Abstract
Ceftaroline is a novel broad-spectrum cephalosporin that exhibits bactericidal activity against many gram-positive and -negative pathogens. However, the activity of ceftaroline cannot be solely relied upon for eradication of multidrug-resistant gram-negative isolates, such as Pseudomonas aeruginosa and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, which represent a current clinical concern. As drug combinations might be beneficial by potential synergy, we evaluated the in vitro activity of ceftaroline combined with meropenem, aztreonam, cefepime, tazobactam, amikacin, levofloxacin, and tigecycline. Susceptibility testing was performed for 20 clinical P. aeruginosa isolates, 10 ESBL-producing Escherichia coli isolates, 10 ESBL-producing Klebsiella pneumoniae isolates, and 10 AmpC-derepressed Enterobacter cloacae isolates. Time-kill experiments were performed for 10 isolates using antimicrobials at one-fourth the MIC. Ceftaroline exhibited a MIC range of 0.125 to 1,024 μg/ml and was reduced 2- to 512-fold by combination with tazobactam (4 μg/ml) for ESBL-producing strains. In time-kill experiments, ceftaroline plus amikacin was synergistic against 90% of the isolates (and indifferent for one P. aeruginosa isolate). Ceftaroline plus tazobactam was indifferent for E. cloacae and P. aeruginosa strains but synergistic against 100% of E. coli and K. pneumoniae isolates. Combinations of ceftaroline plus meropenem or aztreonam were also synergistic for all E. coli and E. cloacae isolates, respectively, but indifferent against 90% of the other isolates. Finally, combinations of ceftaroline plus either tigecycline, levofloxacin, or cefepime were indifferent for 100% of the isolates. No antagonism was observed with any combination. Ceftaroline plus amikacin appeared as the most likely synergistic combination. This represents a promising therapeutic option, and further studies are warranted to elucidate the clinical value of ceftaroline combinations against resistant gram-negative pathogens.
Infections due to multidrug-resistant (MDR) gram-negative pathogens affect both immunocompetent and immunocompromised patients and represent a current and important clinical concern. Over the last decade the incidence of these infections has increased throughout the world, leading to an alarming deficit in effective antimicrobial agents (18, 21). Extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae as well as Pseudomonas aeruginosa are among the most important and frequent nosocomial pathogens and are also resistant to many classes of antibiotics (3, 32). The anti-infective agents currently available to treat Enterobacteriaceae infections include fluoroquinolones and β-lactams, for which the activity has been markedly compromised by the emergence of ESBL enzymes and the spread of plasmid-mediated fluoroquinolone resistance (25). For infections caused by P. aeruginosa, which displays a high rate of multidrug resistance, empirical therapy often requires combination therapy (2, 14). Although it may not always prevent the emergence of resistance, antimicrobial combinations may nevertheless enhance the killing effect and the likelihood of cure, by extending the spectra of activities of drugs active against MDR organisms (3, 5, 24).
Combinations of a β-lactam with an aminoglycoside or a β-lactam inhibitor are the most common and have demonstrated greater efficiencies than monotherapy with a β-lactam in serious infections, including gram-negative sepsis or bacteremia (14, 31). Due to the potential toxicity of aminoglycosides, other combinations, such as a β-lactam plus a fluoroquinolone or double β-lactam combinations, have also been investigated and have demonstrated promising results both in vivo and in vitro (13, 28). Mechanisms responsible for the synergistic effect observed with some of these combinations have been investigated. For example, it has been suggested that penetration of aminoglycosides is increased in the presence of a β-lactam, and the degradation of β-lactams may be considerably reduced in the presence of a β-lactamase inhibitor (19).
Several reviews recently reported anti-infective agents either currently available or in development to treat MDR gram-negative infections, but these reviews also emphasized the urgent need for new therapeutic strategies (3, 21, 22). Ceftaroline (formerly referred to as PPI-0903 M or T-91825) is a novel semisynthetic cephalosporin, discovered and initially synthesized by Takeda Chemical Industries, Ltd., Japan (11). Currently in phase III development by Forest Laboratories, ceftaroline exhibits a broad spectrum of activity, covering most of the resistant gram-positive pathogens as well as many common gram-negative organisms (9, 12, 26, 27). The unique biological activity of this cephalosporin results from its higher affinity for the altered penicillin-binding protein 2, PBP2′ (or PBP2a), which is predominantly expressed in methicillin-resistant Staphylococcus aureus, including strains with reduced susceptibility to glycopeptides (23). Ceftaroline also exhibits excellent activity against Streptococcus pneumoniae isolates, and a clinical trial for community-acquired pneumonia is currently under way (http://clinicaltrials.gov). Like other β-lactams, ceftaroline activity against gram-negative species is limited by its affinity for the PBPs and its susceptibility to β-lactamases, especially the ESBL enzymes and cephalosporinases of Enterobacteriaceae and P. aeruginosa strains (23, 27). Although minimum to no activity was reported against MDR gram-negative bacilli, ceftaroline represents a potential candidate for combination therapy, which may extend its spectrum of activity as well as offer a novel and unique therapeutic option to cover mixed infections due to methicillin-resistant S. aureus and MDR gram-negative organisms (27).
The objective of this study was to evaluate the in vitro activity of ceftaroline against clinical MDR gram-negative isolates and to investigate its potential for synergy in combination with a large panel of antimicrobials, including β-lactams (aztreonam, meropenem, and cefepime), an aminoglycoside (amikacin), a β-lactamase inhibitor (tazobactam), fluoroquinolone (levofloxacin), and glycylcycline (tigecycline), which potentially may offer synergistic combinations.
(A portion of this work was presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy and the 46th Annual Meeting of the Infectious Diseases Society of America, Washington, DC, in 2008.)
