Abstract
Amoxicillin (amoxicilline)-clavulanic acid has promising activity against pathogens that cause bone infections. We present the first evaluation of the bone penetration of a beta-lactam by population pharmacokinetics and pharmacodynamic profiling via Monte Carlo simulations. Twenty uninfected patients undergoing total hip replacement received a single intravenous infusion of 2,000 mg/200 mg amoxicillin-clavulanic acid before surgery. Blood and bone specimens were collected. Bone samples were pulverized under liquid nitrogen with a cryogenic mill, including an internal standard. The drug concentrations in serum and total bone were analyzed by liquid chromatography-tandem mass spectrometry. We used NONMEM and S-ADAPT for population pharmacokinetic analysis and a target time of the non-protein-bound drug concentration above the MIC for ≥50% of the dosing interval for near-maximal bactericidal activity in serum. The median of the ratio of the area under the curve (AUC) for bone/AUC for serum was 20% (10th to 90th percentile for between-subject variability [variability], 16 to 25%) in cortical bone and 18% (variability, 11 to 29%) in cancellous bone for amoxicillin and 15% (variability, 11 to 21%) in cortical bone and 10% (variability, 5.1 to 21%) in cancellous bone for clavulanic acid. Analysis in S-ADAPT yielded similar results. The equilibration half-lives between serum and bone were 12 min for amoxicillin and 14 min for clavulanic acid. For a 30-min infusion of 2,000 mg/200 mg amoxicillin-clavulanic acid every 4 h, amoxicillin achieved robust (≥90%) probabilities of target attainment (PTAs) for MICs of ≤12 mg/liter in serum and 2 to 3 mg/liter in bone and population PTAs above 95% against methicillin-susceptible Staphylococcus aureus in bone and serum. The AUC of amoxicillin-clavulanic acid was 5 to 10 times lower in bone than in serum, and amoxicillin-clavulanic acid achieved a rapid equilibrium and favorable population PTAs against pathogens commonly encountered in bone infections.
Osteomyelitis is difficult to diagnose and treat and may cause irreversible damage. Antibiotic treatment over weeks to months is required, often in addition to surgical debridement. To reduce the incidence of infections after orthopedic surgery, perioperative prophylaxis is standard practice. Each year more than a million hip replacements are done worldwide. Prosthetic devices are particularly susceptible to infections, more than 50% of which are due to Staphylococcus aureus or coagulase-negative staphylococci, such as S. epidermidis (38). It is vitally important that adequate surgical prophylaxis be used and that sufficient concentrations of antibiotic with activity against frequently encountered pathogens in bone be achieved.
Amoxicillin (amoxicilline) in combination with clavulanic acid is active against pathogens commonly found in prosthesis-related bone infections (MICs at which 90% of bacteria are inhibited [MIC90s], 1 mg/liter for methicillin-susceptible S. aureus [MSSA] and 8 mg/liter for S. epidermidis [28]). Successful treatment of infections with amoxicillin-clavulanic acid after molar extraction (22), peri-implantitis (52), osteomyelitis due to diabetic foot infections (40), prophylaxis of infections after orthognathic surgery (6), and staphylococcal osteomyelitis in a rat model (23) has been reported. The combination was recommended for the treatment of osteomyelitis caused by mixed anaerobic and aerobic pathogens (39).
Bone tissue is less vascularized than, for example, the lungs or the skin. Therefore, it is especially important to study the bone penetration of an antimicrobial drug before a clinical effectiveness trial is performed. For the timing of perioperative prophylaxis and surgery, it seems critical to know how fast efficacious concentrations are achieved and how long they are maintained. Modeling of the time course of bone concentrations for penicillins is important, since the shape of the concentration-time curve affects the time above the MIC.
The concentrations of amoxicillin and clavulanic acid in bone were studied in the 1980s (1, 3, 24, 54), and only the bone concentration/serum concentration ratios were reported. As these bone concentration/serum concentration ratios change over time, they are a suboptimal measure of the extent of tissue penetration (36, 47). For patients undergoing joint replacement surgery, only one bone sample is most commonly available per patient. Population pharmacokinetic (PK) modeling offers the advantage that it can fit the full time course of the bone and serum concentrations on the basis of the data for all patients simultaneously. The extent of bone penetration is best described by the ratio of the area under the curve (AUC) for bone/AUC for serum. We are not aware of any reports on population pharmacokinetic-pharmacodynamic (PK-PD) models for beta-lactams in bone.
Our first objective was to investigate the amoxicillin and clavulanic acid concentrations in cancellous and cortical bone in patients undergoing hip replacement by using a standardized and validated analytical method for bone and serum. The second objective was to develop a PK model which describes the time course of the amoxicillin and clavulanic acid concentrations in bone as well as the drug exposure in bone relative to that in serum. The third objective was to evaluate the PD profile of amoxicillin in serum, cortical, and cancellous bone (16, 19) against pathogens commonly encountered in bone infections, such as MSSA and S. epidermidis.
MATERIALS AND METHODS
Study participants.
Twenty patients (9 males, 11 females) who were scheduled to undergo total hip replacement participated in this controlled clinical study. The patients were diagnosed with coxarthosis and had no inflammation of the joints. Their average weight ± standard deviation (SD) was 78 ± 12 kg, their average height ± SD was 169 ± 9 cm, and their average age ± SD was 63 ± 16 years. The study was approved by the Institutional Review Board of the School of Medicine, Friedrich-Alexander-University Erlangen-Nürnberg, and was performed according to the revised version of the Declaration of Helsinki. All subjects gave their written informed consent prior to entry into the study.
Study design and drug administration.
A single dose of 2,000 mg amoxicillin in combination with 200 mg clavulanic acid (Augmentin; GlaxoSmithKline, Munich, Germany) was administered as a short-term intravenous infusion to each patient at the induction of anesthesia. In addition, each patient received a single oral dose of moxifloxacin 2 to 7 h before surgery.
Sampling schedule.
Blood samples were collected predosing and at the time of femoral bone resection. The samples were placed in an ice-water bath and were left to clot before they were centrifuged at 4°C. After centrifugation, the serum samples were immediately frozen and stored at −80°C until the analysis. The hip replacement surgery consisted of resection of the femoral head or both the femoral head and the femoral neck and the subsequent implantation of the prosthetic hip joint. The collected bone specimens were immediately frozen on dry ice and stored at −80°C until analysis.
Determination of serum and bone concentrations.
In cases in which the bone samples included both the femoral head and the femoral neck, these two parts were separated from each other. The samples were then divided into cortical and cancellous tissues. The adhering blood was removed from the samples by swabbing for a short time. The bone samples were subsequently pulverized under liquid nitrogen by use of a cryogenic mill (Freezer/Mill). For preparation of calibration standards and spiked quality controls, appropriate amounts of amoxicillin and clavulanic acid standard solutions were added to serum and bone tissue samples that were shown to be free of the study drugs.
For determination of the amoxicillin and clavulanic acid concentrations in serum, 50 μl of the internal standard solution was added to 100 μl of each sample. The samples were deproteinized by addition of 300 μl acetonitrile. After the samples were thoroughly mixed, they were centrifuged and 50 μl of the clear supernatant was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). For analysis of the bone samples, an aliquot of the bone powder was shaken with six times the amount of buffer for 4 h. The degree of extraction was studied over time to ensure the reproducibility of the results. Amoxicillin and clavulanic acid were stable during the 4-h extraction period. After centrifugation, 50 μl of the internal standard solution was added to 50 μl of the aqueous supernatant. After addition of 175 μl acetonitrile, the samples were thoroughly mixed and centrifuged. The clear supernatant was diluted with twice the amount of buffer.
