Abstract
ST-246, a potent orthopoxvirus egress inhibitor, is safe and effective at preventing disease and death in studies of small-animal models involving challenge by several different pathogenic poxviruses. In this report, the antiviral efficacy of ST-246 in treatment of nonhuman primates infected with variola virus or monkeypox virus was assessed. The data indicate that oral dosing once per day with ST-246 protects animals from poxvirus disease, as measured by reductions in viral load and numbers of lesions and enhancement of survival.
Smallpox, which is caused by variola virus (VAR) infection, is a potentially devastating agent of bioterrorism and a serious threat to human life in the event of its deliberate or accidental release (4, 8, 22). Likewise, monkeypox virus (MPX) causes similar, though less frequently lethal, disease in humans (5, 6, 13, 16). Unlike VAR, MPX has not been eradicated. Rather, it is an emerging pathogen in Africa and it has already made an infectious incursion into North America (1, 16). Infections with either pathogen can be effectively prevented by prophylactic use of a licensed smallpox vaccine; however, potential serious side effects (e.g., eczema vaccinatum, progressive vaccinia, myocarditis, and death) limit its use in the general population in the absence of a verified threat, and the vaccine is contraindicated for use in the treatment of immunocompromised individuals, pregnant women, and individuals with atopic dermatitis. Moreover, the vaccine is only effective when given within a few days after exposure to either VAR or MPX (14). While safer vaccines based on attenuated vaccinia virus strains are being developed, they are not currently on the market. Given the prolonged prodrome of these human infections, if a sentinel case of either disease were to be detected, it is probable that a large number of individuals would be harboring infections for which vaccination would provide no benefit. Thus, it has been imperative to develop an antiviral drug for the prevention and/or treatment of VAR or MPX infections and related orthopoxvirus infections to protect individuals who cannot receive the smallpox vaccine and to protect infected nonvaccinated individuals.
ST-246 is a novel orthopoxvirus egress inhibitor discovered as part of a high-throughput screening procedure designed to identify inhibitors of vaccinia virus-induced cytopathic effects. Following hit-to-lead optimization, ST-246 was identified as a nanomolar inhibitor of poxvirus replication, with a favorable therapeutic index (>5,000) in vitro (23). The antiviral activity of ST-246 is specific for orthopoxviruses, including VAR and MPX, and the compound does not inhibit the replication of other RNA- and DNA-containing viruses or inhibit cell proliferation at concentrations of the compound that exhibit an antiviral effect. ST-246 targets vaccinia virus p37, a highly conserved orthopoxvirus protein required for envelopment and secretion of extracellular forms of virus. Extracellular forms of virus play a central role in viral pathogenesis (20). Preclinical safety pharmacology studies of mice and nonhuman primates (NHP) indicate that ST-246 is readily absorbed following oral administration and well tolerated, with the no-observable-effect level for mice measured at 2,000 mg/kg of body weight and the no-observable-adverse-effect level for NHP measured at 300 mg/kg. Human phase I clinical trials have shown that ST-246 is safe and well tolerated by healthy human volunteers (9). Human studies to assess antiviral efficacy of ST-246 are neither feasible nor ethical; thus, regulatory approval will require the use of appropriate animal models.
To date, ST-246 has been shown to uniformly protect animals from orthopoxvirus disease and death (references 3, 8, 15, 17, 18, 19, and 23 and data not shown). The robust antiviral activity of ST-246 is further established by the fact that the animal model studies have been conducted by multiple investigators in different laboratories with a variety of animal species and by different challenge routes. Models of orthopoxvirus disease were developed using mice (including BALB/c, NMRI, ANC/R, and Nu/nu strains), rabbits, prairie dogs, and ground squirrels. These models provided opportunities to evaluate the antiviral activity of ST-246 against multiple species of orthopoxviruses, including vaccinia virus strains IHD-J, Lister, and WR, ectromelia virus strain Moscow and a highly virulent recombinant strain of ectromelia virus that expressed a mouse interleukin-4 gene, cowpox virus, rabbitpox virus, and MPX. Infections were established by a variety of routes, including intranasal, intravenous (IV), intradermal, subcutaneous, and aerosol delivery of virus. In all cases, ST-246 protected animals from visible disease and death. These models were used to optimize dosing strategies for antiviral efficacy, and studies were conducted to evaluate the effect of adjusting the dose level, dose duration, and time of treatment postinfection on disease outcome. From these studies, we have determined that oral dosing once per day at 300 mg/m2 (the dose level was normalized to body surface area to compare dose levels between animal species) for a period of greater than 10 days appears to be optimal for providing efficacy of protection. In models in which animals succumb to orthopoxvirus disease in 7 to 8 days, treatment with ST-246 can be initiated as late as 72 h postinfection for full protection. While these data are promising, the small-animal models have limitations, some of which include differing disease pathologies and a compressed time course of disease. Furthermore, it is unlikely that the pharmacokinetic behavior of the drug or its tissue distribution in the small animals will mirror the human condition.