MATERIALS AND METHODS
Bacterial strains and media.
Twenty clinical P. aeruginosa isolates, 10 ESBL-producing E. coli isolates, 10 ESBL-producing Klebsiella pneumoniae isolates, and also 10 AmpC-derepressed Enterobacter cloacae isolates were selected from the Anti-Infective Research Laboratory (Detroit, MI) and JMI Laboratories (North Liberty, IA) clinical isolate collections for susceptibility testing. Ten strains (two E. coli, two K. pneumoniae, two E. cloacae, and four P. aeruginosa) with differing susceptibilities to ceftaroline were chosen for time-kill experiments. Mueller-Hinton broth (Difco Laboratories, Detroit, MI) supplemented with magnesium (12.5 μg/ml total concentration) and calcium (25 μg/ml total concentration) was used for all microdilution susceptibility testing and time-kill analyses. Tryptose soy agar (Difco Laboratories, San Jose, CA) was used for growth and colony counts.
Antimicrobial agents.
Ceftaroline (lot CI 170-07) was provided by Forest Laboratories, Inc. (New York, NY). Tigecycline (Wyeth Pharmaceuticals, Inc., Pearl River, NY), meropenem (AstraZeneca Pharmaceuticals LP, Wilmington, DE), and cefepime (Elan Pharmaceuticals, Inc., San Diego, CA) were commercially purchased. Levofloxacin, amikacin, tazobactam, and aztreonam were obtained from Sigma-Aldrich Co. (St. Louis, MO).
Susceptibility testing.
MICs as well as minimum bactericidal concentrations (MBCs) of the tested drugs were determined by using broth microdilution methods according to Clinical and Laboratory Standards Institute guidelines (6). All susceptibility testing was performed in duplicate with inocula of ∼5.5 × 105 CFU/ml.
Time-kill curve analysis.
The potential for synergistic interactions between ceftaroline and other antimicrobials was evaluated in duplicate with an initial inoculum of ∼106 CFU/ml. Ten strains (two E. coli, two K. pneumoniae, two E. cloacae, and four P. aeruginosa) were exposed to each drug alone or in combination at a single concentration equal to one-fourth the MIC, except tazobactam, which was used at a fixed concentration (4 μg/ml) since all organisms were nonsusceptible. Regimens included ceftaroline alone or combined with aztreonam, meropenem, cefepime, amikacin, tazobactam, levofloxacin, or tigecycline. Aliquots (0.1 ml) were removed from 2-ml cultures at 0, 1, 2, 4, 8, and 24 h and serially diluted in cold 0.9% sodium chloride. Bacterial counts were determined by plating 75 to 100 μl of appropriate dilutions using an automatic spiral plating device (WASP; DW Scientific, West Yorkshire, United Kingdom) to enumerate CFU/ml and avoid antibiotic carryover. Colony counts were performed using an automated colony counter (Synoptics Limited, Frederick, MD), and the lower limit of detection was 2 log10 CFU/ml. Time-kill curves were constructed by plotting mean colony counts (log10 CFU/ml) versus time. According to the 2008 guidelines of Antimicrobial Agents and Chemotherapy, synergy was defined as a ≥2-log10 CFU/ml decrease between the combination and the most efficient agent alone at 24 h. The number of surviving organisms in the presence of the combination was also ≥2 log10 CFU/ml and at least one of the drugs alone did not affect the growth curve of the tested organism. Indifference and antagonism were defined at 24 h as a ±1-log10 kill to <2 compared to the most efficient agent alone and >1 log10 growth compared with the less active single agent, respectively. Bactericidal activity of individual drugs alone was defined as ≥3 log10 CFU/ml (99.9%) reduction at 24 h compared to the starting inoculum, while bactericidal activity of drug combinations was defined as a ≥3-log10 CFU/ml (99.9%) reduction compared to the most active drug at 24 h.
RESULTS
Susceptibility.
The susceptibility results for ceftaroline and other agents against selected isolates of Enterobacteriaceae and P. aeruginosa are shown in the Table 1. Ceftaroline MICs ranged from 0.125 to 1,024 μg/ml. Isolates of differing susceptibilities to ceftaroline were chosen for these studies and included 8 with MICs of ≤4 μg/ml (3 E. coli, 1 K. pneumoniae, and 4 E. cloacae), 8 with MICs of 8 μg/ml (2 E. coli, 2 K. pneumoniae, and 4 P. aeruginosa), and 34 with MICs of ≥16 μg/ml (5 E. coli, 7 K. pneumoniae, 6 E. cloacae, and 16 P. aeruginosa). In combination with tazobactam (MIC ≥ 256 mg/liter), the ceftaroline MIC was decreased 2- to 512-fold for ESBL-producing E. coli and K. pneumoniae strains but was unchanged for the majority of AmpC-derepressed E. cloacae and P. aeruginosa isolates (Table 1). Five E. coli and eight K. pneumoniae isolates exhibited significant changes in ceftaroline susceptibility (MICs decreased 8- to 512-fold), but two K. pneumoniae isolates remained highly resistant, with MICs greater than 16 μg/ml. The ceftaroline MIC was slightly decreased (twofold) in the presence of tazobactam for seven E. cloacae and nine P. aeruginosa strains. Other antimicrobials exhibited varied levels of activity against the selected clinical isolates, with MICs ranging from 0.03 to ≥32 μg/ml. All E. coli, K. pneumoniae, and E. cloacae isolates appeared susceptible to meropenem and tigecycline, with MICs less than or equal to 4 and 8 μg/ml, respectively, corresponding to the resistance breakpoints of these species. All clinical P. aeruginosa isolates were susceptible to amikacin, with a MIC range of 2 to 16 μg/ml, and all K. pneumoniae isolates were resistant to aztreonam, with MICs equal to or greater than 32 μg/ml except for one isolate with a MIC of 0.5 μg/ml (Table 1).