For determination of the amoxicillin concentration, 50 μl of each sample was chromatographed on a reversed-phase column (Ultracarb 5 ODS 30) and eluted by use of an isocratic solvent system consisting of 0.001 M ammonium acetate buffer and acetonitrile (90/10, vol/vol). The samples were monitored by LC-MS/MS by the selected reaction monitoring method: precursor → product ion for amoxicillin, m/z 366 → m/z 208; precursor → product ion for the internal standard, m/z 350 → m/z 160. Both analyses were done in the positive mode. Under these conditions, amoxicillin and the internal standard were eluted after approximately 0.8 min.
For determination of the clavulanic acid concentration, 25 μl of each sample was chromatographed on a reversed-phase column (Nucleosil 100 amino) and eluted by use of an isocratic solvent system consisting of 0.01 M ammonium acetate buffer and acetonitrile (40/60, vol/vol). The samples were monitored by LC-MS/MS by the selected reaction monitoring method: precursor → product ion for clavulanic acid, m/z 198 → m/z 108; precursor → product ion for the internal standard, m/z 232 → m/z 140. Both analyses were in the negative mode. Under these conditions, clavulanic acid and the internal standard were eluted after approximately 2 min. MacQuan software (version 1.4-noFPU; Perkin-Elmer, Toronto, Ontario, Canada) was used for evaluation of the chromatograms.
No interference was observed for the study drugs or the internal standards. The precision and the accuracy of the spiked quality controls for amoxicillin in serum ranged from 1.8 to 10% and 97.3 to 107.8%, respectively, and the precision and the accuracy of the spiked quality controls for clavulanic acid in serum ranged from 0.8 to 4.8% and 96.0 to 100.5%, respectively. The precision and the accuracy of the spiked quality controls for amoxicillin in bone homogenate ranged from 5.8 to 8.1% and 97.2 to 99.5%, respectively. The precision and the accuracy of the spiked quality controls for clavulanic acid in bone homogenate ranged from 5.0 to 7.8% and 91.7 to 100.0%, respectively.
Pharmacokinetics. (i) Population PK analysis.
A model with two compartments for amoxicillin and one compartment for clavulanic acid was used to describe the concentrations in serum. A bone compartment was added to model the concentrations in bone. The drug input into the central compartment was described by a time-constrained zero-order process. Standard diagnostic plots for model evaluation were used, and the predictive performance of the final model was tested by the use of visual predictive checks.
For the visual predictive check, serum and bone concentration curves for 10,000 subjects were simulated for amoxicillin and clavulanic acid. We derived the median, the nonparametric 90% prediction interval (5th to 95th percentile), and the nonparametric 50% prediction interval (25th to 75th percentile) from those profiles predicted by validated Perl scripts, as described previously (12). We compared the median predicted concentrations and the prediction intervals with the observed data and performed a visual assessment to determine whether the median and the predicted intervals adequately mirrored the central tendency and the variability of the observed data.
(ii) Structural model.
Observations for amoxicillin and clavulanic acid concentrations in serum, cortical bone, and cancellous bone were available. The differential equations for amoxicillin were as follows:
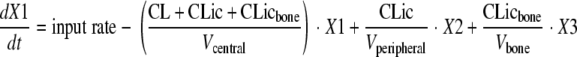 |
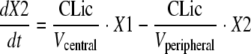 |
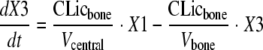 |
where compartment 1 is the central compartment, compartment 2 is the peripheral compartment, and compartment 3 is the bone compartment; X1, X2, and X3 represent the amounts of drug in the central, peripheral, and bone compartments; Vcentral, Vperipheral, and Vbone represent the volumes of distribution in the central, peripheral, and bone compartments. Initial conditions were 0 for all three compartments. CL is the total clearance from the central compartment, CLic is the intercompartmental clearance between the central and the peripheral compartments, and CLicbone is the intercompartmental clearance between the central and the bone compartments. The differential equations for clavulanic acid can be obtained from the above equations by setting CLic equal to 0. The observed bone concentrations and initial modeling showed that the rates of equilibration between serum and cortical bone as well as those between serum and cancellous bone were similar. Therefore, only one compartment was used for bone.
Scale terms were included to describe the equilibrium concentration ratios between cancellous bone and serum (Fcancellous) and between cortical bone and serum (Fcortical). If Fcortical is equal to 1, the AUC from time zero to infinity after the administration of a single dose for bone equals the respective AUC for serum. If Fcortical is less (greater) than 1, the AUC from time zero to infinity for bone is lower (higher) than that for serum.
(iii) PK modeling.
Sparse serum concentration-time data were available for the period from 0 to 1.1 h after the end of the infusion. These data did not allow us to estimate all PK parameters of the population PK model. Therefore, prior knowledge of the structural PK model and the average disposition parameters for the serum concentration profiles and their variability from published studies (4, 5, 29, 30, 49, 51) were incorporated in the present analyses. As those studies were conducted with young healthy volunteers, the amoxicillin clearance reported by Sjovall et al. (53) for elderly subjects was used. This was in agreement with the age-related decrease in renal function predicted by the formula of Cockcroft and Gault (13) on the basis of the CL values from the other studies. For clavulanic acid, the age-related decrease in renal function was accounted for according to the formula of Cockcroft and Gault (13).
As disposition parameters for amoxicillin and clavulanic acid from the literature were determined in the absence of a bone compartment, the amounts of amoxicillin and clavulanic acid in the bone compartment had to be kept minimal. In our model, the serum PK were not affected by the presence of the bone compartment. This was achieved by choosing a small Vbone for the bone compartment.
(iv) BSV and observation model.
Between-subject variability (BSV) was described by an exponential variability model, and residual unidentified variability was described by a proportional error model for concentrations in serum and bone.
(v) Computation.
The first-order conditional estimation method with the interaction estimation option in NONMEM (version V, release 1.1; NONMEM Project Group, University of California, San Francisco) (8) was utilized for population PK modeling. WinNonlin Professional (version 4.0.1; Pharsight Corp., Mountain View, CA) was used for statistical analysis.
(vi) Estimation by three-stage hierarchical population approach.
To independently confirm the results obtained with NONMEM, PK parameters were estimated by the three-stage hierarchical population approach in S-ADAPT (version 1.55) (7). Priors for population means and BSV of the disposition parameters were obtained from previously published studies (4, 5, 29, 30, 49, 51). Informative priors were used for the population mean and variability of CL, Vcentral, Vperipheral, and CLic. Physiologically plausible but uninformative priors were used for the population mean and variability of Fcortical and Fcancellous and the population mean of CLicbone on the basis of data reported in the literature (36). The residual unidentified variability was described by a proportional error model. As only one serum sample and one bone sample were available from each patient, informative priors were used for the residual unidentified variability on the basis of the bioanalytical assay data. A systematic sensitivity analysis was performed to evaluate the effect of the choice of priors on the extent and the rate of bone penetration.
(vii) Extent of drug exposure in bone.