To bridge this gap, we have assessed the ability of ST-246 to protect NHP from VAR or MPX challenges. IV inoculation of NHP with VAR produces a systemic lesional disease that resembles a late viremic stage of human smallpox. Disease severity is related to the amount of virus in the inoculum, with high virus loads (1 × 109 PFU) producing uniformly fatal hemorrhagic disease and lower virus loads (1 × 108 PFU) producing systemic lesional disease with approximately 30% mortality (7), which is similar to the outcome of human disease seen with VAR major infections. The IV challenge route was developed in the absence of a model utilizing a more natural route of transmission (i.e., intranasal, intratracheal, or aerosol) involving the respiratory tract, because preliminary studies have failed to produce appropriate disease through infection by those routes. The pathology of VAR infection in cynomolgus monkeys (Macaca fascicularis) inoculated by this route with 108 PFU produces a systemic lesional disease similar to that seen with smallpox infections in humans (Fig. 1). While the latent phase of infection is shorter than in humans, the disease endpoints (lesions and death) closely resemble those of human smallpox. Likewise, infection of NHP with MPX via IV inoculation with approximately 5 × 107 PFU of virus produces a uniformly lethal disease similar to the lesional disease observed with MPX or VAR infection of humans (Fig. 1). Following IV challenge with MPX, the animals develop a generalized vesiculopustular rash with other characteristics of disease, including fever, elevated white blood cell count, lymphadenopathy, splenomegaly, and pulmonary edema. The disease progresses rapidly, with death occurring between 7 and 15 days postinfection. In many respects, the MPX model represents a more rigorous challenge in that the infected animals develop a higher viral load more quickly than when infected with VAR and that the infection results in a uniformly lethal outcome. Despite the many similarities between the characteristics of these two models and those of natural infections, there are still some important differences, specifically, the large amount of virus in the inoculum (107 to 108 PFU) required to establish infection and an unnatural route entry (IV versus aerosol). However, IV inoculation recapitulates the latter stages of human disease, minus the prodrome and primary viremia, and results in immediate viremia and systemic spread of the virus, thus representing a rigorous test of efficacy for any therapeutic.
FIG. 1.
Comparison of the clinical courses of smallpox infections in humans and infected NHP. DPI, day postinoculation.
The initial study was designed to evaluate the therapeutic benefit of administration of ST-246 for cynomolgus monkeys infected IV with VAR. This study was conducted at the high-containment laboratories at the Centers for Disease Control and Prevention under a protocol approved by the World Health Organization Advisory Committee on Variola Virus Research. An oral dose of 300 mg/per kg of body weight once per day was selected to evaluate efficacy of ST-246 in this model. This dose was selected based upon pharmacokinetic parameters measured in uninfected mice, rabbits, and NHP to produce plasma drug levels predicted to be antiviral in NHP. Drug treatment was initiated at day 0 or day 1 postinoculation to evaluate the potential for therapeutic intervention. IV inoculation produces high viral loads in the blood by day 1 postinoculation, in similarity to the secondary viremia that occurs during the course of human smallpox disease, when rash and lesions first appear by day 3 postinoculation. A 14-day treatment duration was selected to be consistent with the previous preclinical efficacy studies and to ensure adequate suppression of viral replication to allow for the host immune system to react to and begin clearing the infection.
Eight monkeys (five male and three female, with a mean weight of 3.4 ± 0.4 kg) were infected with 1 × 108 PFU of the Harper strain of VAR and randomly assigned into three groups. Two monkeys were designated controls and received vehicle starting immediately after infection (i.e., day 0 postinfection), three monkeys received ST-246 administered at 300 mg/kg/day starting immediately after infection (i.e., day 0 postinfection), and three monkeys received ST-246 administered at 300 mg/kg/day starting 24 h after infection (i.e., day 1 postinfection), for a total of 14 days, followed by 5 ml/kg of a 30% suspension of hydrated homogenized monkey biscuits, because a food effect has been seen in pharmacokinetic studies. Following VAR exposure and over the course of the treatment, the infected animals were to be observed by the investigator at least twice each day for a total of up to 28 days to evaluate signs of illness. Blood samples were collected from the infected animals for virological, hematological, immunological, and chemical analyses. For those moribund animals that were euthanized or died during the study, a full necropsy was performed to collect tissues for pathological examination. At the end of the study, surviving animals were anesthetized, exsanguinated, and euthanized and a full necropsy was performed to collect tissues for pathological examination.
