Abstract
Nonmammalian model systems of infection such as Galleria mellonella (caterpillars of the greater wax moth) have significant logistical and ethical advantages over mammalian models. In this study, we utilize G. mellonella caterpillars to study host-pathogen interactions with the gram-negative organism Acinetobacter baumannii and determine the utility of this infection model to study antibacterial efficacy. After infecting G. mellonella caterpillars with a reference A. baumannii strain, we observed that the rate of G. mellonella killing was dependent on the infection inoculum and the incubation temperature postinfection, with greater killing at 37°C than at 30°C (P = 0.01). A. baumannii strains caused greater killing than the less-pathogenic species Acinetobacter baylyi and Acinetobacter lwoffii (P < 0.001). Community-acquired A. baumannii caused greater killing than a reference hospital-acquired strain (P < 0.01). Reduced levels of production of the quorum-sensing molecule 3-hydroxy-C12-homoserine lactone caused no change in A. baumannii virulence against G. mellonella. Treatment of a lethal A. baumannii infection with antibiotics that had in vitro activity against the infecting A. baumannii strain significantly prolonged the survival of G. mellonella caterpillars compared with treatment with antibiotics to which the bacteria were resistant. G. mellonella is a relatively simple, nonmammalian model system that can be used to facilitate the in vivo study of host-pathogen interactions in A. baumannii and the efficacy of antibacterial agents.
As a consequence of its immense ability to upregulate or acquire antibiotic drug resistance determinants and survive for prolonged periods within the hospital environment, Acinetobacter has emerged as one of the most troublesome pathogens for health care institutions worldwide (16). The organism has a predilection for infecting critically ill patients, with pneumonia and bloodstream infection being the most common infection types (16). In tropical climates, highly pathogenic strains of Acinetobacter infect humans in the community, leading to a distinct and often fulminant clinical syndrome characterized by severe pneumonia and septic shock (11). Thus far, the pathogenic mechanisms of Acinetobacter infection remain poorly understood.
Galleria mellonella is the caterpillar of the greater wax moth and has been utilized to study host-pathogen interactions in a range of organisms including Pseudomonas aeruginosa (8, 12), Burkholderia cepacia (20), Burkholderia mallei (19), Proteus mirabilis (13), Francisella tularensis (2), Bacillus cereus (7), and several pathogenic fungi (6, 14, 18). Importantly, a correlation between the virulence of an organism in G. mellonella and that in mammalian models has been established (8, 14). Recently, the use of an invertebrate model system, in particular Caenorhabditis elegans, for the study of Acinetobacter pathogenesis was described (17, 21). However, certain limitations exist with this model that are resolved with the use of G. mellonella. First, G. mellonella caterpillars can be maintained at temperatures of 37°C and, thus, are well suited to study human pathogens. Second, the infection inoculum can be precisely administered through an injection into the body of the caterpillar. Third, G. mellonella caterpillars have both humoral and cellular immune response pathways mediated by antimicrobial peptides and phagocytic cells (hemocytes), respectively, enabling an assessment of host responses (9). Finally, and of great novelty, the G. mellonella infection model is amenable to antibiotic treatment, and thus, the efficacy of antimicrobial agents can be assessed (2, 14). The objectives of the current study were to utilize G. mellonella caterpillars to study host-pathogen interactions involved in Acinetobacter infection and determine whether the efficacy of antimicrobial treatment can be assessed.
MATERIALS AND METHODS
Strains and media.