The extent of drug exposure in bone was determined for amoxicillin and clavulanic acid by simulating the AUCs in serum and cortical and cancellous bone. On the basis of the final estimates from NONMEM, we simulated 10,000 virtual subjects at steady state and calculated the individual ratios of AUC for bone/AUC for serum as well as their BSV.
(viii) Monte Carlo simulation.
A time that the non-protein-bound drug concentration remained above the MIC (fT>MIC) of ≥50% of the dosing interval was shown to be the PK-PD target for near-maximal bactericidal activity, and an fT>MIC of ≥30% was shown to be the PK-PD target for the bacteriostasis of penicillins on the basis of the concentrations in plasma (16, 19). We used fT>MIC for at least 30% or 50% of the dosing interval as the PK-PD target for serum concentrations. As the PK-PD target for the successful treatment or prophylaxis of bone infections is unknown, we considered a wide range of PK-PD target values for amoxicillin in bone and reported the PK-PD breakpoints for fT>MIC targets of at least 30%, 50%, 70%, or 100% for bone concentrations.
We studied a range of MICs from 0.25 to 64 mg/liter and used a protein binding of 18% for amoxicillin in serum (27). In the absence of data on the protein binding in bone, we assumed a protein binding of 18% for amoxicillin in bone. Additionally, we provided the expected PK-PD breakpoints for various potential scenarios of the distribution of drug and bacteria in bone.
A 30-min infusion of 2,000 mg amoxicillin every 4 h (q4h), q6h, or q8h was studied at steady state. We simulated 10,000 virtual subjects in the absence of residual error. The probabilities of target attainment (PTAs) were derived by calculating the fraction of subjects who attained the PK-PD target at each MIC. The PK-PD breakpoint was defined as the highest MIC for which the PTA was at least 90%.
To determine the clinical relevance of the differences in the PTAs between the dosage regimens, the PTA expectation value was calculated on the basis of several published MIC distributions for amoxicillin-clavulanic acid. These comprised susceptibility data on MSSA and S. epidermidis from North America (28). The PTA expectation value (also called the cumulative fraction of response [46]) was calculated by multiplying the PTA at each MIC with the frequency of how often this MIC occurs in the selected MIC distribution. The individual products at each MIC were summed, and the sum yielded the PTA expectation value for the selected MIC distribution. The PTA expectation value is the PTA in a specific patient population for the treatment of infections caused by bacteria from a specific MIC distribution (ideally, the MIC distribution of each local hospital).
RESULTS
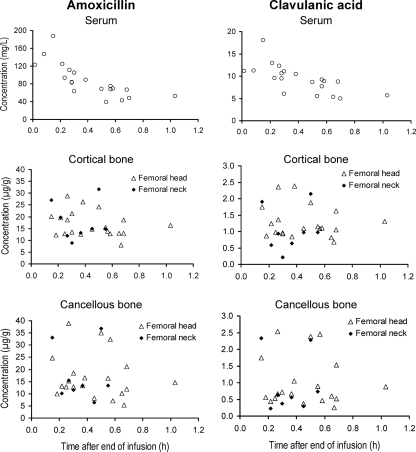
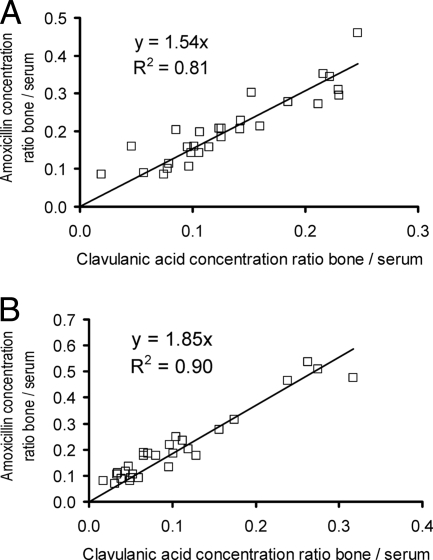
Figure 1 shows the concentrations of amoxicillin and clavulanic acid observed in serum and cortical and cancellous bone. For both drugs, the concentrations were lower in cortical and cancellous bone than in serum. The concentrations in the femoral neck samples were similar to those in the femoral head samples. Figure 2 shows the high correlation of the ratio of the concentration in bone to the concentration in serum within the individual patients.
FIG. 1.
Concentrations in serum and bone of subjects undergoing hip replacement surgery after administration of a single intravenous dose of 2000 mg amoxicillin and 200 mg clavulanic acid as a short-term infusion.
FIG. 2.
Bone concentration-to-serum concentration ratios of amoxicillin versus clavulanic acid in cortical bone (A) and cancellous bone (B).
Population PK.
The population PK parameter estimates from NONMEM and the three-stage hierarchical approach in S-ADAPT were comparable (Table 1). The average estimated drug AUCs for bone relative to that for serum (Fcortical and Fcancellous) ranged from 19 to 21% for amoxicillin and from 12 to 19% for clavulanic acid. The half-life of equilibration between bone and serum was rapid for both drugs (Table 1).
TABLE 1.
Averages and between-subject coefficients of variation (percent) calculated from individual PK parameter estimatesa
| Drug and program | CLb (liter h−1) | Vcentralb (liters) | Vperipheralb (liters) | CLicb (liter h−1) | t1/2,equilibrationc,d (min) | Fcorticalc | Fcancellousc |
|---|---|---|---|---|---|---|---|
| Amoxicillin | |||||||
| NONMEM | 12.4 (19)e | 11.3 (12) | 6.17 (3.7) | 11.1 (19) | 11.8 | 0.199 (11) | 0.192 (39) |
| S-ADAPT | 12.0 (15) | 11.3 (9.8) | 6.14 (3.1) | 11.2 (17.6) | 11.1 (4.4) | 0.210 (30) | 0.204 (49) |
| Clavulanic acid | |||||||
| NONMEM | 11.0 (6.2) | 16.7 (25) | 14 | 0.156 (17) | 0.119 (57) | ||
| S-ADAPT | 11.0 (6.6) | 16.2 (22) | 21.4 (12) | 0.188 (34) | 0.147 (61) |
The Vbone was fixed to 0.177 liter for amoxicillin and to 0.172 liter for clavulanic acid (see Materials and Methods for details).
An informative prior was used.
An uninformative but physiologically plausible prior was used.
t1/2,equilibration, half-life of equilibration between serum and bone.
Values in parentheses are the between-subject coefficients of variation (in percent).
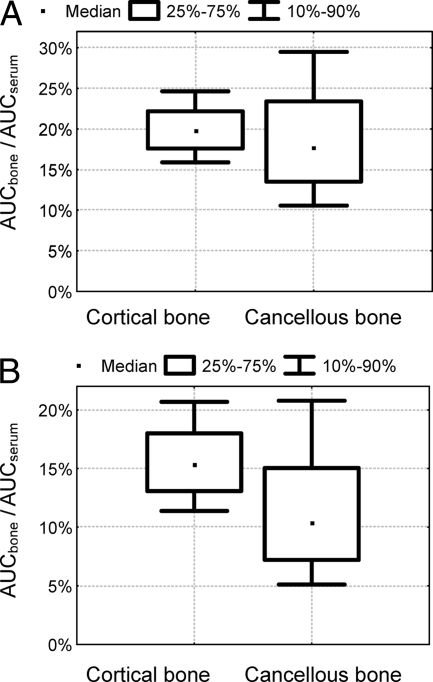
Figure 3 shows the simulated extent of amoxicillin and clavulanic acid penetration into cortical and cancellous bone and the BSV of penetration, calculated from the ratios of AUCcortical/AUCserum and AUCcancellous/AUCserum at steady state for 10,000 virtual subjects on the basis of the results obtained with NONMEM. The median AUC ratios for amoxicillin were 20% (10th to 90th percentile, 16% to 25%) for cortical bone and 18% (10th to 90th percentile, 11% to 29%) for cancellous bone. The median AUC ratios for clavulanic acid were 15% (10th to 90th percentile, 11% to 21%) for cortical bone and 10% (10th to 90th percentile, 5.1% to 21%) for cancellous bone. Therefore, for both drugs the AUC ratio for cancellous bone was more variable (see also the variability for Fcortical and Fcancellous in Table 1) and (slightly) lower than that for cortical bone.
