Abstract
Antibiotic resistance genes are spread mostly through plasmids, integrons (as a form of gene cassettes), and transposons in gram-negative bacteria. We describe here a novel genetic structure, named the integron mobilization unit (IMU), that has characteristics similar to those of miniature inverted transposable elements (MITEs). Two IMUs (288 bp each) were identified from a carbapenem-resistant Enterobacter cloacae isolate that formed a composite structure encompassing a defective class 1 integron containing the carbapenem resistance gene blaGES-5. This ß-lactamase gene was located on a 7-kb IncQ-type plasmid named pCHE-A, which was sequenced completely. The plasmid pCHE-A was not self conjugative but was mobilizable, and it was successfully transferred from E. cloacae to Pseudomonas aeruginosa. The in silico analysis of the extremities of the IMU elements identified similarities with those of insertion sequence ISSod9 from Shewanella oneidensis MR-1. The mobilization of the IMU composite structure was accomplished by using the transposase activity of ISSod9 that was provided in trans. This is the first identification of MITE-type structures as a source of gene mobilization, implicating here a clinically relevant antibiotic resistance gene.
The mobilization of antibiotic resistance genes in gram-negative bacteria occurs through several mechanisms. The transfer of plasmids (via transformation or conjugation) or of phages (via transduction) is known to be the key factor for the horizontal transfer of resistance genes. Additionally, their acquisition is related mainly to different mechanisms, including recombination, the integron-mediated mobilization of gene cassettes, classical transposition, and rolling-circle transposition mediated by the recently described ISCR elements (3, 19, 49, 54, 55). A large variety of antibiotic resistance determinants (resistance to ß-lactams, aminoglycosides, chloramphenicol, rifampin, trimethoprim, and sulfonamides) have been identified as gene cassettes located in class 1 integrons. An integron is a non-self-transferable genetic unit that is capable of capturing and expressing gene cassettes. The dissemination of class 1 integrons usually is related to their location in transposon structures, being mostly of Tn21 and Tn402 types in gram-negative bacteria (26, 30).
Among the ß-lactamases identified in Enterobacteriaceae, the carbapenemases have the broadest spectrum of activity and belong to Ambler classes A, B, and D (22, 43, 46, 56). The class A carbapenemases are inhibited by clavulanic acid, and their genes are located on chromosomes (e.g., blaNMC-A or blaSME) or plasmids (e.g., blaIMI, blaGES, or blaKPC). The blaGES genes have been identified in Enterobacteriaceae and Pseudomonas aeruginosa, and the ß-lactamases GES-2, GES-4, GES-5, and GES-8 possess carbapenemase activity (35, 43). All of the blaGES-type genes have been identified as part of the class 1 integrons, with the exception of one report describing the blaGES-1 gene as part of a class 3 integron in Klebsiella pneumoniae (13). The only class A carbapenemase reported to date in Enterobacter cloacae is NMC-A (34).
This work was initiated by the isolation of a carbapenem-resistant E. cloacae strain harboring the blaGES-5 gene that was located in a defective class 1 integron structure, in association with a novel genetic element, termed the integron-mobilization unit (IMU), which lacks self-transposition ability. However, transposition events were obtained using a transposase from Shewanella oneidensis provided in trans. That finding represents a novel mechanism of the transfer of antibiotic resistance genes that may be of more general interest.
MATERIALS AND METHODS
Bacterial strains.
E. cloacae CHE-2 was identified according to biochemical testing (API20E system; bioMérieux, Marcy-l'Etoile, France). A GES-1-positive control was Klebsiella pneumoniae isolate ORI-1 (42). Escherichia coli TOP10 was used for cloning experiments, whereas azide-resistant E. coli J53 was used as the recipient for conjugation and transformation experiments (32). A wild-type E. cloacae isolate (strain 5390) and P. aeruginosa PU21 (reference strain) were used as recipients for transformation experiments (32). E. coli JF703 and JF701 (kindly provided by G. Jacoby) expressing either OmpC or OmpF, respectively, were used as reference strains for outer membrane protein (OMP) analysis (23). S. oneidensis strain MR-1 was used to obtain the DNA template for cloning the transposase gene of insertion sequence element ISSod9 as a source of transposase activity in transposition experiments (20, 39).
Genetic support and plasmid analysis.
Mating-out assays were performed between E. cloacae CHE-2 as a donor and azide-resistant E. coli J53 as a recipient strain, as described previously (32). Plasmid DNAs of E. cloacae CHE-2 and of the E. coli transformants were extracted using the Kieser method (27). They were separated on a 0.8% agarose gel, transferred onto a nylon membrane (Hybond N+; GE Healthcare, Orsay, France), and hybridized with blaGES-5 and blaSHV-5 internal probes. The labeling of the probe and signal detection were carried out using a nonradioactive ECL labeling and detection kit according to the manufacturer's instructions (GE Healthcare).