The bacterial strains used in this study are shown in Table 1. Bacteria were cultured on Luria-Bertani (LB) agar or broth aerobically at 37°C. Heat-killed Acinetobacter cells were prepared by exposure to 80°C for 90 min. For assessments of Acinetobacter supernatant, bacterial cells were grown overnight in LB broth at 37°C in a roller drum. The cells were spun down, and the resulting supernatant was filter sterilized using a 0.22-μm filter (Millipore). Organism identification and susceptibility testing were performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines (5). Green fluorescent protein (GFP)-linked A. baumannii (reference strain ATCC 19606) was constructed by the introduction of broad-host-range plasmid pHC60 through triparental mating (4). In brief, 100 μl of a culture of Escherichia coli containing pHC60 (tetracycline resistance marker) grown overnight was mixed with 100 μl of E. coli strain MT616 containing helper plasmid pRK600 and 100 μl of A. baumannii ATCC 19606. One hundred microliters of this mixture was spotted onto LB agar and incubated overnight at 37°C. The following day, the mating mixture was streaked onto LB agar containing tetracycline (10 μg/ml) and ampicillin (100 μg/ml) to select for A. baumannii colonies containing pHC60.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristic | Reference or source |
|---|---|---|
| A. baumannii ATCC 17978 | Reference strain | |
| A. baylyi PS8004 | Reference strain | Schimmel Laboratory, The Scripps Research Institute, CA |
| A. lwoffii ATCC 15309 | Reference strain | |
| A. baumannii strain A9844 | Recent clinical strain, carbapenem susceptible | 17 |
| A. baumannii strain A3587 | Recent clinical strain, carbapenem resistant | This study |
| A. baumannii strain HK4620 | Community-acquired A. baumannii clinical strain | Ho Laboratory, Department of Microbiology, University of Hong Kong |
| A. baumannii strain M2 | Hospital-acquired clinical strain | 15 |
| A. baumannii aba1::Km mutant | Targeted knockout mutant of the abaI gene from parent strain M2 | 15 |
G. mellonella killing assay.
G. mellonella caterpillars in the final-instar larval stage (Vanderhorst, Inc., St. Mary's, OH) were stored in the dark and used within 7 days from shipment. Caterpillars 250 mg to 350 mg in weight were employed in all assays. Sixteen randomly chosen caterpillars were used for each group of an experiment. Prior to inoculation into G. mellonella caterpillars, bacterial cells were washed with phosphate-buffered saline (PBS) and then diluted to an appropriate cell density, as determined by the optical density at 600 nm. A 10-μl Hamilton syringe was used to inject 10-μl aliquots of the inoculum into the hemocoel of each caterpillar via the last left proleg (14). Bacterial colony counts on LB agar were used to confirm all inocula. Experiments comparing two or more bacterial strains in which the inoculum of one of the strains was >0.5 log CFU/larva different from its comparator were discarded and repeated. After injection, caterpillars were incubated in plastic containers, and the number of dead caterpillars was scored daily for 6 days. Caterpillars were considered dead when they displayed no movement in response to touch.
For all experiments, two control groups were used: the first group included caterpillars that were inoculated with PBS to monitor for killing due to physical trauma; the second group included caterpillars that received no injection. Experiments that had more than two dead caterpillars in either control group were discarded and repeated. For simplicity, the control groups were not included in the figures. Survival curves were plotted using the Kaplan-Meier method, and differences in survival were calculated by using the log-rank test (STATA 6). A P value of <0.05 was considered to be statistically significant. All G. mellonella killing experiments were performed at least twice. Representative experiments are presented.
Microscopy of hemocytes.
To determine the association between host phagocytic cells (hemocytes) and GFP-linked A. baumannii, the hemolymph was collected from 6 to 10 caterpillars 24 h after infection and was fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA). Light microscopy and fluorescent microscopy were then performed using an Olympus BX51 microscope and Vectashield mounting medium (Vector Laboratories, Youngstown, OH).
Administration of antibiotics.
G. mellonella caterpillars were infected with a lethal inoculum of A. baumannii, and antibiotics were then administered by injection into a different proleg within 30 min. G. mellonella caterpillars that were injected twice with PBS were used as a further control group for these experiments. The antibiotics and doses included cefotaxime (150 mg/kg of body weight), tetracycline (50 mg/kg), gentamicin (6 mg/kg), and meropenem (60 mg/kg). Doses were based on those used for humans. Antibiotics were purchased from Sigma and were prepared according to the manufacturer's guidelines.