FIG. 3.
Simulated ratios for the AUC between bone and serum for amoxicillin (A) and clavulanic acid (B) during one dosing interval at steady state (based on the results obtained with NONMEM [Table 1]).
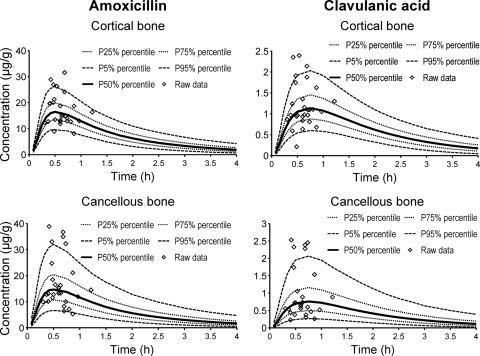
The visual predictive checks showed a highly sufficient predictive performance of the final NONMEM model for both drugs (Fig. 4). This qualified our model for use in the Monte Carlo simulation.
FIG. 4.
Visual predictive check after treatment with 2,000 mg amoxicillin and 200 mg clavulanic acid. The plots show the observed data, the 90% prediction (P) intervals (5th to 95th percentiles), and the interquartile ranges (25th to 75th percentiles). Ideally, 50% of the raw data points should fall inside the interquartile range at each time point and 90% of the raw data should fall inside the 90% prediction interval. Visual predictive checks are based on the results obtained with NONMEM.
Monte Carlo simulation of amoxicillin PK-PD.
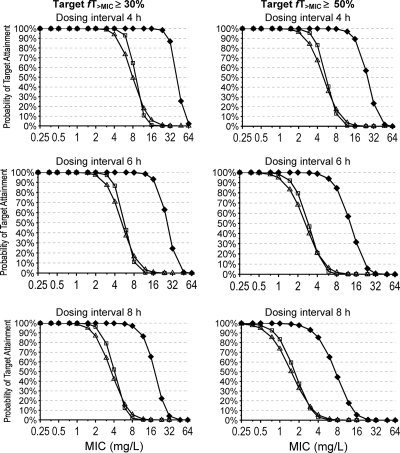
The PTA-versus-MIC plots based on the estimates from NONMEM are shown in Fig. 5; and the respective PK-PD breakpoints for serum, cortical bone, and cancellous bone are listed in Table 2. Monte Carlo simulations based on parameters from S-ADAPT yielded comparable results. The PK-PD breakpoints were about four times higher in serum than in cortical bone and about six times higher in serum than in cancellous bone due to the low extent of bone penetration (Fcortical and Fcancellous). The PTA expectation values for the MIC distributions of two different pathogens are shown in Table 3. For 4-h or 6-h dosing intervals, PTA expectation values above 90% were achieved against MSSA in serum and cortical and cancellous bone, based on the target fT>MIC of ≥50%. For S. epidermidis, a dosing interval of 4 h was required to reach PTA expectation values above 80% in bone in these simulations.
FIG. 5.
PTAs for serum (⧫), cortical bone (□), and cancellous bone (▵) after the administration of 2,000 mg amoxicillin (and 200 mg clavulanic acid) as a 30-min infusion at steady state (based on the results obtained with NONMEM [Table 1]).
TABLE 2.
PK-PD breakpoints for amoxicillin in serum and cortical and cancellous bone for 30-min infusions of 2,000 mg amoxicillin and 200 mg clavulanic acid q4h, q6h, or q8h at steady state
| Target fT>MIC (%) | Dosing interval (h) | PK-PD breakpoint concna
|
||
|---|---|---|---|---|
| Serum (mg/liter) | Cortical bone (μg/g) | Cancellous bone (μg/g) | ||
| 30 | 4 | 24 | 6 | 4 |
| 30 | 6 | 16 | 3 | 2 |
| 30 | 8 | 8 | 2 | 1.5 |
| 50 | 4 | 12 | 3 | 2 |
| 50 | 6 | 6 | 1.5 | 1 |
| 50 | 8 | 3 | 0.75 | 0.5 |
| 70 | 4 | 2.0 | 1.5 | |
| 70 | 6 | 0.5 | 0.5 | |
| 70 | 8 | 0.25 | 0.1875 | |
| 100 | 4 | 0.75 | 0.5 | |
| 100 | 6 | 0.1875 | 0.125 | |
| 100 | 8 | 0.03125 | 0.03125 | |
The breakpoints are based on the estimates from NONMEM (Table 1). A level of protein binding of 18% in both serum and bone was assumed.
TABLE 3.
Comparison of the expectation value for target attainment against MSSA and S. epidermidis (28) for amoxicillina
| Compartment and dosing interval (h) | Expected value (%) for the indicated target values of fT>MIC
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MSSA (n = 196)
|
S. epidermidis (n = 119)
|
|||||||
| 30% | 50% | 70% | 100% | 30% | 50% | 70% | 100% | |
| Serum | ||||||||
| 4 | 99.5 | 97.2 | 96.2 | 95.4 | 99.7 | 97.9 | 96.1 | 90.5 |
| 6 | 97.2 | 96.0 | 95.1 | 86.9 | 98.0 | 95.1 | 87.6 | 68.0 |
| 8 | 96.4 | 95.3 | 90.4 | 58.9 | 96.8 | 89.4 | 73.1 | 45.3 |
| Cortical bone | ||||||||
| 4 | 95.7 | 95.3 | 94.4 | 88.3 | 93.4 | 87.2 | 77.8 | 63.5 |
| 6 | 95.3 | 93.6 | 83.2 | 47.4 | 87.7 | 72.9 | 57.4 | 38.0 |
| 8 | 94.8 | 86.5 | 56.4 | 17.7 | 80.9 | 60.3 | 42.0 | 22.2 |
| Cancellous bone | ||||||||
| 4 | 95.6 | 95.1 | 93.5 | 84.5 | 91.5 | 84.4 | 75.3 | 61.1 |
| 6 | 95.1 | 92.0 | 78.9 | 44.3 | 85.1 | 70.4 | 55.4 | 36.9 |
| 8 | 94.2 | 81.8 | 52.1 | 16.5 | 77.9 | 57.7 | 40.6 | 20.9 |
Simulations are based on the estimates from NONMEM (Table 1) and a 30-min infusion of 2,000 mg amoxicillin and 200 mg clavulanic acid at steady state.
