T50 is the time to achieve 50% of the AImax. The time represents time after the first efavirenz dose. To examine if the magnitude of autoinduction associated with CLiday1, AImax was further tested as a function of CLiday1 with a proportional constant of A:
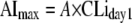 |
(6) |
Interaction models for efavirenz.
Data from the both single- and multiple-dose studies were used for developing the interaction model. A first-order absorption and two-compartment disposition model was selected as the structural model. To describe the change of efavirenz clearance during the interaction, two methods were employed (models A and B).
(i) Model A.
For model A, the efavirenz CLi was described as a function of time, composed of CLiday1, change in CLi during autoinduction, and change in CLi during efavirenz-carbamazepine interactions:
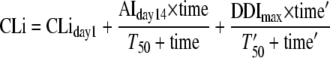 |
(7) |
where AIday14 is the maximum change in CLi up to day 14, the last day that efavirenz was given alone prior to coadministration of carbamazepine, and DDImax is the maximum change in CLi from day 14 to the steady state of the interaction. T50 is time to achieve 50% of AIday14, and T50′ is time′ to achieve 50% of the DDImax. In this model, time was restricted between days 1 and 14 to estimate the efavirenz autoinduction when administered alone. Time′ was set to zero prior to day 15 and then computed starting from day 15 to estimate the combined inductive effects caused by the coadministration of efavirenz and carbamazepine.
(ii) Model B.
The efavirenz CLi was described as a function of time during autoinduction and as a function of the carbamazepine concentration in the presence of carbamazepine:
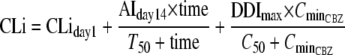 |
(8) |
where CminCBZ is the trough concentration of carbamazepine on day 35 in the presence of efavirenz and C50 is the CminCBZ yielding 50% of the DDImax. Since the trough carbamazepine concentration closely correlated with daily carbamazepine exposure (AUC), this model was designed to examine the effect of daily carbamazepine exposure on enzyme induction dynamics. Trough concentrations following the last dose were used as a surrogate measure for daily carbamazepine exposure under near-steady-state conditions.
Model evaluation.
Model diagnostic plots were used to evaluate the goodnesses of fit of tested models. Model-based simulation was performed to generate concentration-time profiles of efavirenz on selected days and the exposure parameters. The estimated exposures from the simulated profiles were compared to the parameters estimated by a noncompartmental analysis. The time-dependent interaction model (model A) was further evaluated by visual predictive check (32) to determine if the model-simulated efavirenz plasma concentrations were comparable with the observed concentrations in the absence and in the presence of carbamazepine. The 5th, 50th, and 95th percentiles of the simulated data at each time point (1,000 simulations per time point) were calculated, and the percentage of observed concentration data within the 5th and 95th percentiles of the simulated data (i.e., 90% prediction interval) was assessed.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Adkins, J. C., and S. Noble. 1998. Efavirenz. Drugs 56:1055-1064. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, J. S., A. S. Joshi, T. M. Ludden, W. D. Fiske, and H. J. Pieniaszek, Jr. 2002. Population pharmacokinetic meta-analysis with efavirenz. Int. J. Clin. Pharmacol. Ther. 40:507-519. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, J. S., L. Labbe, and M. Pfister. 2005. Application and impact of population pharmacokinetics in the assessment of antiretroviral pharmacotherapy. Clin. Pharmacokinet. 44:591-625. [DOI] [PubMed] [Google Scholar]
- 4.Beal, S. L., L. B. Sheiner, and A. J. Boeckmann (ed.). 1989-2006. NONMEM user's guides. Icon Development Solutions, Ellicott City, MD.