RESULTS AND DISCUSSION
Lethal G. mellonella infection by A. baumannii.
To determine whether G. mellonella was a suitable model to study Acinetobacter pathogenesis, we first defined its infection characteristics. As shown in Fig. 1A, inoculation of G. mellonella with a reference strain of A. baumannii (ATCC 17978) resulted in the rapid killing of the caterpillars. Killing was significantly dependent on the number of A. baumannii cells injected (Fig. 1A). For example, more than 75% of the G. mellonella caterpillars were killed within 48 h of infection with at least 3.7 × 105 CFU/larva, whereas very few G. mellonella caterpillars were killed with 3.7 × 104 CFU/larva or less (P < 0.01) (Fig. 1A). Of interest, it was previously shown that nonpathogenic microbial species, including the auxotrophic Escherichia coli strain OP50, Saccharomyces cerevisiae, and some strains of Aspergillus fumigatus, do not kill G. mellonella at inocula of up to 1 × 107 CFU/larva (2). These data show the pathogenic potential of A. baumannii against G. mellonella and indicate the viability of studying the G. mellonella-Acinetobacter infection model further.
FIG. 1.
G. mellonella killing by A. baumannii is dependent on the inoculum and the incubation temperature postinfection. Killing was more pronounced at 37°C (A) than at 30°C (B) (P = 0.01 for comparison of an inoculum of 105 CFU/larva at 37°C and 30°C).
An important benefit of G. mellonella over other invertebrate hosts such as C. elegans is its ability to be incubated at temperatures of up to 37°C, a more suitable thermal environment to study human pathogens. We observed that G. mellonella killing by A. baumannii (ATCC 17978) was dependent on the incubation temperature following infection, whereby the rate of killing was slower at 30°C (Fig. 1B) than at 37°C (Fig. 1A). For example, fewer than 50% of the G. mellonella caterpillars were killed at 3.7 × 105 CFU/larva when incubated at 30°C, compared with greater than 80% when incubated at 37°C (P = 0.01) (Fig. 1). No difference in A. baumannii growth kinetics was observed at these temperatures (data not shown). These data suggest that either A. baumannii has temperature-sensitive virulence traits or that G. mellonella caterpillars are less susceptible to infection at the lower temperature. Similar findings were described previously for other pathogens (14).
To evaluate whether G. mellonella killing by A. baumannii was due to a noninfectious process, we determined the rate of survival of G. mellonella caterpillars after inoculation with washed, heat-killed A. baumannii cells (ATCC 17978). No killing was observed (data not shown). Furthermore, to determine whether a bacterial secretory product was involved in G. mellonella killing, we inoculated G. mellonella caterpillars with an A. baumannii culture filtrate taken from stationary-phase growth. Similarly, no G. mellonella killing was observed (data not shown). These data suggest that infection with live bacterial cells is required for A. baumannii pathogenesis in G. mellonella caterpillars. Given the above-described killing characteristics, for further G. mellonella experiments, we used inocula of 105 to 106 CFU/larva and incubated the infected caterpillars at 37°C.
Differences in pathogenicities of Acinetobacter species.
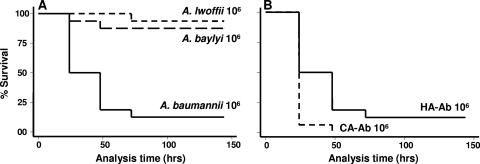
Thus far, there have been 31 validly described species of Acinetobacter (16). Of all species, A. baumannii has proven to be the most clinically important, with the greatest potential for outbreaks (16). Despite a recent report of human infection with Acinetobacter baylyi (3), it is considered to be significantly less pathogenic to humans. Also, other species such as Acinetobacter lwoffii, which commonly colonize the skin of humans, rarely cause serious infection. To determine whether such differences in pathogenicity to humans are represented in the G. mellonella-Acinetobacter infection model, we infected G. mellonella caterpillars with these species. As shown in Fig. 2A, a similar pattern of species pathogenicity was observed in G. mellonella, whereby A. baumannii caused significantly greater killing than did A. baylyi and A. lwoffii (P < 0.001). These functional pathogenicity differences between Acinetobacter species have been characterized further in comparative genomic studies with A. baumannii (ATCC 17978) and A. baylyi, whereby unique pathogenicity islands were found in the former (21). The correlation between species pathogenicity in humans and G. mellonella further supports the utility of this infection model system.