The breakpoints in Table 2 and Table 3 are based on the assumption that amoxicillin and clavulanic acid distribute homogeneously in bone (scenario A in Table 4). If amoxicillin and clavulanic acid distribute only into certain parts of the bone (Table 4), the total concentration in bone homogenate will be higher than the total concentration at these sites in the bone. Therefore, breakpoints for the other scenarios in Table 4 are higher than those for scenario A, assuming that the binding of antibiotics in bone is similar to the binding of antibiotics in serum and that bacteria distribute to the same sites as amoxicillin-clavulanic acid. If bacteria distribute intracellularly or reside in sequestered areas into which antibiotics do not penetrate or penetrate only poorly, the risk for relapse after the termination of antibiotic therapy (scenario D) or the failure of therapy (scenario F) seems high.
TABLE 4.
Estimated breakpoints for various scenarios of distribution of amoxicillin-clavulanic acid and bacteriaf
| Scenario | Presence of drug or drug/bacteria inf:
|
PK-PD breakpoint for cortical bonea | ||||||
|---|---|---|---|---|---|---|---|---|
| Vascular space | Interstitial fluid | Total bone fluidc | Bone cells | Organic matrix | Hydroxyapatite | Sequestered area | ||
| Ae | Yes | Yes/yes | Yes/yes | Yes/yes | Yes/yes | Yes/yes | No/no | Breakpoint, 1.5 μg/g (as reported in Table 2); binding of β-lactams to hydroxyapatite disagrees with binding studies in literature (1, 55) |
| B | Yes | Yes/yes | No/no | No/no | No/no | No/no | No/no | Breakpoint, 28 μg/g,d as concentrations in bone homogenate are lower than those in interstitial fluid and the vascular space |
| C | Yes | Yes/yes | Yes/yes | No/no | No/no | No/no | No/no | Breakpoint, 7.5 μg/g, as concentrations in total body fluid are higher than those in bone homogenated |
| D | Yes | Yes/yes | Yes/yes | No/yes | No/no | No/no | No/no | Breakpoint, 7.5 μg/gd for acute infection but risk of relapse,b unless treatment is combined with an antibiotic with high intracellular penetration |
| E | Yes | Yes/yes | Yes/yes | No/no | Yes/yes | No/no | No/no | Breakpoint, 4.3 μg/gd |
| F | Yes | Yes/yes | Yes/yes | No/no | No/no | No/no | No/yes | Antibiotic therapy alone not effective, surgery needed |
| G | Yes | Yes/yes | Yes/yes | Yes/yes | Yes/yes | No/no | No/no | Breakpoint, 4.3 μg/gd |
The target is an fT>MIC of ≥50% (on the basis of a level of protein binding in bone of 18% and treatment with 2,000 mg amoxicillin-200 mg clavulanic acid q6h). Further binding studies, e.g., for binding of β-lactams to collagen, are needed.
Risk for relapse due to the survival of bacteria, e.g., S. aureus as small-colony variants (2, 31, 35, 38) in osteoblasts.
Whereas the total fluid space in bone has been reported to be 0.245 ml/ml (45) and 0.287 ml/ml (33), some is bound in pores of the hydroxyapatite crystals (45).
The breakpoint is higher than that reported in Table 2, since the total concentrations in bone homogenate are lower than the antibiotic concentrations in the part of the bone into which the antibiotic is distributed in this scenario.
This scenario was used for the simulations reported in Table 2.
The volumes of the vascular space, interstitial fluid, total bone fluid, bone cells, organic matrix, and hydroxyapatite crystals are assumed to be 1.3% (17, 45), 4.0% (17, 41, 45), 20% (33, 44, 45), 1.5% (14), 35% (14, 25, 48), and 65% (14, 25, 32, 48, 54) of the total bone volume. The density of bone is assumed to be 1.0 g/ml. Reported values are between 1.3 and 1.9 g/ml (10, 11, 18, 42, 43). For a bone density of 1.5 g/ml, breakpoints would be 42, 11.3, and 2.5 for scenarios B, C, and A and 6.5 μg/ml for scenarios E and G. The binding of amoxicillin-clavulanic acid to hydroxyapatite crystals is unlikely on the basis of studies described in the literature (1, 55); therefore, this scenario was included only in scenario A.
DISCUSSION
The amoxicillin and clavulanic acid concentrations in bone that have been published previously vary widely (1, 3, 24, 50, 54). Average bone concentration/serum concentration ratios differ up to 3-fold for amoxicillin and 25-fold for clavulanic acid between various studies. Possible reasons might be that the determination of concentrations in bone is methodologically more complex than the determination of concentrations in serum and the use of microbiological assays in studies performed in the 1980s. The assay developed and validated in the present study relies on a highly standardized LC-MS/MS method. The pulverization of bone tissue under liquid nitrogen with a cryogenic mill allowed the efficient, reproducible, and rapid extraction of the drug from the resulting bone powder, which may be critical for unstable drugs like clavulanic acid. The degree of extraction was tested over time to ensure that it was reproducible. Amoxicillin-clavulanic acid was stable during the extraction. Calibration standards for bone concentrations were prepared in blank bone tissue and not in buffer or serum, as is often reported by other authors. We recently reviewed the limitations of bone sample preparation and analysis (36).
The vast majority of bone penetration studies published to date, including those for amoxicillin-clavulanic acid (1, 3, 24, 54), only report bone concentration/serum concentration ratios (36). Grimer et al. (24) report average bone concentrations of 3.6 mg/liter for amoxicillin and 0.54 mg/liter for clavulanic acid about 0.5 h after the intravenous injection of 1,000/200 mg amoxicillin-clavulanic acid q6h on day 2. The concentrations in bone were at least 10-fold lower than those in serum (24). Weismeier et al. (54) administered 2,000/200 mg amoxicillin-clavulanic acid as an intravenous infusion. They related the concentrations determined to the organic bone mass, which accounts for about 30 to 40% of the total bone mass. The slightly higher concentrations in cortical bone than in cancellous bone that they found are in agreement with the findings of our study. When calculated in relation to total bone, the average amoxicillin concentrations were about 8 mg/kg of bone and the average clavulanic acid concentrations were 0.7 to 0.8 mg/kg at up to 2 h after administration of the dose, and the average amoxicillin concentrations were about 2.5 mg/kg of bone and the average clavulanic acid concentrations were about 0.3 mg/kg 2 to 4 h after administration of the dose. This corresponds to average ratios for total bone concentration/serum concentration of approximately 8 to 14% and 4 to 8% for amoxicillin and clavulanic acid during the first 2 h and 15 to 18% and 6 to 7% for amoxicillin and clavulanic acid at 2 to 4 h (54).
Akimoto et al. (3) studied amoxicillin bone penetration in 26 patients on average 2 h (range, 1 to 2.5 h) after the administration of a single oral dose of 500 mg. The average bone concentration-to-serum concentration ratios were 16% (range, 3.7% to 28%) for mandibular bone and 26% (range, 5.6% to 55%) for maxillary bone, which were similar to the findings of our study. Pignanelli et al. (50) reported an average bone concentration/serum concentration ratio of 8.2% in the jaw bones of nine patients who were receiving 500 mg amoxicillin every 8 h for 2 days and to whom the last dose was given at about 2 h before surgery. Adam et al. (1) found 9.8 mg/kg clavulanic-acid in cancellous bone and 15 mg/kg in cortical bone at 0.5 to 1 h after the end of a 20-min infusion of 200 mg clavulanic acid. The bone amoxicillin-clavulanic acid concentrations from Grimer et al. (24) and Weismeier et al. (54) were approximately 2 to 3 times lower than those from our study, whereas Adam et al. (1) reported clavulanic acid concentrations about 10 times higher than those obtained in our study (Fig. 1). This pronounced difference (20- to 30-fold for averages) for clavulanic acid calls for standardized methods of sample preparation, drug analysis, and PK evaluation.