Plasmids were classified according to their incompatibility group using the PCR-based replicon typing method described by Carattoli et al. (6). This technique discriminates 18 types of enterobacterial plasmids according to incompatibility groups. Positive control strains were based on E. coli strain DH5α and contained replicons of the incompatibility groups cloned into a TA cloning vector. PCR products were sequenced to confirm the specificity of the amplicon.
Since GES-type enzymes have been identified in unrelated gram-negative species, including Pseudomonas spp. (8, 16, 38, 44), electrotransformation assays of natural plasmid pCHE-A were performed in electrocompetent P. aeruginosa PU21 and in the recipient strain E. cloacae 5390, as described previously (38, 44). The selection of E. cloacae and P. aeruginosa transformants harboring plasmid pCHE-A was performed on ticarcillin (100 μg/ml)-containing agar plates.
Susceptibility testing.
Antibiotic-containing disks were used for routine antibiograms that were performed by disk diffusion testing (Sanofi-Diagnostic Pasteur, Marnes-la-Coquette, France). MICs were determined by an agar dilution technique, except for colistin susceptibility, which was determined with an Etest strip (AB Biodisk, Solna, Sweden). MICs of ß-lactams were determined alone or in combination with a fixed concentration of clavulanic acid (4 μg/ml), and results were interpreted according to the guidelines of the Clinical Laboratory Standards Institute (CLSI) (10).
PCR and hybridization experiments.
Total DNA of enterobacterial isolates were extracted as described previously (42) and were used as templates in standard PCR conditions with a series of primers designed for the detection of Ambler class A ß-lactamase genes: blaTEM, blaSHV, blaCARB, blaVEB, blaPER, blaGES, and blaCTX-M (44). Southern hybridizations were performed using the ECL nonradioactive labeling and detection kit (GE Healthcare, Orsay, France). An internal probe for blaGES-5 obtained by PCR was used for Southern hybridization experiments.
Cloning experiments, recombinant plasmid analysis, and DNA sequencing.
Total DNA of E. cloacae CHE-2 was EcoRI restricted, ligated into the EcoRI site of plasmid pBK-CMV, and then transformed in E. coli TOP10, as described previously (42), giving rise to recombinant plasmid pBCHE-2. Strains containing recombinant plasmids were selected onto Trypticase soy (TS) agar plates containing amoxicillin (50 μg/ml) and kanamycin (30 μg/ml) and sequenced on both strands with an Applied Biosystems sequencer (ABI 3100) (Applied Biosystems, Foster City, CA).
In addition, PCR amplicons encompassing the entire sequence of the blaGES-5 gene with or without the upstream-located IMU element were obtained with primers PRE-1 (5′-CAG AAA TGC CTC GAC TTC GC-3′) and PRE2 (5′-AAT GAG AAT CAG ATC CGC GC-3′), respectively, in combination with primer 3′-CS (29) from whole-cell DNA of E. cloacae CHE-2, and subsequently were cloned using the ZeroBluntTOPOPCR cloning kit (Invitrogen, Cergy-Pontoise, France), giving rise to recombinant plasmids p1 and p2, respectively. Recombinant strains E. coli TOP10 (p1) and E. coli TOP10 (p2) were used for the comparison of MICs to evaluate the possible role of the IMU in enhancing blaGES-5 expression.
The entire sequence of the natural pCHE-A plasmid was determined by a primer-walking approach. The nucleotide and deduced amino acid sequences were analyzed and compared to sequences available over the Internet at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov). The analyses of the IMU genetic features also were performed using the ISBiotoul website (http://www-is.biotoul.fr/).
PCR amplicons encompassing the entire sequence of the ISSod9 transposase gene were obtained with primers PreTnA-Fw (5′-TGT GTC CGA GAG CTC TTG TC-3′) and Pre-TnA-Rev (5′-AAA ATC GTA CGC TAA GCC GG-3′) from whole-cell DNA of S. oneidensis MR-1 and subsequently were cloned using ZeroBluntTOPOPCR, giving rise to recombinant plasmid pTnpA-ISSod9.
Transposition experiments.
In vivo transposition experiments were performed to determine the mobility of the IMU-blaGES-5-IMU structure. The transposition of IMU-blaGES-5-IMU onto the pOX38-Gen conjugative plasmid was investigated with a mating-out technique in liquid medium, as described previously (41). First, natural and non-self-conjugative plasmid pCHE-A was electroporated into E. coli RZ211(pOX38-Gen) (selection was based on amoxicillin [100 μg/ml]). Second, nonconjugative recombinant plasmid pTnpA-ISSod9 was electroporated into E. coli RZ211(pOX38-Gen, pCHE-A) to provide the ISSod9 transposase in trans activity (selection was based on kanamycin [30 μg/ml]) that is necessary for transposition experiments. The transfer of the recombinant plasmids with the pOX38 backbone into E. coli J53AZR then was performed by conjugation. Transconjugants were selected on agar plates containing 7 μg per ml of gentamicin (pOX38 plasmid marker), 100 μg per ml of amoxicillin (GES-5 marker), and 100 μg per ml of azide (E. coli J53 chromosomal marker). The transposition frequency was calculated by dividing the number of transconjugants by the number of donors.