FIG. 2.
Acinetobacter is important for pathogenesis to G. mellonella (A), whereby the most important human pathogen, A. baumannii, killed significantly (P < 0.001) more than the less-common human pathogens A. baylyi and A. lwoffii. (B) Furthermore, the highly virulent community-acquired A. baumannii (CA-Ab) strain was more pathogenic than the reference hospital strain (HA-Ab) (ATCC 17978) (P = 0.005).
Pathogenic community-acquired A. baumannii infection.
Apart from interspecies differences in pathogenicity, within-species differences have also been observed with A. baumannii strains from different geographic regions. The most pronounced of these is the infectious syndrome caused by community-acquired A. baumannii, a distinct clinical entity reported from tropical climates characterized by high rates of bacteremia, acute respiratory distress syndrome, disseminated intravascular coagulation, and death (1, 11). Interestingly, we observed greater G. mellonella killing with a community-acquired A. baumannii strain (HK4620) than with our reference hospital-acquired A. baumannii strain (ATCC 17978) (P < 0.01) (Fig. 2B), indicating a further similarity of A. baumannii pathogenesis in G. mellonella and humans. Thus far, the pathogenic mechanisms of community-acquired A. baumannii remain unknown.
Quorum sensing and virulence of A. baumannii.
Recently, a quorum-sensing gene known as abaI in A. baumannii was described and was found to control the production of the homoserine lactone molecule 3-hydroxy-C12-homoserine lactone (15). In other gram-negative bacteria such as Pseudomonas aeruginosa, quorum sensing is an important virulence determinant that has been associated with biofilm formation and the production of pathogenic toxins (10). A knockout mutant of the A. baumannii abaI gene was found to be impaired in the later stages of biofilm development (15). To explore whether this quorum-sensing gene is an important virulence determinant for G. mellonella, we assessed the virulence of the recently described A. baumannii aba1::Km quorum-sensing mutant compared to that of its isogenic parent strain (M2) (15). No difference in G. mellonella killing between the aba1::Km mutant and the M2 strain was observed (data not shown). Whether this quorum-sensing gene is important for A. baumannii virulence to other hosts, including a mammalian system, is currently unknown.
Host response to A. baumannii.
Apart from the structural barrier formed by the cuticle of the caterpillar, G. mellonella mounts a multifaceted host response to pathogens (9). Hemocytes form the cellular component of this defense and function as phagocytic cells. By use of a GFP-A. baumannii strain, we observed that G. mellonella hemocytes are part of the immune response to infection by A. baumannii (Fig. 3). Increased levels of green fluorescence were observed within the G. mellonella hemocytes (Fig. 3A and B), compared with infection with an isogenic, non-GFP-expressing A. baumannii strain (Fig. 3C and D). These appearances likely represent the accumulation of bacteria within the phagolysosomes of the hemocytes. Remarkable similarities between vertebrate and insect innate immune responses to infection exist (9), and these data indicate the value of using G. mellonella to further study such responses to A. baumannii. Understanding the surface antigenic properties of A. baumannii required for phagocytosis is an area of future research.
FIG. 3.
A. baumannii is engulfed by G. mellonella hemocytes after infection. Light and fluorescent microscopy images are shown of GFP-A. baumannii (A and B) and the non-GFP parent strain (ATCC 19606) (C and D). Increased green fluorescence of hemocytes is seen with GFP-A. baumannii. Low-level autofluorescence is seen in D.