The concentrations of amoxicillin and clavulanic acid showed a high correlation in cortical bone (r = 0.90; observed data) and cancellous bone (r = 0.95; Fig. 2). This suggests that both drugs were stable and that sample preparation and drug analysis were precise and reproducible. The concentrations in cortical and cancellous bone were correlated for both drugs (r = 0.75). This agrees with the data from Weismeier et al. (54) and indicates that the equilibration rates between cortical bone and serum and between cancellous bone and serum were probably similar. This was confirmed by population PK modeling. Consequently, the equilibration half-lives for both types of bone were assumed to be the same in the final model. Short equilibration half-lives between bone and serum were estimated for both drugs (Table 1; Fig. 4). This suggests that concentrations above the PK-PD breakpoints were reached within less than 30 min after the end of a 30-min infusion. As serum and bone concentrations are decreasing after the end of a short-term infusion, these fast equilibration half-lives suggest from a PK point of view that the surgery should start within the first 30 min after the end of a 30-min infusion. As the breakpoints reported here are based on PK-PD targets for the treatment of infections and as these targets were derived on the basis of the plasma or serum concentrations, PK-PD targets for surgical prophylaxis need to be established and our simulation results should be interpreted conservatively.
The determination of total antibiotic concentrations in bone homogenate, as reported in virtually all studies on bone penetration published to date (36), has several limitations. Bone tissue consists of an organic matrix (30 to 35% of total bone mass) and an inorganic matrix (65 to 70%) (14, 25, 48). The organic matrix mainly consists of collagen fibrils, glycoproteins, proteoglycans, extracellular fluid (25), and bone cells (1 to 2% of the total bone mass) (14). The inorganic matrix is formed by hydroxyapatite crystals (calcium phosphate) deposited within the organic matrix (14). Neither antibiotics nor bacteria are expected to distribute homogeneously in bone. No techniques for the separation of bone samples into extracellular fluid, hydroxyapatite, collagen fibrils, and bone cells are currently available. In addition, only the unbound fraction of drug is considered to be microbiologically active, and the extent of binding in bone is not known. Binding experiments suggest that beta-lactams do not bind (or bind only to a minor extent) to bone powder or hydroxyapatite crystals (1, 55).
Beta-lactams likely distribute mainly within the vascular and extracellular fluid spaces in bone (17, 26, 34, 41). They pass the capillary walls of blood vessels located in the Haversian and Volkmann canals in bone, diffuse into the interstitial fluid space, and likely distribute in the lacunocanalicular system (Table 4, scenarios B and C) (15, 34). Furthermore, bacteria are not expected to distribute into the inorganic matrix or collagen fibrils but mainly distribute in the interstitial and extracellular fluid spaces (scenarios B and C). Therefore, scenarios B and C in Table 4 seem to be the most likely, and the breakpoints reported in Table 2 appear to be conservative (i.e., low) estimates, if the bacteria do not distribute intracellularly and do not reside in sequestered areas.
As it is not known how drug and bacteria distribute within bone, Table 4 lists potential breakpoints for a dose of 2,000/200 mg amoxicillin-clavulanic acid q6h for various distribution scenarios. The most realistic potential scenario might be scenario B or a scenario in which amoxicillin distributes in interstitial fluid plus part of the total bone fluid outside the interstitial space (a mix of scenarios B and C). As total bone fluid likely includes fluid in small pores of the hydroxyapatite crystals from which interstitial fluid markers (e.g., sucrose) are excluded (45), distribution throughout total bone fluid (scenario C) seems less likely. The ability of S. aureus to enter and survive in osteoblasts (31, 35) was suggested to be a possible reason for relapses of osteomyelitis (Table 4, scenario D). As beta-lactams are not expected to penetrate cells well, an intracellular bone infection might relapse (38). In any case, when bacteria reside in a part of the bone which is inaccessible to amoxicillin, treatment failure or relapse seems likely.
The volumes described in Table 4 refer to those in healthy bone. In patients with acute osteomyelitis, it has been suggested that the blood supply to the bone is increased, capillary permeability is higher, and potentially greater antibiotic concentrations reach bone (34). In osteomyelitic canine bone, the volume of distribution of cefazolin was increased to 0.572 ml per ml bone, whereas in uninfected bone, it was 0.0662 ml per ml bone (17). The results of few PK studies with patients with osteomyelitis are available. In patients with chronic osteomyelitis, dead bone (a location where bacteria may be sequestered) which is not reached by the blood circulation is often present. Beta-lactams most likely cannot penetrate into sequestered areas, and therefore, surgical debridement, in addition to antibiotic therapy, is necessary.
Another limitation of our analysis is that the target for beta-lactams in bone is unknown. Data on the efficacies of amoxicillin and other beta-lactams for the treatment of osteomyelitis are sparse. Therefore, the present analysis could not apply reverse engineering to determine the PK-PD target, as was done previously (9, 37). To consider a wide range of potential target values, we reported the results for fT>MIC targets of at least 30%, 50%, 70%, or 100% (Tables 2 and 3). Should a future study determine that amoxicillin needs to achieve a free concentration of greater than 4× the MIC for 50% of the time, for example, one can directly calculate the breakpoints on the basis of the results of our analysis by dividing our breakpoint (on the basis of fT>1× MIC) by 4, since amoxicillin displays linear PK after intravenous administration.
Although beta-lactams likely distribute mainly in the interstitial space in bone and the bacteria residing there might encounter concentrations similar to those that they would encounter in plasma, the time course of the concentration in bone is different from that in serum. For the beta-lactam target fT>MIC, the shape of the concentration-time curve affects the PTA, unless the drug is given by continuous infusion. Simulations with our model confirmed that fT>MIC depends on the equilibration half-life between serum and bone, even for dosing at steady state. For single doses, such as for perioperative prophylaxis, the equilibration half-life has an even greater impact. Therefore, it is important to determine the half-life of equilibration by modeling and to derive breakpoints on the basis of the concentration-time profiles in bone. However, clinical trials are needed to determine the PK-PD target for bone.
Like virtually all studies of drug bone penetration whose findings have been published, we had one bone sample per patient. The collection of multiple bone samples is not feasible in joint replacement studies. In contrast to previous studies, we applied population PK analysis in which all data from all patients were considered simultaneously. In addition, a full Bayesian analysis, which is the latest method for data analysis and which is particularly valuable for use with sparse data, was applied. Our analysis considered between-patient variability and the PK in both serum and bone and therefore provides information in addition to the comparison of bone concentrations to MICs usually applied. The latter method was also applied to amoxicillin bone penetration studies (3, 24, 36, 54) and was recently criticized by Mouton et al. (47) as “meaningless,” and they also considered its use to be “potentially harmful in patient care.”
Despite the activity of amoxicillin-clavulanic acid against pathogens commonly encountered in bone infections (28), its PK profile and PK-PD breakpoints in bone have not been determined by population PK and Monte Carlo simulations. Drusano et al. used population PK and Monte Carlo simulations to analyze the penetration of levofloxacin into the prostate (21) and epithelial lining fluid (20). We used these techniques, which consider the full time course of tissue and serum concentrations and their BSVs, to estimate the extent and rate of bone penetration and to evaluate the PK-PD profile. Even though the data were sparse, the estimates from NONMEM and S-ADAPT were comparable (Table 1).