ß-Lactamase activity.
The specific activity of the crude ß-lactamase from culture extracts of E. cloacae CHE-2 was obtained by UV spectrophotometry, as described previously (42), using benzylpenicillin and imipenem as substrates. One unit of enzyme activity was defined as the activity that hydrolyzed 1 μmol of substrate per min per mg of protein. The total protein content was measured with the DC protein assay kit (Bio-Rad, Ivry-sur-Seine, France).
OMP analysis.
OMPs were extracted by the sarcosyl extraction of total membrane preparations, as described previously (40). Briefly, cell cultures were harvested in logarithmic phase and lysed by sonication. OMPs were obtained after the treatment of cell membranes with sodium lauryl sarcosylate (Sigma, Saint-Quentin-Fallavier, France) and subsequent ultracentrifugation. The proteins were examined by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis.
Nucleotide sequence accession number.
The nucleotide sequences reported in this work and corresponding to the entire sequence of plasmid pCHE-A have been deposited in the GenBank nucleotide database under accession no. EU266532.
RESULTS
E. cloacae CHE-2 harbors a carbapenemase-encoding gene.
E. cloacae CHE-2 was isolated from the sputum of a patient admitted for pneumonia to an acute-care center in March 2006 in Calgary, Canada. This isolate was resistant to all β-lactam molecules, including carbapenems (Table 1). Clavulanic acid addition decreased the MIC of ceftazidime (Table 1), suggesting the production of an extended-spectrum ß-lactamase (ESBL). E. cloacae CHE-2 also was resistant to kanamycin, netilmicin, tobramycin, chloramphenicol, tetracycline, nalidixic acid, fluoroquinolones, rifampin, and sulfonamides, remaining susceptible only to gentamicin, amikacin, and colistin.
TABLE 1.
MICs of β-lactams for E. cloacae CHE-2 clinical isolate, E. coli J53 harboring plasmids pCHE-A and pCHE-B expressing β-lactamases GES-5 and SHV-5, respectively, P. aeruginosa PU21 harboring pCHE-A, and reference strains E. coli J53 and P. aeruginosa PU21
| β-Lactam(s) | MIC for:
|
|||||
|---|---|---|---|---|---|---|
| E. cloacae CHE-2 (GES-5 + SHV-5) | E. coli J53(pCHE-A) (GES-5) | E. coli J53(pCHE-B) (SHV-5) | E. coli J53 | P. aeruginosa PU21(pCHE-A) (GES-5) | P. aeruginosa PU21 | |
| Amoxicillin | >512 | >512 | >512 | 2 | >512 | >512 |
| Amoxicillin + CLAa | >256 | 16 | 4 | 2 | >512 | >512 |
| Ceftazidime | >512 | 128 | 256 | 0.06 | 256 | 1 |
| Ceftazidime + CLA | 256 | 16 | 4 | 0.06 | 64 | 1 |
| Cefoxitin | >512 | 128 | 4 | 4 | >512 | >512 |
| Imipenem | >32 | 1 | 0.06 | 0.06 | 16 | 2 |
| Meropenem | >32 | 0.25 | 0.06 | 0.03 | >32 | 0.5 |
| Ertapenem | >32 | 0.12 | 0.06 | 0.03 | >32 | 8 |
CLA, clavulanic acid at 4 μg/ml.
To determine whether resistance to carbapenems was related to any β-lactamase, imipenem hydrolysis was tested using a culture extract of E. cloacae CHE-2, which revealed a significant carbapenemase activity (20 mU/mg of protein−1). Subsequent PCR screening using primers specific for ß-lactamase genes identified a blaGES-type gene using whole-cell DNA of E. cloacae CHE-2 as the template. The cloning of EcoRI-restricted whole-cell DNA of E. cloacae CHE-2 into pBK-CMV gave recombinant plasmid pBCHE2. E. coli TOP10 (pBCHE2) expressed an ESBL phenotype with reduced susceptibility to imipenem (Table 1) that was consistent with the resistance profile of the clinical isolate. The DNA sequence analysis of plasmid pBCHE-2 identified a 1,620-bp insert containing the blaGES-5 gene, encoding a 287-amino-acid protein, GES-5. It differs from GES-1 by a serine instead of a glycine residue at Ambler position 170, and it is known to hydrolyze carbapenems (1, 24, 28). PCR screening followed by sequencing showed that E. cloacae CHE-2 also was positive for the blaSHV gene, encoding the ESBL SHV-5, which does not hydrolyze carbapenems. Since both ß-lactamases GES-5 and SHV-5 could not explain the high MIC of imipenem for E. cloacae CHE-2 (Table 1), the outer membrane profile of E. cloacae CHE-2 was studied and compared to that of wild-type E. cloacae 5390 and to that of two E. coli reference strains. E. cloacae CHE-2 lacked two porins of 37 and 38 kDa, corresponding to OmpD and OmpF in E. coli (data not shown), respectively, therefore explaining the additional degree of resistance to carbapenems (12, 47, 53).