TABLE 1.
Susceptibility profiles (MIC and MBC ranges) of the 30 tested clinical Enterobacteriaceae and 20 P. aeruginosa isolates
| Antimicrobial | MIC/MBC ranges (μg/ml) for species (n)
|
|||
|---|---|---|---|---|
| E. coli (10) | K. pneumoniae (10) | E. cloacae (10) | P. aeruginosa (20) | |
| Ceftaroline | 2-512/4-1,024 | 8-1,024/32-1,024 | 0.125-512/0.125-1,024 | 8-256/16-256 |
| Ceftaroline-tazobactam | 0.5-4/1-8 | 1-64/1-64 | 0.125-256/0.125-1,024 | 4-128/16-128 |
| Meropenem | 0.03-0.06/0.06-0.125 | 0.03-0.06/0.06-0.125 | 0.03-0.25/0.03-0.5 | 0.125-16/0.125-32 |
| Cefepime | 0.125-256/0.25-512 | 0.5-16/0.5 to >64 | 0.06-32/0.125-64 | 2-32/4-64 |
| Aztreonam | 0.125 to >64/0.125 to >64 | 0.25 to >64/0.5 to >64 | 0.125 to >64/0.125 to >64 | 2-64/8-64 |
| Amikacin | 1-16/4-64 | 1-32/1-128 | 0.5-4/1-8 | 2-16/2-64 |
| Levofloxacin | 0.03-32/0.06-32 | 0.25 to >32/0.25 to >32 | 0.03-64/0.03-128 | 0.25-32/0.5-32 |
| Tigecycline | 0.06-0.5/0.06-4 | 0.125-1/0.5-8 | 0.25-2/0.5-4 | 2-32/8-256 |
Time-kill analysis.
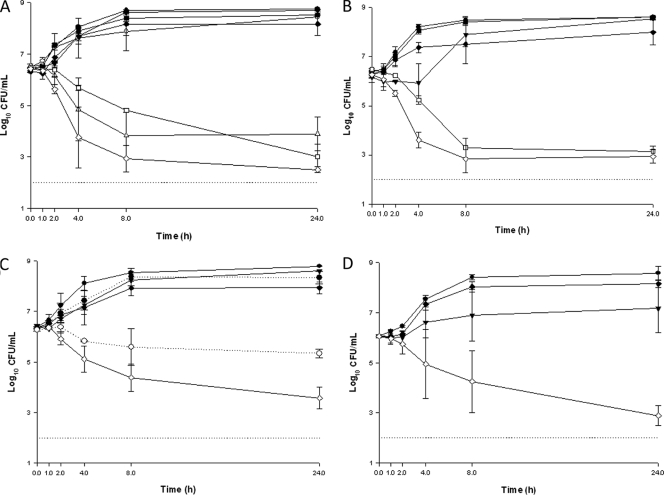
Antimicrobial susceptibility values of each strain run in time-kill experiments are presented in Table 2. In time-kill experiments, ceftaroline and the other agents alone were not bactericidal at one-fourth the MIC and did not significantly affect the growth curve of the organisms (data not shown). In combination, ceftaroline with tigecycline, levofloxacin, or cefepime was only indifferent (mean decrease from 0.01 to 1.8 ± 0.40 log10 CFU/ml) (Table 3) In contrast, ceftaroline plus amikacin demonstrated a synergistic effect against 90% of the tested strains, with decreases in viable organisms at 24 h of ∼5.65, 4.4, 5.1, and 3.6 log10 CFU/ml for E. coli, E. cloacae, K. pneumoniae, and P. aeruginosa, respectively (Fig. 1A to D; Table 3). Ceftaroline combined with meropenem, aztreonam, or tazobactam led to variable effects, depending on the combination and the species, none of which was antagonistic. Ceftaroline plus tazobactam was synergistic against both E. coli and K. pneumoniae isolates, with similar mean differences (∼5.51 and 5.44 log10 CFU/ml), but indifferent for E. cloacae and P. aeruginosa isolates (Fig. 1A to D; Table 3). Combinations of ceftaroline plus meropenem or aztreonam were synergistic against the two ESBL-producing E. coli isolates (mean difference of ∼4.45 log10 CFU/ml) or the two AmpC-derepressed E. cloacae isolates (mean difference of ∼3.03 log10 CFU/ml), respectively (Fig. 1A and C; Table 3). No antagonism was observed in these studies for any ceftaroline combination with any of the tested isolates.
TABLE 2.
In vitro activities of ceftaroline and tested antimicrobials (MICs and MBCs) against 10 selected clinical isolates
| Antimicrobial | MIC/MBC (μg/ml) for indicated species and isolate no.
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli
|
K. pneumoniae
|
E. cloacae
|
P. aeruginosa
|
|||||||
| 5401 | 5411 | 5427 | 5436 | 4073 | 5420 | 796 | 956 | 1019 | 1037 | |
| Ceftaroline | 4/8 | 64/128 | 4/16 | 1,024/1,024 | 256/512 | 64/128 | 16/32 | 128/256 | 32/64 | 8/32 |
| Meropenem | 0.06/0.06 | 0.06/0.06 | 4/16 | 0.06/0.06 | 0.25/0.5 | 0.125/0.125 | 1/2 | 0.25/2 | 1/2 | 0.5/1 |
| Cefepime | 4/4 | 2/4 | 0.5/1 | 16/32 | 4/16 | 0.25/1 | 8/16 | 8/32 | 2/4 | 1/2 |
| Ceftaroline-tazobactam | 0.5/2 | 0.5/2 | 1/1 | 2/8 | 128/512 | 32/64 | 16/32 | 64/128 | 32/64 | 4/16 |
| Aztreonam | 0.25/0.25 | 8/32 | 0.5/1 | 64/64 | 32/64 | 8/16 | 4/32 | 8/64 | 4/4 | 4/8 |
| Amikacin | 2/4 | 8/16 | 2/2 | 1/2 | 1/8 | 1/2 | 16/32 | 4/64 | 4/4 | 2/4 |
| Levoflaxacin | 32/32 | 8/16 | 0.25/2 | 4/4 | 0.06/0.06 | 0.06/0.06 | 1/2 | 0.5/1 | 0.5/1 | 0.25/0.5 |
| Tigecycline | 0.5/1 | 0.125/0.5 | 0.5/2 | 0.125/1 | 0.5/2 | 0.5/2 | 32/32 | 16/128 | 2/8 | 8/32 |
TABLE 3.