Given the limitations pointed out above, the PK-PD breakpoints (Table 2) predicted by population PK and Monte Carlo simulations compared favorably to the MICs for clinically relevant pathogens. Amoxicillin achieved PTA expectation values of >90% against MSSA in bone and serum for 30-min infusions of 2,000/200 mg amoxicillin-clavulanic acid q4h and q6h and for both an fT>MIC of ≥30% and an fT>MIC of ≥50% (Table 3). The susceptibility patterns of the local hospital should be used to determine if amoxicillin-clavulanic acid is a promising choice for the treatment of bone infections.
In conclusion, the median ratios of the AUC for bone/AUC for serum were 20% (80% prediction interval for BSV, 16% to 25%) for cortical bone and 18% (80% prediction interval for BSV, 11% to 29%) for cancellous bone for amoxicillin and 15% (80% prediction interval for BSV, 11% to 21%) for cortical bone and 10% (80% prediction interval for BSV, 5.1% to 21%) for cancellous bone for clavulanic acid. Equilibration between serum and bone was rapid for both drugs. For dosing q4h, amoxicillin achieved robust (≥90%) PTAs for MICs of ≤12 mg/liter in serum and 2 to 3 mg/liter in cortical and cancellous bone for the nearly maximal kill target (fT>MIC, ≥50%). Amoxicillin achieved PTA expectation values of >90% against MSSA for both targets in bone and serum for 30-min infusions of 2,000/200 mg amoxicillin-clavulanic acid q4h and q6h (fT>MIC, ≥50%). The PTA expectation values were slightly lower for S. epidermidis. As the PK-PD target in bone will need to be established in future studies, we considered a wide range of PK-PD targets, and our simulation results should be interpreted conservatively.
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Adam, D., H. D. Heilmann, and K. Weismeier. 1987. Concentrations of ticarcillin and clavulanic acid in human bone after prophylactic administration of 5.2 g of timentin. Antimicrob. Agents Chemother. 31:935-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, S., S. Meghji, R. J. Williams, B. Henderson, J. H. Brock, and S. P. Nair. 2001. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect. Immun. 69:2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akimoto, Y., K. Kaneko, and T. Tamura. 1982. Amoxicillin concentrations in serum, jaw cyst, and jawbone following a single oral administration. J. Oral Maxillofac. Surg. 40:287-293. [DOI] [PubMed] [Google Scholar]
- 4.Allen, G. D., P. E. Coates, and B. E. Davies. 1988. On the absorption of clavulanic acid. Biopharm. Drug Dispos. 9:127-136. [DOI] [PubMed] [Google Scholar]
- 5.Arancibia, A., J. Guttmann, G. Gonzalez, and C. Gonzalez. 1980. Absorption and disposition kinetics of amoxicillin in normal human subjects. Antimicrob. Agents Chemother. 17:199-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baqain, Z. H., N. Hyde, A. Patrikidou, and M. Harris. 2004. Antibiotic prophylaxis for orthognathic surgery: a prospective, randomised clinical trial. Br. J. Oral Maxillofac. Surg. 42:506-510. [DOI] [PubMed] [Google Scholar]
- 7.Bauer, R. J. 2007. S-ADAPT/MCPEM user's guide, version 1.55. Software for pharmacokinetic, pharmacodynamic and population data analysis.
- 8.Beal, S. L., A. J. Boeckmann, L. B. Sheiner, and NONMEM Project Group. 1999. NONMEM users guides, version 5. University of California at San Francisco, San Francisco.
- 9.Blumer, J. L., M. D. Reed, E. L. Kaplan, and G. L. Drusano. 2005. Explaining the poor bacteriologic eradication rate of single-dose ceftriaxone in group A streptococcal tonsillopharyngitis: a reverse engineering solution using pharmacodynamic modeling. Pediatrics 116:927-932. [DOI] [PubMed] [Google Scholar]
- 10.Boselli, E., D. Breilh, J. C. Bel, R. Debon, M. C. Saux, D. Chassard, and B. Allaouchiche. 2002. Diffusion of isepamicin into cancellous and cortical bone tissue. J. Chemother. 14:361-365. [DOI] [PubMed] [Google Scholar]
- 11.Breilh, D., E. Boselli, J. C. Bel, D. Chassard, M. C. Saux, and B. Allaouchiche. 2003. Diffusion of cefepime into cancellous and cortical bone tissue. J. Chemother. 15:134-138. [DOI] [PubMed] [Google Scholar]
- 12.Bulitta, J. B., S. B. Duffull, M. Kinzig-Schippers, U. Holzgrabe, U. Stephan, G. L. Drusano, and F. Sorgel. 2007. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 51:2497-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 14.Cotran, R. S., V. Kumar, T. Collins, et al. 1994. Robbins pathological basis of disease. W. B. Saunders Co., Philadelphia, PA.
- 15.Cowin, S. C. 2001. Bone mechanics handbook, 2nd ed. CRC Press, Inc., Boca Raton, FL.
- 16.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Daly, R. C., R. H. Fitzgerald, Jr., and J. A. Washington II. 1982. Penetration of cefazolin into normal and osteomyelitic canine cortical bone. Antimicrob. Agents Chemother. 22:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djabarouti, S., E. Boselli, B. Allaouchiche, B. Ba, A. T. Nguyen, J. B. Gordien, J. M. Bernadou, M. C. Saux, and D. Breilh. 2004. Determination of levofloxacin in plasma, bronchoalveolar lavage and bone tissues by high-performance liquid chromatography with ultraviolet detection using a fully automated extraction method. J. Chromatogr. B 799:165-172. [DOI] [PubMed] [Google Scholar]
- 19.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat. Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 20.Drusano, G. L., S. L. Preston, M. H. Gotfried, L. H. Danziger, and K. A. Rodvold. 2002. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 46:586-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drusano, G. L., S. L. Preston, M. Van Guilder, D. North, M. Gombert, M. Oefelein, L. Boccumini, B. Weisinger, M. Corrado, and J. Kahn. 2000. A population pharmacokinetic analysis of the penetration of the prostate by levofloxacin. Antimicrob. Agents Chemother. 44:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueiredo, R., E. Valmaseda-Castellon, D. M. Laskin, L. Berini-Aytes, and C. Gay-Escoda. 2008. Treatment of delayed-onset infections after impacted lower third molar extraction. J. Oral Maxillofac. Surg. 66:943-947. [DOI] [PubMed] [Google Scholar]
- 23.Gisby, J., A. S. Beale, J. E. Bryant, and C. D. Toseland. 1994. Staphylococcal osteomyelitis—a comparison of co-amoxiclav with clindamycin and flucloxacillin in an experimental rat model. J. Antimicrob. Chemother. 34:755-764. [DOI] [PubMed] [Google Scholar]
- 24.Grimer, R. J., M. R. Karpinski, J. M. Andrews, and R. Wise. 1986. Penetration of amoxycillin and clavulanic acid into bone. Chemotherapy 32:185-191. [DOI] [PubMed] [Google Scholar]
- 25.Guyton, A. C., and J. E. Hall. 1996. Textbook of medical physiology, 9th ed. W. B. Saunders Co., Philadelphia, PA.