The blaGES-5 ß-lactamase gene is located on an IncQ-type plasmid.
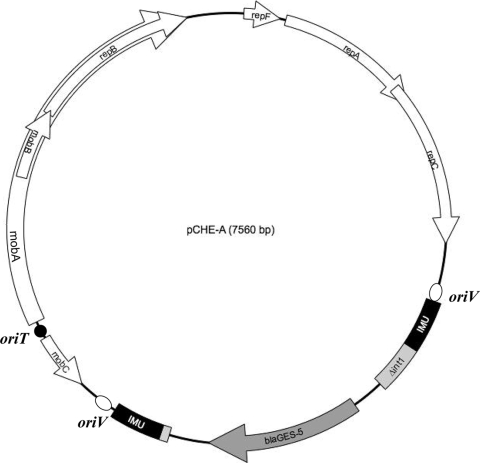
The plasmid analysis of E. cloacae CHE-2 identified three plasmids, pCHE-A, pCHE-B, and pCHE-C, being ca. 7, 150, and 50 kb in size, respectively (data not shown). Mating-out assays gave two types of transconjugants, both expressing an ESBL phenotype. Transconjugant E. coli J53 (pCHE-A) expressed the ESBL GES-5, whereas the E. coli J53 (pCHE-B) transconjugant expressed the ESBL SHV-5 (Table 1). Plasmid pCHE-B belonged to the IncA/C2 incompatibility group, whereas plasmid pCHE-A was not typeable using a PCR-based technique for incompatibility grouping (6). The direct sequencing of the entire 7,560-bp-long plasmid pCHE-A by a primer-walking technique showed that it contained eight open reading frames (ORFs): RepF, RepA, RepB, RepC, MobA, MobB, MobC, and GES-5 (Fig. 1). The identification of three ORFs encoding RepF, RepA, RepB, and RepC proteins (68, 279, 323, and 312 amino acids long, respectively) (Fig. 1) classified plasmid pCHE-A as a member of the MOBQ family of plasmids, also known as mobilizable IncQ-type plasmids (they are not included in the PCR-based replicon typing scheme) (6). RepF, RepA, RepB, and RepC proteins shared 63, 93, 71, and 85% amino acid identity, respectively, with those of the reference and broad-host-range plasmid pRSF1010, which was identified in E. coli (50). A mobA gene was identified that encoded a putative 701-amino-acid MobA mobilization protein sharing 90% amino acid identity with that identified on a plasmid conferring resistance to florfenicol from Pasteurella multocida (25). The repB gene mentioned above corresponds to the same reading frame as mobA, but it has a different start codon that gives rise to a shorter protein (Fig. 1). Considering another reading frame overlapping the mobA sequence, an mobB gene was identified that encoded a 136-amino-acid-long MobB protein sharing 62% identity with that of plasmid pRSF1010 (50). A mobC gene was identified that encoded a putative 105-amino-acid MobC mobilization protein sharing 84% amino acid identity with that of plasmid pRFS1010 (50). Those Mob proteins are part of a plasmid mobilization system consisting of multifunctional proteins acting as relaxases and DNA primases, in which an oriT region was identified (18, 48). The sixth ORF corresponded to that of the blaGES-5 gene (Fig. 1). The oriV sequence, corresponding to the origin of replication of plasmid pCHE-A, also was identified downstream of the repB gene (Fig. 1).
FIG. 1.
Map of natural plasmid pCHE-A. A genetic map of plasmid pCHE-A is shown. Coding regions are indicated by arrows giving the direction of transcription. The plasmid is made of the replication initiation genes repF, repA, repB, and repC, the putative mobilization module genes mobA, mobB, and mobC, and the ß-lactamase blaGES-5 gene bracketed by the two IMUs. In addition, the oriT region that is required for mobilization and the oriV region that is required for replication are indicated.
The broad-host-range property of plasmid pCHE-A was assessed by obtaining transformants using P. aeruginosa PU21 and E. cloacae 5390 as recipient strains. It was not self conjugative, as indicated by the negative results of mating-out assays. However, it was mobilizable in trans by the IncA/C2 plasmid pCHE-B (blaSHV-5), as shown by the obtention of E. coli J53 transconjugants harboring both plasmids pCHE-A and pCHE-B.
The blaGES-5 gene is associated with novel genetic structures.