In vitro activities of drug combinations at one-fourth the MIC (or 4 mg/liter for tazobactam) against 10 randomly selected clinical isolatesa
| Drug combination | Species | Isolate no. | Decrease in log10 CFU/ml (mean ± SD) at:
|
Effect of combinationb | |||
|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 8 h | 24 h | ||||
| CPT + AMK | E. coli | 5401 | 1.01 ± 0.00 | 4.78 ± 0.08 | 5.30 ± 0.08 | 5.32 ± 0.02 | S |
| 5411 | 0.93 ± 0.80 | 2.88 ± 0.32 | 5.10 ± 0.06 | 5.98 ± 0.37 | S | ||
| K. pneumoniae | 5427 | 0.65 ± 0.24 | 3.42 ± 0.08 | 4.43 ± 0.38 | 4.81 ± 0.34 | S | |
| 5436 | 0.26 ± 0.36 | 0.87 ± 0.14 | 3.31 ± 0.01 | 5.31 ± 0.14 | S | ||
| E. cloacae | 4073 | 1.38 ± 0.01 | 1.68 ± 0.01 | 3.19 ± 0.16 | 4.65 ± 0.02 | S | |
| 5420 | 0.02 ± 0.02 | 1.12 ± 0.80 | 3.27 ± 0.36 | 4.44 ± 0.72 | S | ||
| P. aeruginosa | 796 | 0.07 ± 0.05 | 0.63 ± 0.31 | 2.14 ± 0.41 | 5.23 ± 0.32 | S | |
| 956 | 0.36 ± 0.05 | 2.61 ± 0.02 | 2.66 ± 0.50 | 3.60 ± 0.44 | S | ||
| 1019 | 0.16 ± 0.11 | 1.32 ± 0.07 | 3.84 ± 0.81 | 0.67 ± 0.28 | I | ||
| 1037 | 0.09 ± 0.08 | 2.13 ± 0.07 | 2.71 ± 0.30 | 3.51 ± 0.27 | S | ||
| CPT + TAZ | E. coli | 5401 | 0.94 ± 0.26 | 2.00 ± 0.02 | 3.37 ± 0.17 | 4.99 ± 0.09 | S |
| 5411 | 0.66 ± 0.18 | 2.43 ± 0.09 | 3.79 ± 0.01 | 6.02 ± 0.04 | S | ||
| K. pneumoniae | 5427 | 1.13 ± 0.02 | 2.78 ± 0.16 | 4.89 ± 0.28 | 5.27 ± 0.50 | S | |
| 5436 | 0.48 ± 0.07 | 2.92 ± 0.14 | 5.31 ± 0.05 | 5.62 ± 0.56 | S | ||
| E. cloacae | 4073 | 0.57 ± 0.13 | 0.65 ± 0.08 | 0.83 ± 0.09 | 0.47 ± 0.04 | I | |
| 5420 | 0.14 ± 0.38 | 0.43 ± 0.18 | 0.29 ± 0.08 | 0.46 ± 0.04 | I | ||
| P. aeruginosa | 796 | 0.12 ± 0.28 | 0.32 ± 0.28 | 0.08 ± 0.01 | 0.31 ± 0.06 | I | |
| 956 | 0.08 ± 0.08 | 0.01 ± 0.08 | 0.51 ± 0.27 | 0.52 ± 0.13 | I | ||
| 1019 | 0.02 ± 0.50 | 0.43 ± 0.47 | 0.95 ± 0.09 | 1.22 ± 0.09 | I | ||
| 1037 | 0.01 ± 0.19 | 0.06 ± 0.16 | 0.73 ± 0.07 | 0.56 ± 0.24 | I | ||
| CPT + MEM | E. coli | 5401 | 0.87 ± 0.20 | 3.12 ± 0.14 | 4.20 ± 0.72 | 4.93 ± 0.75 | S |
| 5411 | 0.08 ± 0.58 | 1.78 ± 0.15 | 3.91 ± 0.13 | 4.17 ± 0.47 | S | ||
| K. pneumoniae | 5427 | 0.06 ± 0.13 | 1.13 ± 0.46 | 0.12 ± 0.05 | 0.12 ± 0.03 | I | |
| 5436 | 0.59 ± 0.05 | 1.16 ± 0.15 | 3.66 ± 0..32 | 0.04 ± 0.12 | I | ||
| E. cloacae | 4073 | 0.86 ± 0.13 | 1.39 ± 0.28 | 1.21 ± 0.09 | 0.72 ± 0.20 | I | |
| 5420 | 0.10 ± 0.02 | 1.03 ± 0.73 | 1.44 ± 0.74 | 0.12 ± 0.11 | I | ||
| P. aeruginosa | 796 | 0.07 ± 0.08 | 0.03 ± 0.04 | 0.05 ± 0.03 | 0.14 ± 0.16 | I | |
| 956 | 0.02 ± 0.03 | 0.21 ± 0.27 | 0.10 ± 0.01 | 0.31 ± 0.31 | I | ||
| 1019 | 0.01 ± 0.01 | 0.27 ± 0.10 | 0.04 ± 0.05 | 0.05 ± 001 | I | ||
| 1037 | 0.20 ± 0.04 | 0.32 ± 0.27 | 0.28 ± 0.15 | 1.71 ± 0.14 | I | ||
| CPT + ATM | E. coli | 5401 | 0.32 ± 0.06 | 1.36 ± 0.01 | 0.12 ± 0.05 | 0.08 ± 0.01 | I |
| 5411 | 0.63 ± 0.25 | 0.99 ± 0.04 | 0.16 ± 0.03 | 0.33 ± 0.08 | I | ||
| K. pneumoniae | 5427 | 0.01 ± 0.07 | 0.64 ± 0.02 | 0.06 ± 0.08 | 0.02 ± 0.09 | I | |
| 5436 | 0.44 ± 0.01 | 0.37 ± 0.02 | 2.00 ± 0.09 | 0.14 ± 0.07 | I | ||