- 26.Hall, B. B., and R. H. Fitzgerald, Jr. 1983. The pharmacokinetics of penicillin in osteomyelitic canine bone. J. Bone Joint Surg. Am. 65:526-532. [PubMed] [Google Scholar]
- 27.Hardman, J. G., L. E. Limbird, P. B. Molinoff, R. W. Ruddon, and A. Goodman Gilman. 1996. Goodman & Gilman's the pharmacological basis of therapeutics, 9th ed. McGraw-Hill, New York, NY.
- 28.Hoban, D. J., S. K. Bouchillon, J. L. Johnson, G. G. Zhanel, D. L. Butler, K. A. Saunders, L. A. Miller, and J. A. Poupard. 2003. Comparative in vitro potency of amoxycillin-clavulanic acid and four oral agents against recent North American clinical isolates from a global surveillance study. Int. J. Antimicrob. Agents 21:425-433. [DOI] [PubMed] [Google Scholar]
- 29.Hoffken, G., H. Tetzel, P. Koeppe, and H. Lode. 1985. Pharmacokinetics and serum bactericidal activity of ticarcillin and clavulanic acid. J. Antimicrob. Chemother. 16:763-771. [DOI] [PubMed] [Google Scholar]
- 30.Horber, F. F., F. J. Frey, C. Descoeudres, A. T. Murray, and F. C. Reubi. 1986. Differential effect of impaired renal function on the kinetics of clavulanic acid and amoxicillin. Antimicrob. Agents Chemother. 29:614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson, M. C., W. K. Ramp, N. C. Nicholson, A. S. Williams, and M. T. Nousiainen. 1995. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb. Pathog. 19:409-419. [DOI] [PubMed] [Google Scholar]
- 32.Hughes, S., R. Davies, R. Khan, and P. Kelly. 1978. Fluid space in bone. Clin. Orthop. Relat. Res., issue 134, p. 332-341. [PubMed]
- 33.Hughes, S. P. 1992. Antibiotic penetration into bone in relation to the immediate management of open fractures: a review. Acta Orthop. Belg. 58(Suppl. 1):217-221. [PubMed] [Google Scholar]
- 34.Hughes, S. P., and F. M. Anderson. 1985. Penetration of antibiotics into bone. J. Antimicrob. Chemother. 15:517-519. [DOI] [PubMed] [Google Scholar]
- 35.Jevon, M., C. Guo, B. Ma, N. Mordan, S. P. Nair, M. Harris, B. Henderson, G. Bentley, and S. Meghji. 1999. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect. Immun. 67:2677-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landersdorfer, C. B., J. B. Bulitta, M. Kinzig, U. Holzgrabe, and F. Sorgel. 2009. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharmacokinet. 48:89-124. [DOI] [PubMed] [Google Scholar]
- 37.Landersdorfer, C. B., M. Kinzig, F. F. Hennig, J. B. Bulitta, U. Holzgrabe, G. L. Drusano, F. Sorgel, and J. Gusinde. 17 February 2009. Penetration of moxifloxacin into bone evaluated by Monte Carlo simulation. Antimicrob. Agents Chemother. doi: 10.1128/AAC.01056-08. [DOI] [PMC free article] [PubMed]
- 38.Lew, D. P., and F. A. Waldvogel. 2004. Osteomyelitis. Lancet 364:369-379. [DOI] [PubMed] [Google Scholar]
- 39.Lew, D. P., and F. A. Waldvogel. 1997. Osteomyelitis. N. Engl. J. Med. 336:999-1007. [DOI] [PubMed] [Google Scholar]
- 40.Lipsky, B. A., P. D. Baker, G. C. Landon, and R. Fernau. 1997. Antibiotic therapy for diabetic foot infections: comparison of two parenteral-to-oral regimens. Clin. Infect. Dis. 24:643-648. [DOI] [PubMed] [Google Scholar]
- 41.Lunke, R. J., R. H. Fitzgerald, Jr., and J. A. Washington II. 1981. Pharmacokinetics of cefamandole in osseous tissue. Antimicrob. Agents Chemother. 19:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malincarne, L., M. Ghebregzabher, M. V. Moretti, A. M. Egidi, B. Canovari, G. Tavolieri, D. Francisci, G. Cerulli, and F. Baldelli. 2006. Penetration of moxifloxacin into bone in patients undergoing total knee arthroplasty. J. Antimicrob. Chemother. 57:950-954. [DOI] [PubMed] [Google Scholar]
- 43.Meissner, A., K. Borner, and P. Koeppe. 1990. Concentrations of ofloxacin in human bone and in cartilage. J. Antimicrob. Chemother. 26(Suppl. D):69-74. [DOI] [PubMed] [Google Scholar]
- 44.Meissner, A., R. Haag, and R. Rahmanzadeh. 1989. Adjuvant fosfomycin medication in chronic osteomyelitis. Infection 17:146-151. [DOI] [PubMed] [Google Scholar]
- 45.Morris, M. A., J. A. Lopez-Curto, S. P. Hughes, K. N. An, J. B. Bassingthwaighte, and P. J. Kelly. 1982. Fluid spaces in canine bone and marrow. Microvasc. Res. 23:188-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mouton, J. W., M. N. Dudley, O. Cars, H. Derendorf, and G. L. Drusano. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601-607. [DOI] [PubMed] [Google Scholar]
- 47.Mouton, J. W., U. Theuretzbacher, W. A. Craig, P. M. Tulkens, H. Derendorf, and O. Cars. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235-237. [DOI] [PubMed] [Google Scholar]
- 48.Mutschler, E., G. Thews, and P. Vaupel. 1999. Anatomie, Physiologie, Pathophysiologie des Menschen, 5th ed. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart, Germany.
- 49.Nilsson-Ehle, I., H. Fellner, S. A. Hedstrom, P. Nilsson-Ehle, and J. Sjovall. 1985. Pharmacokinetics of clavulanic acid, given in combination with amoxycillin, in volunteers. J. Antimicrob. Chemother. 16:491-498. [DOI] [PubMed] [Google Scholar]
- 50.Pignanelli, M., F. Santoro, F. Fraschini, and F. Scaglione. 1981. Concentration of antibiotics in various jaw tissues: concentration of amoxicillin. Dent. Cadmos. 49:17-22. (In Italian.) [PubMed] [Google Scholar]
- 51.Piotrovskij, V. K., G. Paintaud, G. Alvan, and T. Trnovec. 1994. Modeling of the saturable time-constrained amoxicillin absorption in humans. Pharm. Res. 11:1346-1351. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez-Garces, M. A., and C. Gay-Escoda. 2004. Periimplantitis. Med. Oral Patol. Oral Cir. Bucal. 9(Suppl.):69-74; 63-69. [PubMed] [Google Scholar]
- 53.Sjovall, J., G. Alvan, and B. Huitfeldt. 1986. Intra- and inter-individual variation in pharmacokinetics of intravenously infused amoxycillin and ampicillin to elderly volunteers. Br. J. Clin. Pharmacol. 21:171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weismeier, K., D. Adam, H. D. Heilmann, and P. Koeppe. 1989. Penetration of amoxycillin/clavulanate into human bone. J. Antimicrob. Chemother. 24(Suppl. B):93-100. [DOI] [PubMed] [Google Scholar]
- 55.Wittmann, D. H. 1980. Chemotherapeutic principles of difficult-to-treat infections in surgery. II. Bone and joint infections. Infection 8:330-333. [Google Scholar]