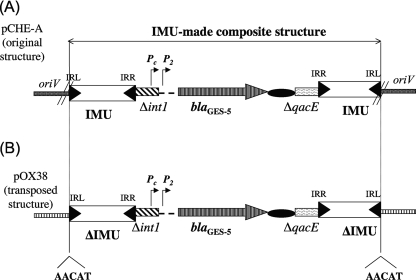
The analysis of the DNA sequences flanking the blaGES-5 gene showed that it formed a gene cassette, as previously observed for blaGES-like genes and some other ESBL-encoding genes (33, 38). In particular, it possessed core and inverse core sites, together with a 59-base element (52). The blaGES-5 gene cassette was preceded by a class 1 integrase gene and followed by a qacE gene, which are two classical features of class 1 integrons, but both were truncated in that present structure (Fig. 1 and 2A). Further analysis identified two repeated and perfectly identical 288-bp elements (termed IMUs) encompassing this class 1 integron remnant. These elements were located in opposite orientations on each side of the blaGES-5 gene (Fig. 1 and 2A). Their GC content was 50%; it was 62.3% for the overall plasmid backbone. A Blast analysis did not show any significant identity between those elements and any known sequences. However, a detailed analysis performed on the ISBiotoul website (http://www-is.biotoul.fr/) revealed that the first 38 bp of one end of the IMU element (defined arbitrarily as being its right end and consequently named the right inverted repeat [IRR]) were 95% identical (only two base pair mismatches) with the IRR of insertion sequence ISSod9, which belongs to the Tn3 family of transposons. Two copies of ISSod9 previously had been identified on a 160-kb plasmid of Shewanella oneidensis MR-1 (20). A putative left inverted repeat (IRL) sequence was identified at the opposite end of the IMU, sharing 28 out of the 38 bp of the IRR (Fig. 3). However, as opposed to insertion sequence (IS) elements, no ORF (and therefore no transposase) was identified in the IMU elements.
FIG. 2.
Structure of the IMU-made composite structure harboring the blaGES-5 gene. (A) Schematic map of the putative mobilized structure on natural plasmid pCHE-A corresponding to the two IMUs bracketing the blaGES-5-borne integron, with a truncated int1 integrase gene and a truncated qacE gene. The IRR and IRL identified in the IMU are indicated by black triangles. The 59-bp sequence of the blaGES-5 gene cassette is indicated by a black oval, as is the oriV coding sequence region. The integron-mediated Pc and P2 promoter sequences are indicated. (B) Schematic map of the fragment mobilized by the in trans transposition process onto recipient plasmid pOX38.
FIG. 3.
Sequence of the 288-bp-long IMU element. The arbitrarily defined extremities are indicated. The IRR and IRL 38-bp sequences are shaded in gray.
IMUs possess features similar to MITEs. These elements belong to a large family of small, repeat, and nonautonomous sequences identified in several bacterial genomes, archaebacteriacae, and eukaryotes (5). Notably, no target site duplication was identified on either extremity of the IMU-Δint/Δqac-IMU structure that could have suggested any transposition-mediated acquisition.
Interestingly, the integration of the IMU-Δint/Δqac-IMU structure occurred within the oriV region of plasmid pCHE-A, disrupting the so-called single-strand initiation (ssi) signal sequences that serve for initiating the priming of single-stranded DNA synthesis during plasmid replication (18, 48). A detailed sequence analysis showed that part of these ssi sequences were duplicated and identified on both extremities of the IMU-Δint/Δqac-IMU structure, but in opposite orientations.
IMU elements may mobilize the antibiotic resistance gene by transposition.
To determine whether the IMU-Δint/blaGES-5/Δqac-IMU structure could be mobilized by transposition, experiments were conducted by providing in trans the ISSod9 transposase activity of S. oneidensis. Several recA-E. coli J53 transconjugants contained two plasmids, whereas others harbored only the plasmid pOX38 backbone. The identification of two plasmids in a single host strain corresponded to a mobilization of the natural blaGES-5-positive plasmid pCHE-A by plasmid pOX38. The transposition of the IMU-Δint/blaGES-5/Δqac-IMU overall fragment was identified in transconjugants that had a single pOX38-like plasmid. On those plasmids, the blaGES-5 gene was bracketed by the two IMU elements. A 5-bp target site duplication was identified on both ends of the ΔIMU-Δint/Δqac-ΔIMU structures, which is the signature of an in vivo transposition process (Fig. 2B). This result was in accordance with the in silico identification of 5-bp-long duplicated sequences identified on both extremities of an ISSod9-made composite transposon in S. oneidensis MR-1 (data not shown). Since all transconjugants did not harbor recombinant pOX38 plasmids, it was not possible to deduce the transposition frequency.
The IMU element does not enhance expression of the antibiotic resistance gene.
Since multiple IS elements may enhance the expression of downstream-located genes, the IMU-dependent expression of the blaGES-5 gene was evaluated. The IMU location truncated the int1 integrase gene (by introducing an immediate stop codon in its corresponding frame), whereas the Pc and P2 promoter sequences specific to class 1 integrons still were clearly identified (Fig. 2A) (11). Clonings of the blaGES-5+ PCR fragments with or without the IMU structure located upstream of the blaGES-5 gene showed identical ß-lactam MICs for E. coli recombinant strains, indicating that the IMU structure did not enhance the expression of the blaGES-5 gene (data not shown).
DISCUSSION
This study described a carbapenem-resistant E. cloacae isolate producing the ß-lactamase GES-5 and is the first evidence of the dissemination of this carbapenemase in North America. The blaGES-5 gene was located on a small-size, broad-host-range, IncQ-type plasmid named pCHE-A. This plasmid was not self conjugative but was mobilizable by a broad-host-range IncA/C2 plasmid (named pCHE-B). Plasmid pCHE-A was transferred to and was able to replicate into enterobacterial and Pseudomonas species.