| E. cloacae | 4073 | 0.69 ± 0.32 | 1.71 ± 0.25 | 1.73 ± 0.29 | 3.08 ± 0.13 | I | |
| 5420 | 0.03 ± 0.04 | 0.90 ± 0.88 | 3.33 ± 0.91 | 2.99 ± 0.12 | I | ||
| P. aeruginosa | 796 | 0.06 ± 0.11 | 0.04 ± 0.26 | 0.97 ± 0.18 | 0.85 ± 0.15 | I | |
| 956 | 0.18 ± 0.15 | 0.12 ± 0.17 | 0.20 ± 0.53 | 0.73 ± 0.68 | I | ||
| 1019 | 0.03 ± 0.05 | 0.15 ± 0.22 | 0.15 ± 0.03 | 0.26 ± 0.27 | I | ||
| 1037 | 0.17 ± 0.09 | 0.12 ± 0.36 | 0.22 ± 0.16 | 1.01 ± 0.54 | I | ||
| CPT + LEV | E. coli | 5401 | 0.11 ± 0.11 | 1.00 ± 0.21 | 0.05 ± 0.02 | 0.04 ± 0.06 | I |
| 5411 | 0.63 ± 0.58 | 0.58 ± 0.01 | 0.08 ± 0.14 | 0.01 ± 0.05 | I | ||
| K. pneumoniae | 5427 | 0.15 ± 0.05 | 0.18 ± 0.07 | 1.68 ± 0.22 | 1.70 ± 0.20 | I | |
| 5436 | 0.09 ± 0.07 | 1.05 ± 0.09 | 0.47 ± 0.08 | 0.08 ± 0.01 | I | ||
| E. cloacae | 4073 | 0.10 ± 0.07 | 0.24 ± 0.02 | 0.10 ± 0.02 | 0.16 ± 0.01 | I | |
| 5420 | 0.01 ± 0.10 | 0.41 ± 0.38 | 0.05 ± 0.14 | 0.04 ± 0.06 | I | ||
| P. aeruginosa | 796 | 0.03 ± 0.02 | 0.21 ± 0.42 | 0.35 ± 0.09 | 0.04 ± 0.14 | I | |
| 956 | 0.08 ± 0.09 | 0.25 ± 0.23 | 0.14 ± 0.00 | 0.98 ± 0.06 | I | ||
| 1019 | 0.21 ± 0.15 | 0.03 ± 0.01 | 1.21 ± 0.13 | 0.00 ± 0.00 | I | ||
| 1037 | 0.02 ± 0.10 | 0.10 ± 0.15 | 0.04 ± 0.01 | 0.10 ± 0.36 | I | ||
| CPT + CPM | E. coli | 5401 | 0.37 ± 0.04 | 1.54 ± 0.12 | 0.04 ± 0.01 | 0.03 ± 0.02 | I |
| 5411 | 0.56 ± 0.51 | 0.38 ± 0.01 | 0.07 ± 0.00 | 0.01 ± 0.00 | I | ||
| K. pneumoniae | 5427 | 0.08 ± 0.03 | 0.82 ± 0.19 | 0.38 ± 0.32 | 0.20 ± 0.30 | I | |
| 5436 | 0.01 ± 0.07 | 0.51 ± 0.11 | 0.16 ± 0.06 | 0.31 ± 0.01 | I | ||
| E. cloacae | 4073 | 0.17 ± 0.12 | 0.57 ± 0.14 | 0.55 ± 0.14 | 0.03 ± 0.02 | I | |
| 5420 | 0.41 ± 0.33 | 0.12 ± 1.09 | 0.03 ± 0.06 | 0.07 ± 0.11 | |||
| P. aeruginosa | 796 | 0.02 ± 0.04 | 0.27 ± 0.30 | 0.08 ± 0.01 | 0.02 ± 0.01 | I | |
| 956 | 0.25 ± 0.24 | 0.01 ± 0.19 | 0.35 ± 0.37 | 0.52 ± 0.38 | I | ||
| 1019 | 0.13 ± 0.11 | 0.08 ± 0.08 | 0.03 ± 0.01 | 0.05 ± 0.29 | I | ||
| 1037 | 0.52 ± 0.18 | 1.17 ± 0.19 | 1.66 ± 0.47 | 1.81 ± 0.41 | I | ||
| CPT + TIG | E. coli | 5401 | 0.12 ± 0.08 | 0.45 ± 0.78 | 0.02 ± 0.11 | 0.13 ± 0.07 | I |
| 5411 | 0.23 ± 0.11 | 0.17 ± 0.09 | 0.08 ± 0.15 | 0.01 ± 0.02 | I | ||
| K. pneumoniae | 5427 | 0.32 ± 0.07 | 0.59 ± 0.02 | 0.62 ± 0.59 | 0.25 ± 0.31 | I | |
| 5436 | 0.13 ± 0.16 | 0.40 ± 0.52 | 0.22 ± 0.17 | 0.03 ± 0.02 | I | ||
| E. cloacae | 4073 | 0.05 ± 0.06 | 0.43 ± 0.00 | 0.95 ± 0.00 | 0.14 ± 0.03 | I | |
| 5420 | 0.13 ± 0.11 | 0.15 ± 0.11 | 0.08 ± 0.08 | 0.10 ± 0.12 | I | ||
| P. aeruginosa | 796 | 0.05 ± 0.03 | 0.48 ± 0.08 | 0.01 ± 0.03 | 0.17 ± 0.28 | I | |
| 956 | 0.12 ± 0.06 | 0.22 ± 0.28 | 0.28 ± 0.07 | 0.33 ± 0.38 | I | ||
| 1019 | 0.00 ± 0.02 | 0.10 ± 0.11 | 0.11 ± 0.01 | 0.14 ± 0.16 | I | ||
| 1037 | 0.10 ± 0.03 | 0.06 ± 0.31 | 1.07 ± 0.25 | 0.54 ± 0.12 | I | ||
The starting inoculum used was ∼106 CFU/ml. Abbreviations: CPT, ceftaroline; AMK, amikacin; TAZ, tazobactam; MEM, meropenem; ATM, aztreonam; LEV, levofloxacin; CPM, cefepime; TIG, tigecycline.