- 5.Bernus, I., R. G. Dickinson, W. D. Hooper, and M. J. Eadie. 1996. Dose-dependent metabolism of carbamazepine in humans. Epilepsy Res. 24:163-172. [DOI] [PubMed] [Google Scholar]
- 6.Bondareva, L. B., R. W. Jelliffe, E. I. Gusev, A. B. Guekht, E. G. Melikyan, and Y. B. Belousov. 2006. Population pharmacokinetic modeling of carbamazepine in epileptic elderly patients: implications for dosage. J. Clin. Pharm. Ther. 31:211-221. [DOI] [PubMed] [Google Scholar]
- 7.Burger, D., I. van der Heiden, C. Porte, M. van der Ende, P. Groeneveld, and C. Richter. 2005. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race and CYP2B6 polymorphism. Br. J. Clin. Pharmacol. 61:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csajka, C., C. Marzolini, K. Fattinger, L. A. Décosterd, J. Fellay, A. Telenti, J. Biollaz, and T. Buclin. 2003. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin. Pharmacol. Ther. 73:20-30. [DOI] [PubMed] [Google Scholar]
- 9.Desta Z., D. A. Flockhart, et al. 2003. CYP2B6 and drug interaction: FDA Advisory Committee for Pharmaceutical Sciences. FDA, Washington, DC.
- 10.Desta, Z. 2007. Rifampin enhances efavirenz metabolism in healthy volunteers: implication for HIV therapy. Clin. Pharmacol. Ther. 81(Suppl.):S99. [Google Scholar]
- 11.Ekins, S., M. Vandenbranden, B. J. Ring, J. S. Gillespie, T. J. Yang, H. V. Gelboin, and S. A. Wrighton. 1998. Further characterization of the expression in liver and catalytic activity of CYP2B6. J. Pharmacol. Exp. Ther. 286:1253-1259. [PubMed] [Google Scholar]
- 12.Faucette, S. R., H. Wang, G. A. Hamilton, S. L. Jolley, D. Gilbert, C. Lindley, B. Yan, M. Negishi, and E. L. LeCluyse. 2004. Regulation of CYP2B6 in primary human hepatocytes by prototypic inducers. Drug Metab. Dispos. 32:348-358. [DOI] [PubMed] [Google Scholar]
- 13.Faucette, S. R., T. C. Zhang, R. Moore, T. Sueyoshi, C. J. Omiecinski, E. L. LeCluyse, M. Negishi, and H. Wang. 2007. Relative activation of human pregnane X receptor versus constitute androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J. Pharmcol. Exp. Ther. 320:72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellay, J., C. Marzolini, E. R. Meaden, D. J. Back, T. Buclin, J. P. Chave, L. A. Decosterd, H. Furrer, M. Opravil, G. Pantaleo, D. Retelska, L. Ruiz, A. H. Schinkel, P. Vernazza, C. B. Eap, A. Telenti, and the Swiss HIV Cohort Study. 2001. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet 359:30-36. [DOI] [PubMed] [Google Scholar]
- 15.Hariparsad, N., S. C. Nallani, R. S. Sane, D. J. Buckley, A. R. Buckley, and P. B. Desai. 2004. Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J. Clin. Pharmcol. 44:1273-1281. [DOI] [PubMed] [Google Scholar]
- 16.Ji, P., B. Damle, J.-D. Xie, S. E. Unger, D. M. Grasela, and S. Kaul. 2008. Pharmacokinetic interaction between efavirenz and carbamazepine after multiple-dose administration in healthy subjects. J. Clin. Pharmacol. 48:948-956. [DOI] [PubMed] [Google Scholar]
- 17.Klein, K., T. Lang, T. Saussele, E. Barbosa-Sicard, W. H. Schunck, M. Eichelbaum, M. Schwab, and U. M. Zanger. 2005. Genetic variability of CPY2B6 in population of African and Asian origin: allele frequencies, novel functional variants, and possible implication for anti-HIV therapy with efavirenz. Pharamcogenet. Genomics 15:861-873. [DOI] [PubMed] [Google Scholar]
- 18.Lamba, V., J. Lamba, K. Yasuda, S. Strom, J. Davila, M. L. Hancock, J. D. Fackenthal, P. K. Rogan, B. Ring, S. A. Wrighton, and E. G. Schuetz. 2003. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR expression. J. Pharmacol. Exp. Ther. 307:906-922. [DOI] [PubMed] [Google Scholar]