By far, the most interesting result of the study is the identification of a totally novel DNA element, IMU, of which two copies were identified, in opposite orientations, encompassing a defective class 1 integron containing the carbapenem resistance gene blaGES-5. The IMU elements did not code for any ORF but possessed two 39-bp imperfect inverted repeats. The IMU structure resembles MITEs, which have been identified in eukaryotes such as plants (7) and also in bacteria (5, 17). However, the IMUs differ from other MITEs in the following features: IMUs are larger (MITEs usually are <200 bp) and have a higher GC content (MITEs usually are AT rich) (14). Nevertheless, some MITEs can be large, such as SMN1 from the archaebacterium Sulfolobus islandicus (321 bp in size) (4). In addition, as opposed to what has been observed with the MITE-type elements from Neisseria spp. (Correia element) (14, 33), no integration host factor binding site was identified in the IMU sequence. The only known MITE identified in Enterobacteriaceae corresponded to the enterobacterial intergenic consensus, which likely is involved in the regulation of mRNA stability (21). Other bacterial MITEs are RUPs (repeat unit of Pneumococcus) from Streptococcus pneumoniae or RPE (Rickettsia palindromic element) in Rickettsia spp., and all are known to be naturally present at multiple copies in their host (31, 36, 37).
The two IMUs identified in this study formed a composite element and likely were responsible for the mobilization of this defective class 1 integron structure. We have shown that the mobility of the IMU elements is mediated by a transposition mechanism once a transposase activity (such as ISSod9 transposase) is provided in trans. The trans-mobilization of deleterious IS elements previously had been reported with, e.g., IS911 (45) or IS231 (15). Also, an in silico analysis of the whole genome of Geobacter uraniireducens Rf4 allowed us to hypothesize that the Chunjie MITE had been mobilized in trans by the transposase of ISGur4, which shared similar inverted repeat sequences (9). Interestingly, we identified that the IMU-Δint/Δqac-IMU structure inserted within the ssi repeats of the oriV region, which was duplicated in part. In silico analysis showed that the duplication of this part of the oriV sequence may be observed in other IncQ-type plasmids (i.e., plasmids pIE1130 and pIE1115 [51]).
Since the absence of target site duplication on plasmid pCHE-A does not correlate with the integration of the IMU-Δint/Δqac-IMU structure by a transposition mechanism, several hypotheses can be made. One is that the oriV duplication observed on each side of the two IMUs has been at the origin of a homologous recombination process, leading to the acquisition of the IMU-Δint/Δqac-IMU structure.
Although all known MITEs are located on chromosomes, we report in this study a plasmid location of the IMUs associated with a clinically relevant antibiotic resistance gene. Finally, we have shown that the IMUs at least can be mobilized by the transposase of an unrelated IS element. The discovery of IMUs provides a new piece of evidence in the evolutionary engineering of bacterial pathogens, playing an adaptive role for survival against selective pressure (2). Since the genetic elements have been identified in a broad-host-range plasmid, future studies will evaluate the prevalence and possible spread of IMUs among clinically relevant pathogens.
Acknowledgments
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (grant UPRES-EA3539), Université Paris XI, Paris, France, and mostly by a grant from the European Community (6th PCRD, LSHM-CT-2005-018705) and by the INSERM, France.
We thank M. Chandler and T. Naas for fruitful discussions.
Footnotes
Published ahead of print on 30 March 2009.
REFERENCES
- 1.Bae, I. K., Y. N. Lee, S. H. Jeong, S. G. Hong, J. H. Lee, S. H. Lee, H. J. Kim, and H. Youn. 2007. Genetic and biochemical characterization of GES-5, an extended-spectrum class A ß-lactamase from Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 58:465-468. [DOI] [PubMed] [Google Scholar]
- 2.Baquero, F. 2004. From pieces to patterns: evolutionary engineering in bacterial pathogens. Nat. Rev. Microbiol. 2:510-518. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, P. M. 2008. Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br. J. Pharmacol. 153(Suppl. 1):S347-S357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkner, S., and G. Lipps. 2007. An active nonautonomous mobile element in Sulfolobus islandicus REN1H1. J. Bacteriol. 189:2145-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buisine, N., C. M. Tang, and R. Chalmers. 2002. Transposon-like Correia elements: structure, distribution and genetic exchange between pathogenic Neisseria sp. FEBS Lett. 522:52-58. [DOI] [PubMed] [Google Scholar]
- 6.Carattoli, A., A. Bertini, L. Villa, V. Falbo, K. L. Hopkins, and E. J. Threlfall. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 7.Casacuberta, J. M., and N. Santiago. 2003. Plant LTR-retrotransposons and MITEs: control of transposition and impact on the evolution of plant genes and genomes. Gene 311:1-11. [DOI] [PubMed] [Google Scholar]
- 8.Castanheira, M., R. E. Mendes, T. R. Walsh, A. C. Gales, and R. N. Jones. 2004. Emergence of the extended-spectrum ß-lactamase GES-1 in a Pseudomonas aeruginosa strain from Brazil: report from the SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemother. 48:2344-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Y., F. Zhou, G. Li, and Y. Xu. 2008. A recently active miniature inverted-repeat transposable element, Chunjie, inserted into an operon without disturbing the operon structure in Geobacter uraniireducens Rf4. Genetics 179:2291-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement M100-S18. Clinical and Laboratory Standards Institute, Baltimore, MD.