S, synergy; I, indifference.
FIG. 1.
Synergistic combinations observed in time-kill experiments using drugs at one-fourth the MIC, or 4 mg/liter for tazobactam. Results for each time-kill curve are presented as the mean log10 CFU/ml ± the standard deviation at each time point for two ESBL-producing E. coli (A), two ESBL-producing K. pneumoniae (B), two AmpC-derepressed E. cloacae (C), and three P. aeruginosa (D) isolates (796, 956, 1019). Symbols: -•-, growth control; -▾-, ceftaroline; -○-, meropenem; -▵-, ceftaroline plus meropenem; -▪-, tazobactam; -□-, ceftaroline plus tazobactam; -⧫-, amikacin; -⋄-, ceftaroline plus amikacin; ···•···, aztreonam; ···○···, ceftaroline plus aztreonam. The straight dotted line at the bottom of each graph shows the limit of detection.
DISCUSSION
Because of a limited number of efficacious anti-infective drugs or novel therapeutic strategies, MDR gram-negative pathogens currently represent a serious clinical concern (22). The use of antimicrobial combinations is considered one of the best options available to treat infections caused by these pathogens, despite controversial opinions based on potential increased toxicity and lack of clinical evidence of higher efficiency compared to monotherapy (14, 29). Indeed, in addition to the improvement of the killing effect of the drugs, combination therapy may also reduce the emergence of resistance and improve the spectrum of activity (5).
This preliminary study was designed to investigate the potential for synergy of ceftaroline combinations, which may extend its bactericidal activity against resistant gram-negative bacilli, as previously demonstrated for other β-lactams (7, 15, 17, 28). We selected a large panel of drugs, including an aminoglycoside, β-lactams, and fluoroquinolones, which were likely to provide synergy in combination with a β-lactam (31). Ceftaroline and combinations were evaluated against a broad selection of resistant gram-negative strains, which included Enterobacteriaceae isolates exhibiting ceftaroline MICs ranging from 0.125 to 1,024 μg/ml and clinical P. aeruginosa isolates, selected at random, with a range of MICs for ceftaroline from 8 to 256 μg/ml. Although it has not been clearly defined yet, the low activity of ceftaroline against gram-negative bacilli may be the result of various parameters. Thus, the affinity of the drug for specific penicillin-binding proteins of each species, as well as the expression of β-lactamase enzymes (such as ESBL and cephalosporinases in Enterobacteriaceae and P. aeruginosa isolates, respectively) and β-lactamase-related inoculum effects, may be suggested (20). High MICs, reversible in combination with a β-lactamase inhibitor such as clavulanate, were previously reported for ceftaroline against isolates expressing β-lactamases of the class A type, including ESBL-producing Enterobacteriaceae (20). A preliminary correlation between ESBL type and ceftaroline activity was suggested by Mushtaq et al., who reported higher ceftaroline MICs for CTX-M-producing isolates (greater than 128 μg/ml) compared to classical β-lactamases, such as TEM-1, TEM-2, or SHV-1 (MICs from 2 to 16 mg/ml) (20). In the present study, ceftaroline MICs were reduced by 2- to 512-fold for ESBL-producing E. coli and K. pneumoniae isolates by the addition of tazobactam. In contrast, the wide range of ceftaroline MICs observed for the AmpC-derepressed E. cloacae isolates was unchanged by the addition of the tazobactam component, which is known to be inactive against AmpC β-lactamases. Further investigations are required to clarify the sensitivity of ceftaroline to different β-lactamases, as well as to assess the affinity of ceftaroline for the PBPs of Enterobacteriaceae. However, we confirmed the potential benefits of combinations of ceftaroline plus β-lactamase inhibitor therapy, and these promising results warrant further study of alternative β-lactamase inhibitor agents. A new β-lactamase inhibitor, NXL104 (Novexel, Romainville, France), is currently under development and has been studied with cephalosporin combinations in an attempt to restore activity against class A and class C β-lactamase-producing organisms (16). According to Forest Laboratories, studies of ceftaroline in combination with NXL104 are currently in progress (1). For P. aeruginosa isolates, against which ceftaroline has limited activity, high levels of resistance to ceftaroline and other β-lactam drugs may be due to (i) a decreased affinity for the PBPs, (ii) a failure to achieve inhibitory concentrations due to impermeability or efflux (via the MexXY efflux pump, for example), and/or (iii) enzymatic hydrolysis resistant to β-lactamase inhibitors (4, 8, 10). Studies of the mechanisms of resistance responsible for the reduced activity of ceftaroline against P. aeruginosa isolates are still warranted.