Melanization in G. mellonella is also thought to be a key part of the defense against a range of pathogens (9). Melanin is deposited around microbes within the hemolymph and is thought to facilitate pathogen killing (9). Over the course of A. baumannii infection, caterpillars demonstrated obvious signs of melanization (Fig. 4).
FIG. 4.
Melanization of G. mellonella is part of the infection process with A. baumannii. The image was taken 24 h after infection with 1.6 × 106 CFU/larva of A. baumannii ATCC 17978. Two dead caterpillars were removed prior to photograph.
Antibacterial efficacy in the G. mellonella-A. baumannii infection model.
It was previously shown that the administration of antimicrobials can successfully treat a lethal G. mellonella infection (2, 14). This in vivo system has obvious logistical and ethical advantages over testing antimicrobial agents in mammals. To determine whether the G. mellonella-A. baumannii infection model could be used to study the in vivo activity of antibacterial agents, we investigated the effect of treating A. baumannii-infected G. mellonella caterpillars with a single dose of gentamicin (6 mg/kg), cefotaxime (150 mg/kg), tetracycline (50 mg/kg), or meropenem (60 mg/kg). Initially, we infected G. mellonella caterpillars with a clinical A. baumannii strain (A9844) that was known to be susceptible to meropenem and gentamicin but resistant to tetracycline and cefotaxime. Treatment of a lethal A. baumannii infection with antibiotics that had in vitro activity against the infecting A. baumannii strain significantly prolonged the rate of survival of G. mellonella caterpillars compared with treatment with antibiotics for which the bacteria were resistant (P < 0.001) (Fig. 5). In contrast, when G. mellonella caterpillars were infected with a carbapenem-resistant A. baumannii strain (A3587), meropenem was not effective at prolonging G. mellonella survival (≤12% survival) (data not shown). Thus, these data indicate that the G. mellonella-A. baumannii infection model can be used to study the efficacy of antibacterial agents and, importantly, may provide a more simplistic system than mammalian models.
FIG. 5.
Antibacterials that are active against A. baumannii (Ab) can prolong the survival of A. baumannii-infected G. mellonella caterpillars. After infection with a lethal dose of A. baumannii strain A9844 (5 × 105 CFU/larva), meropenem (MER) and gentamicin (Gm), to which the strain was susceptible, significantly prolonged the survival of G. mellonella caterpillars (P < 0.001 for comparison with no antibiotics). However, cefotaxime (CTX) and tetracycline (TET), to which the strain was resistant, caused no difference in killing compared with no antibiotic treatment (P = 0.41). A. baumannii (Ab) with no antibiotic designation was the untreated control.
The contributions to biomedical science of using invertebrate hosts to study microbial pathogenesis and host responses have been substantial over the last 10 years. In this study, we describe the utility of G. mellonella to not only study host-pathogen interactions in A. baumannii but also assess the efficacy of antibiotic treatment. Very little is known about the pathogenic mechanisms of A. baumannii, and thus, the infection model system described herein serves as a useful tool for future research. Interestingly, we observed a correlation between the pathogenicity of Acinetobacter in G. mellonella and that which we observed in humans. The infection model described here also has the potential to be used to study novel compounds for in vivo activity against A. baumannii without the logistical, ethical, and financial barriers that exist with mammalian models.
Acknowledgments
We thank Beth Fuchs for her support and guidance with Galleria mellonella experiments.
Support was provided by NIH K08 award AI63084 and R01 award AI075286 (E.M.), a new scholar award in global infectious diseases from the Ellison Medical Foundation (E.M.), and a University of Queensland postgraduate scholarship award (A.Y.P.).
A.Y.P. has served as a consultant for Abbott Molecular. G.M.E. has served as a consultant for and received research funding support from Cubist Pharmaceuticals and Pfizer, Inc. R.C.M. has served as a consultant to Pfizer, Cubist, and Wyeth and received research funding support from Cubist Pharmaceuticals. E.M. has served as a consultant for Biogen Idec., received research support from Astellas Pharma Inc., and is a member of the Speaker's Bureau for Pfizer, Inc. All other authors have no conflicts of interest.