- 11.Collis, C. M., and R. M. Hall. 1995. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob. Agents Chemother. 39:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornaglia, G., K. Russell, G. Satta, and R. Fontana. 1995. Relative importances of outer membrane permeability and group 1 ß-lactamase as determinants of meropenem and imipenem activities against Enterobacter cloacae. Antimicrob. Agents Chemother. 39:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correia, M., F. Boavida, F. Grosso, M. J. Salgado, L. M. Lito, J. M. Cristino, S. Mendo, and A. Duarte. 2003. Molecular characterization of a new class 3 integron in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 47:2838-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delihas, N. 2008. Small mobile sequences in bacteria display diverse structure/function motifs. Mol. Microbiol. 67:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Palmenaer, D., C. Vermeiren, and J. Mahillon. 2004. IS231-MIC231 elements from Bacillus cereus sensu lato are modular. Mol. Microbiol. 53:457-467. [DOI] [PubMed] [Google Scholar]
- 16.Dubois, V., L. Poirel, C. Marie, C. Arpin, P. Nordmann, and C. Quentin. 2002. Molecular characterization of a novel class 1 integron containing blaGES-1 and a fused product of aac3-Ib/aac6′-Ib′ gene cassettes in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filée, J., P. Siguier, and M. Chandler. 2007. Insertion sequence diversity in Archaea. Microbiol. Mol. Biol. Rev. 71:121-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francia, M. V., A. Varsaki, M. P. Garcillan-Barcia, A. Latorre, C. Drainas, and F. de la Cruz. 2004. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 28:79-100. [DOI] [PubMed] [Google Scholar]
- 19.Hall, R. M., and C. M. Collis. 1995. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15:593-600. [DOI] [PubMed] [Google Scholar]
- 20.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson, and C. M. Fraser. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 21.Hulton, C. S., C. F. Higgins, and P. M. Sharp. 1991. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol. Microbiol. 5:825-834. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby, G. A., and L. S. Munoz Price. 2005. The new ß-lactamases. N. Engl. J. Med. 352:380-391. [DOI] [PubMed] [Google Scholar]
- 23.Jacoby, G. A., and L. Sutton. 1985. β-Lactamases and β-lactam resistance in Escherichia coli. Antimicrob. Agents Chemother. 28:703-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong, S. H., I. K. Bae, D. Kim, S. G. Hong, J. S. Song, J. H. Lee, and S. H. Lee. 2005. First outbreak of Klebsiella pneumoniae clinical isolates producing GES-5 and SHV-12 extended-spectrum ß-lactamases in Korea. Antimicrob. Agents Chemother. 49:4809-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehrenberg, C., and S. Schwarz. 2005. Plasmid-borne florfenicol resistance in Pasteurella multocida. J. Antimicrob. Chemother. 55:773-775. [DOI] [PubMed] [Google Scholar]
- 26.Kholodii, G. Y., S. Z. Mindlin, I. A. Bass, O. V. Yurieva, S. V. Minakhina, and V. G. Nikiforov. 1995. Four genes, two ends, and a res region are involved in transposition of Tn5053: a paradigm for a novel family of transposons carrying either a mer operon or an integron. Mol. Microbiol. 17:1189-1200. [DOI] [PubMed] [Google Scholar]
- 27.Kieser, T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19-36. [DOI] [PubMed] [Google Scholar]
- 28.Lee, S. H., and S. H. Jeong. 2005. Nomenclature of GES-type extended-spectrum ß-lactamases. Antimicrob. Agents Chemother. 49:2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lévesque, C., S. Brassard, J. Lapointe, and P. H. Roy. 1994. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene 142:49-54. [DOI] [PubMed] [Google Scholar]
- 30.Liebert, C. A., R. M. Hall, and A. O. Summers. 1999. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loot, C., N. Santiago, A. Sanz, and J. M. Casacuberta. 2006. The proteins encoded by the pogo-like Lemi1 element bind the TIRs and subterminal repeated motifs of the Arabidopsis emigrant MITE: consequences for the transposition mechanism of MITEs. Nucleic Acids Res. 34:5238-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mammeri, H., M. Van De Loo, L. Poirel, L. Martinez-Martinez, and P. Nordmann. 2005. Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrob. Agents Chemother. 49:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naas, T., Y. Mikami, T. Imai, L. Poirel, and P. Nordmann. 2001. Characterization of In53, a class 1 plasmid- and composite transposon-located integron of Escherichia coli which carries an unusual array of gene cassettes. J. Bacteriol. 183:235-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naas, T., and P. Nordmann. 1994. Analysis of a carbapenem-hydrolyzing class A ß-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc. Natl. Acad. Sci. USA 91:7693-7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naas, T., L. Poirel, and P. Nordmann. 2008. Minor extended-spectrum ß-lactamases. Clin. Microbiol. Infect. 14(Suppl. 1):42-52. [DOI] [PubMed] [Google Scholar]