As expected, MBCs of ceftaroline alone were similar or onefold higher than the MICs, suggesting that ceftaroline bactericidal activity is close to the inhibitory concentration. In order to observe synergistic effects, we selected a concentration that was one-fourth the MIC for the time-kill experiments. Effects of the combined treatments depended on the species and the drugs tested, but in no cases were instances of antagonism observed. Against ESBL-producing E. coli and K. pneumoniae, ceftaroline plus amikacin or tazobactam were the most synergistic combinations, followed by ceftaroline plus meropenem for E. coli isolates and ceftaroline plus aztreonam for E. cloacae strains. With all other antimicrobials, ceftaroline combinations were indifferent. Our results appeared consistent with those presented in an earlier study that used checkerboard assays to investigate the ceftaroline potential for synergy in combination against two E. coli and two K. pneumoniae isolates, including one ESBL-producing strain of each species (30). Indeed, synergy was reported for ceftaroline plus amikacin against the only ESBL-producing E. coli tested. However, meropenem and tazobactam were not evaluated. The combination of ceftaroline plus meropenem was also synergystic against the only ESBL-producing K. pneumoniae tested, but amikacin, aztreonam, and levofloxacin were not investigated (30).
Some limitations for this work can be pointed out. First, we used antimicrobials at a single sub-MIC level equal to one-fourth the MIC for both agents. Synergy or antagonism might occur at different concentrations, and further investigations are therefore warranted to clarify the potential for synergy of ceftaroline in combination. Additionally, although we evaluated strains exhibiting different susceptibility levels for all antimicrobials, this study provides merely preliminary data and additional experiments would be of benefit. Finally, another limitation of this study might be the potential instability of the drugs tested. Although it is not usual to take this into account for a such short-term experiment, degradation of a drug might explain the absence of in vitro synergy for several combinations. Further investigations would therefore clarify the role of degradation in the in vitro activities of several combined treatments.
Conclusions.
These studies demonstrated the potential benefit for use of combination therapy for extending the spectrum of in vitro activity of ceftaroline against multidrug-resistant gram-negative pathogens. In the present work, several antimicrobials, including aminoglycosides or β-lactamase inhibitors such as tazobactam, led to synergistic effects in combination with ceftaroline, and none of them demonstrated antagonistic effects. Although the mechanisms that contribute to synergy are not well understood and the fact that combination therapy remains a debated topic, it appears to be used often in clinical practice, especially during treatment of polymicrobial infections. Based on our findings, further studies of drug combinations with ceftaroline, including in vivo studies in animal models, are warranted to better understand the potential utility for ceftaroline combination therapy against resistant gram-negative pathogens.
Acknowledgments
This study was funded in part by a research/educational grant from Forest Laboratories, Inc.
Footnotes
Published ahead of print on 6 April 2009.
REFERENCES
- 1.Anonymous. 2008. Novexel and Forest Laboratories announce license agreement for NXL 104, a novel broad-spectrum beta lactamase inhibitor. Forest Laboratories, New York, NY. http://www.frx.com/news/PressRelease.aspx?ID=1098399.
- 2.Anonymous. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 3.Bassetti, M., E. Righi, and C. Viscoli. 2008. Pseudomonas aeruginosa serious infections: mono or combination antimicrobial therapy? Curr. Med. Chem. 15:517-522. [DOI] [PubMed] [Google Scholar]
- 4.Baum, E. Z., S. M. Crespo-Carbone, B. D. Foleno, E. Wira, H. Wenping, and B. Morrow. 2008. MexXY expression in Pseudomomas aeruginosa (PsA) and susceptibility to cephalosporins, including ceftobiprole. Progr. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., poster C1-158.
- 5.Bouza, E., and P. Munoz. 2000. Monotherapy versus combination therapy for bacterial infections. Med. Clin. North Am. 84:1357-1389. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Critchley, I. A., D. F. Sahm, L. J. Kelly, and J. A. Karlowsky. 2003. In-vitro synergy studies using aztreonam and fluoroquinolone combinations against six species of gram-negative bacilli. Chemotherapy 49:44-48. [DOI] [PubMed] [Google Scholar]
- 8.Dubois, V., C. Arpin, V. Dupart, A. Scavelli, L. Coulange, C. Andre, I. Fischer, F. Grobost, J. P. Brochet, I. Lagrange, B. Dutilh, J. Jullin, P. Noury, G. Larribet, and C. Quentin. 2008. Beta-lactam and aminoglycoside resistance rates and mechanisms among Pseudomonas aeruginosa in French general practice (community and private healthcare centres). J. Antimicrob. Chemother. 62:316-323. [DOI] [PubMed] [Google Scholar]