- 19.Levy, R. M., D. E. Bredesen, M. L. Rosenblum, and R. L. Davis. 1989. Central nervous system disorders in AIDS. Immunol. Ser. 44:371-401. [PubMed] [Google Scholar]
- 20.Mouly, S., K. S. Lown, D. Kornhauser, J. L. Joseph, W. D. Fiske, I. H. Benedek, and P. B. Watkins. 2002. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin. Pharmacol. Ther. 72:1-9. [DOI] [PubMed] [Google Scholar]
- 21.Oscarson, M., U. M. Zanger, O. F. Rifki, K. Klein, M. Eichelbaum, and U. A. Meyer. 2006. Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin. Pharmacol. Ther. 80:440-456. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Sedano, B., A. Iranzo, J. Marti-Fabregas, P. Domingo, A. Escartin, M. Fuster, J. L. Barrio, and M. A. Sambeat. 1999. Prospective study of new-onset seizures in patients with human immunodeficiency virus infection: etiologic and clinical aspects. Arch. Neurol. 56:609-612. [DOI] [PubMed] [Google Scholar]
- 23.Pfister, M., L. Labbé, J. F. Lu, S. M. Hammer, J. Mellors, K. K. Bennett, S. Rosenkranz, L. B. Sheiner, and the Adult AIDS Clinical Trial Group Study 398 Investigators. 2002. Effect of coadministration of nelfinavir, indinavir, and saquinavir on the pharmacokinetics of amprenavir. Clin. Pharmacol. Ther. 72:133-141. [DOI] [PubMed] [Google Scholar]
- 24.Pfister, M., L. Labbé, S. M. Hammer, J. Mellors, K. K. Bennett, S. Rosenkranz, L. B. Sheiner, and the Adult AIDS Clinical Trial Group Protocol 398 Investigators. 2003. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trail Group study 398. Antimicrob. Agents Chemother. 47:130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reith, D. M., W. D. Hooper, J. Parke, and B. Charles. 2001. Population pharmacokinetic modeling of steady state carbamazepine clearance in children, adolescents, and adults. J. Pharmacokinet. Phrmacodynam. 28:79-92. [DOI] [PubMed] [Google Scholar]
- 26.Rotger, M., H. Tegude, S. Colombo, M. Cavassini, H. Furrer, L. Décosterd, J. Blievernicht, T. Saussele, H. F. Günthard, M. Schwab, M. Eichelbaum, A. Telenti, and U. M. Zanger. 2007. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin. Pharmacol. Ther. 81:557-566. [DOI] [PubMed] [Google Scholar]
- 27.Rowland. M., and T. N. Tozer. 1989. Clinical pharmacokinetics: concepts and application, 2nd ed. Lea & Febiger, Philadelphia, PA.
- 28.Sheiner, L. B., B. Rosenberg, and V. V. Marathe. 1977. Estimation of population characteristics of pharmacokinetics parameters from routine clinical data. J. Pharmacokinet. Biopharm. 5:445-479. [DOI] [PubMed] [Google Scholar]
- 29.Smith, P. F., R. Dicenzo, and G. D. Morse. 2001. Clinical pharmacokinetics of non-nucleoside reverse transcriptase inhibitors. Clin. Pharmacokinet. 40:893-905. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya, K., H. Gatanaga, N. Tachikawa, K. Teruya, Y. Kikuchi, M. Yoshino, T. Kuwahara, T. Shirasaka, S. Kimura, and S. Oka. 2004. Homozygous CYP2B6*6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem. Biophys. Res. Commun. 319:1322-1326. [DOI] [PubMed] [Google Scholar]
- 31.Ward, B. A., J. C. Gorski, D. R. Jones, S. D. Hall, D. A. Flockhart, and Z. Desta. 2003. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J. Pharmacol. Exp. Ther. 306:287-300. [DOI] [PubMed] [Google Scholar]
- 32.Yano, Y., S. L. Beal, and L. B. Sheiner. 2001. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J. Pharmacokinet. Pharmacodynam. 28:171-191. [DOI] [PubMed] [Google Scholar]
- 33.Zhu, M., P. Nandy, S. Kaul, D. M. Grasela, and M. Pfister. 2006. Model-based approach to characterize auto-induction: experience with efavirenz. Clin. Pharmacol. Ther. 79:46. [Google Scholar]