- 36.Ogata, H., S. Audic, V. Barbe, F. Artiguenave, P. E. Fournier, D. Raoult, and J. M. Claverie. 2000. Selfish DNA in protein-coding genes of Rickettsia. Science 290:347-350. [DOI] [PubMed] [Google Scholar]
- 37.Oggioni, M. R., and J. P. Claverys. 1999. Repeated extragenic sequences in prokaryotic genomes: a proposal for the origin and dynamics of the RUP element in Streptococcus pneumoniae. Microbiology 145:2647-2653. [DOI] [PubMed] [Google Scholar]
- 38.Poirel, L., L. Brinas, N. Fortineau, and P. Nordmann. 2005. Integron-encoded GES-type extended-spectrum ß-lactamase with increased activity toward aztreonam in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3593-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poirel, L., C. Héritier, and P. Nordmann. 2004. Chromosome-encoded ambler class D ß-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob. Agents Chemother. 48:348-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poirel, L., C. Héritier, C. Spicq, and P. Nordmann. 2004. In vivo acquisition of high-level resistance to imipenem in Escherichia coli. J. Clin. Microbiol. 42:3831-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poirel, L., M.-F. Lartigue, J.-W. Decousser, and P. Nordmann. 2005. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob. Agents Chemother. 49:447-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poirel, L., I. Le Thomas, T. Naas, A. Karim, and P. Nordmann. 2000. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum ß-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poirel, L., J. D. Pitout, and P. Nordmann. 2007. Carbapenemases: molecular diversity and clinical consequences. Future Microbiol. 2:501-512. [DOI] [PubMed] [Google Scholar]
- 44.Poirel, L., G. F. Weldhagen, T. Naas, C. De Champs, M. G. Dove, and P. Nordmann. 2001. GES-2, a class A ß-lactamase from Pseudomonas aeruginosa with increased hydrolysis of imipenem. Antimicrob. Agents Chemother. 45:2598-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polard, P., B. Ton-Hoang, L. Haren, M. Bétermier, R. Walczak, and M. Chandler. 1996. IS911-mediated transpositional recombination in vitro. J. Mol. Biol. 264:68-81. [DOI] [PubMed] [Google Scholar]
- 46.Queenan, A. M., and K. Bush. 2007. Carbapenemases: the versatile ß-lactamases. Clin. Microbiol. Rev. 20:440-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raimondi, A., A. Traverso, and H. Nikaido. 1991. Imipenem- and meropenem-resistant mutants of Enterobacter cloacae and Proteus rettgeri lack porins. Antimicrob. Agents Chemother. 35:1174-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawlings, D. E., and E. Tietze. 2001. Comparative biology of IncQ and IncQ-like plasmids. Microbiol. Mol. Biol. Rev. 65:481-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice, L. B. 2002. Association of different mobile elements to generate novel integrative elements. Cell Mol. Life Sci. 59:2023-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scholz, P., V. Haring, B. Wittmann-Liebold, K. Ashman, M. Bagdasarian, and E. Scherzinger. 1989. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 75:271-288. [DOI] [PubMed] [Google Scholar]
- 51.Smalla, K., H. Heuer, A. Götz, D. Niemeyer, E. Krögerrecklenfort, and E. Tietze. 2000. Exogenous isolation of antibiotic resistance plasmids from piggery manure slurries reveals a high prevalence and diversity of IncQ-like plasmids. Appl. Environ. Microbiol. 66:4854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stokes, H. W., D. B. O'Gorman, G. D. Recchia, M. Parsekhian, and R. M. Hall. 1997. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol. Microbiol. 26:731-745. [DOI] [PubMed] [Google Scholar]
- 53.Szabó, D., F. Silveira, A. M. Hujer, R. A. Bonomo, K. M. Hujer, J. W. Marsh, C. R. Bethel, Y. Doi, K. Deeley, and D. L. Paterson. 2006. Outer membrane protein changes and efflux pump expression together may confer resistance to ertapenem in Enterobacter cloacae. Antimicrob. Agents Chemother. 50:2833-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toleman, M. A., P. M. Bennett, and T. R. Walsh. 2006. ISCR elements: novel gene-capturing systems of the 21st century. Microbiol. Mol. Biol. Rev. 70:296-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh, T. R. 2006. Combinatorial genetic evolution of multiresistance. Curr. Opin. Microbiol. 9:476-482. [DOI] [PubMed] [Google Scholar]
- 56.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-ß-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]