- 9.Ge, Y., D. Biek, G. H. Talbot, and D. F. Sahm. 2008. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:3398-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotoh, N., K. Nunomura, and T. Nishino. 1990. Resistance of Pseudomonas aeruginosa to cefsulodin: modification of penicillin-binding protein 3 and mapping of its chromosomal gene. J. Antimicrob. Chemother. 25:513-523. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa, T., Y. Nakayama, M. Tomimoto, S. I. Niwa, K. Kamiyama, S. Hashiguchi, Y. Iizawa, K. Okonogi, and A. Miyake. 2001. Studies on anti-MRSA parenteral cephalosporins. IV. A novel water-soluble N-phosphono type prodrug for parental administration. J. Antibiot. (Tokyo) 54:364-374. [DOI] [PubMed] [Google Scholar]
- 12.Jones, R. N., T. R. Fritsche, Y. Ge, K. Kaniga, and H. S. Sader. 2005. Evaluation of PPI-0903M (T91825), a novel cephalosporin: bactericidal activity, effects of modifying in-vitro testing parameters and optimization of disc diffusion tests. J. Antimicrob. Chemother. 56:1047-1052. [DOI] [PubMed] [Google Scholar]
- 13.Joshi, J. H., K. A. Newman, B. W. Brown, R. S. Finley, R. L. Ruxer, M. A. Moody, and S. C. Schimpff. 1993. Double beta-lactam regimen compared to an aminoglycoside/beta-lactam regimen as empiric antibiotic therapy for febrile granulocytopenic cancer patients. Support. Care Cancer 1:186-194. [DOI] [PubMed] [Google Scholar]
- 14.Klibanov, O. M., R. H. Raasch, and J. C. Rublein. 2004. Single versus combined antibiotic therapy for gram-negative infections. Ann. Pharmacother. 38:332-337. [DOI] [PubMed] [Google Scholar]
- 15.Kresken, M., and M. Heep. 2005. In vitro activity of ceftobiprole in combination with ciprofloxacin, levofloxacin, amikacin, and tobramycin against clinical isolates of Pseudomonas aeruginosa, poster E314. Progr. Abstr. 45th Annu. Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 16.Livermore, D. M., S. Mushtaq, M. Warner, C. Miossec, and N. Woodford. 2008. NXL104 combinations versus Enterobacteriaceae with CTX-M extended-spectrum β-lactamases and carbapenemases. J. Antimicrob. Chemother. 62:1053-1056. [DOI] [PubMed] [Google Scholar]
- 17.Louie, A., C. Fregeau, W. Liu, K. Bush, G. Noel, and G. L. Drusano. 2008. Ceftobiprole and levofloxacin are synergistic against an isolate of Pseudomonas aeruginosa as evaluated in a neutropenic mouse thigh infection model, abstr. 0144. Progr. Abstr. 18th Eur. Congr. Clin. Microbiol. Infect. Dis., Barcelona, Spain, 19 to 22 April 2008.
- 18.Millar, M., A. Philpott, M. Wilks, A. Whiley, S. Warwick, E. Hennessy, P. Coen, S. Kempley, F. Stacey, and K. Costeloe. 2008. Colonization and persistence of antibiotic-resistant Enterobacteriaceae strains in infants nursed in two neonatal intensive care units in East London, United Kingdom. J. Clin. Microbiol. 46:560-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moellering, R. C. 1983. Rationale for use of antimicrobial combinations. Am. J. Med. 75:4-8. [DOI] [PubMed] [Google Scholar]
- 20.Mushtaq, S., M. Warner, Y. Ge, K. Kaniga, and D. M. Livermore. 2007. In vitro activity of ceftaroline (PPI-0903M, T-91825) against bacteria with defined resistance mechanisms and phenotypes. J. Antimicrob. Chemother. 60:300-311. [DOI] [PubMed] [Google Scholar]
- 21.Nicasio, A. M., J. L. Kuti, and D. P. Nicolau. 2008. The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy 28:235-249. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill, A. J. 2008. New antibacterial agents for treating infections caused by multi-drug resistant gram-negative bacteria. Expert Opin. Investig. Drugs 17:297-302. [DOI] [PubMed] [Google Scholar]
- 23.Parish, D., and N. Scheinfeld. 2008. Ceftaroline fosamil, a cephalosporin derivative for the potential treatment of MRSA infection. Curr. Opin. Investig. Drugs 9:201-209. [PubMed] [Google Scholar]
- 24.Paterson, D. L. 2008. Impact of antibiotic resistance in gram-negative bacilli on empirical and definitive antibiotic therapy. Clin. Infect. Dis. 47(Suppl. 1):S14-S20. [DOI] [PubMed] [Google Scholar]
- 25.Pitout, J. D. 2008. Multiresistant Enterobacteriaceae: new threat of an old problem. Expert Rev. Anti Infect. Ther. 6:657-669. [DOI] [PubMed] [Google Scholar]
- 26.Sader, H. S., T. R. Fritsche, and R. N. Jones. 2008. Antimicrobial activities of ceftaroline and ME1036 tested against clinical strains of community-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 52:1153-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sader, H. S., T. R. Fritsche, K. Kaniga, Y. Ge, and R. N. Jones. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sader, H. S., H. K. Huynh, and R. N. Jones. 2003. Contemporary in vitro synergy rates for aztreonam combined with newer fluoroquinolones and beta-lactams tested against gram-negative bacilli. Diagn. Microbiol. Infect. Dis. 47:547-550. [DOI] [PubMed] [Google Scholar]
- 29.Safdar, N., J. Handelsman, and D. G. Maki. 2004. Does combination antimicrobial therapy reduce mortality in gram-negative bacteraemia? A meta-analysis. Lancet Infect. Dis. 4:519-527. [DOI] [PubMed] [Google Scholar]
- 30.Schaadt, R. D., D. A. Sweeney, D. Biek, and G. E. Zurenko. 2007. In vitro evaluation of the antibacterial activity of ceftaroline in combination with other antibacterial agents, abstr. E-279. Progr. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL. American Society for Microbiology, Washington, DC.
- 31.Schentag, J. J., L. C. Strenkoski-Nix, D. E. Nix, and A. Forrest. 1998. Pharmacodynamic interactions of antibiotics alone and in combination. Clin. Infect. Dis. 27:40-46. [DOI] [PubMed] [Google Scholar]
- 32.Waterer, G. W., and R. G. Wunderink. 2001. Increasing threat of gram-negative bacteria. Crit. Care Med. 29:N75-N81. [DOI] [PubMed] [Google Scholar]