Footnotes
Published ahead of print on 30 March 2009.
REFERENCES
- 1.Anstey, N. M., B. J. Currie, and K. M. Withnall. 1992. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin. Infect. Dis. 14:83-91. [DOI] [PubMed] [Google Scholar]
- 2.Aperis, G., B. B. Fuchs, C. A. Anderson, J. E. Warner, S. B. Calderwood, and E. Mylonakis. 2007. Galleria mellonella as a model host to study infection by the Francisella tularensis live vaccine strain. Microbes Infect. 9:729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, T. L., L. K. Siu, Y. T. Lee, C. P. Chen, L. Y. Huang, R. C. Wu, W. L. Cho, and C. P. Fung. 2008. Acinetobacter baylyi as a pathogen for opportunistic infection. J. Clin. Microbiol. 46:2938-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180:5183-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2007. Dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A7. CLSI, Wayne, PA.
- 6.Cotter, G., S. Doyle, and K. Kavanagh. 2000. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol. Med. Microbiol. 27:163-169. [DOI] [PubMed] [Google Scholar]
- 7.Fedhila, S., N. Daou, D. Lereclus, and C. Nielsen-LeRoux. 2006. Identification of Bacillus cereus internalin and other candidate virulence genes specifically induced during oral infection in insects. Mol. Microbiol. 62:339-355. [DOI] [PubMed] [Google Scholar]
- 8.Jander, G., L. G. Rahme, and F. M. Ausubel. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 182:3843-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavanagh, K., and E. P. Reeves. 2004. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol. Rev. 28:101-112. [DOI] [PubMed] [Google Scholar]
- 10.Kirisits, M. J., and M. R. Parsek. 2006. Does Pseudomonas aeruginosa use intercellular signalling to build biofilm communities? Cell. Microbiol. 8:1841-1849. [DOI] [PubMed] [Google Scholar]
- 11.Leung, W. S., C. M. Chu, K. Y. Tsang, F. H. Lo, K. F. Lo, and P. L. Ho. 2006. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest 129:102-109. [DOI] [PubMed] [Google Scholar]
- 12.Miyata, S., M. Casey, D. W. Frank, F. M. Ausubel, and E. Drenkard. 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 71:2404-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton, D. B., R. I. Barnett, and J. S. Chadwick. 1984. Structural alterations to Proteus mirabilis as a result of exposure to haemolymph from the larvae of Galleria mellonella. Microbios 39:177-185. [PubMed] [Google Scholar]
- 14.Mylonakis, E., R. Moreno, J. B. El Khoury, A. Idnurm, J. Heitman, S. B. Calderwood, F. M. Ausubel, and A. Diener. 2005. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 73:3842-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niu, C., K. M. Clemmer, R. A. Bonomo, and P. N. Rather. 2008. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J. Bacteriol. 190:3386-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peleg, A. Y., H. Seifert, and D. L. Paterson. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peleg, A. Y., E. Tampakakis, B. B. Fuchs, G. M. Eliopoulos, R. C. Moellering, Jr., and E. Mylonakis. 2008. Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105:14585-14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves, E. P., C. G. Messina, S. Doyle, and K. Kavanagh. 2004. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 158:73-79. [DOI] [PubMed] [Google Scholar]
- 19.Schell, M. A., L. Lipscomb, and D. DeShazer. 2008. Comparative genomics and an insect model rapidly identify novel virulence genes of Burkholderia mallei. J. Bacteriol. 190:2306-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seed, K. D., and J. J. Dennis. 2008. Development of Galleria mellonella as an alternative infection model for the Burkholderia cepacia complex. Infect. Immun. 76:1267-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, M. G., T. A. Gianoulis, S. Pukatzki, J. J. Mekalanos, L. N. Ornston, M. Gerstein, and M. Snyder. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21:601-614. [DOI] [PMC free article] [PubMed] [Google Scholar]