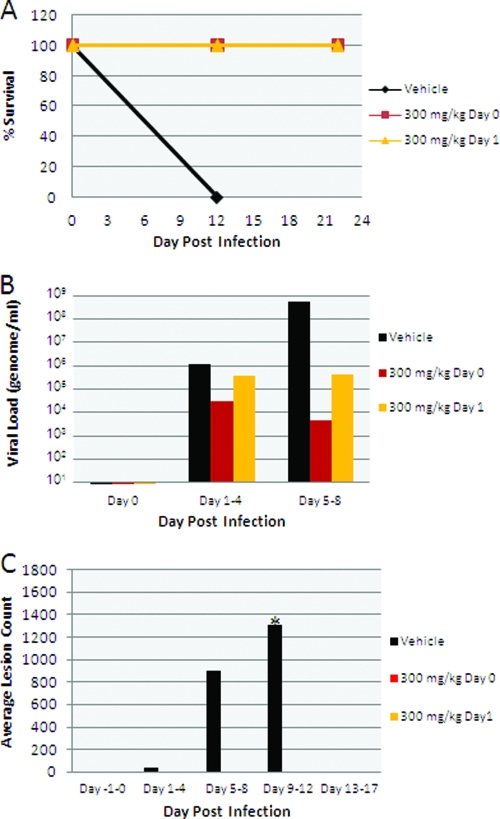
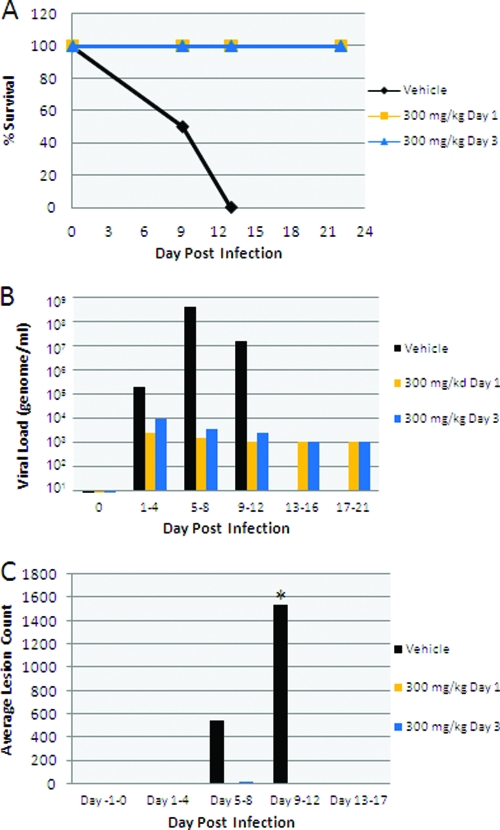
Both animals receiving vehicle alone required euthanasia on day 12 during the study period due to disease severity, while all animals receiving ST-246 at 300 mg/kg/day survived infection whether the drug was administered at the time of infection or at 24 h postinfection (Fig. 2A). Viral DNA levels in blood were significantly reduced in ST-246 treatment groups compared to vehicle treatment groups (Fig. 2B). An approximately 3- to 5-log reduction in blood DNA levels was observed for ST-246 treatment groups in the interval between days 5 and 8. ST-246 treatment resulted in a complete inhibition of lesion formation (Fig. 2C). In vehicle-treated animals, poxvirus lesions were first observed during the interval between days 1 and 4 following inoculation with the virus, with lesions first observable on the head, arm, and leg, with spreading to the rest of the body by the assessments during days 5 to 8. Animals administered ST-246 on day 0 or day 1 following inoculation did not develop any poxvirus lesions. VAR (and MPX) infection resulted in surprisingly small changes in hematology and clinical chemistry values given the severity of infection. These changes were less pronounced among animals administered vehicle compared with animals administered ST-246, as is consistent with a reduction in orthopoxvirus-induced disease as the result of ST-246 treatment (Tables 1 and 2). Reference hematology and clinical chemistry values for noninfected cynomolgus monkeys are as follows: hematocrit range, 37.3% to 48.4%; platelet count range, 236 × 103 to 665 × 103/μl; mean corpuscular hemoglobin concentration range, 28.6 to 32.9 g/dl; white blood cell count range, 7.1 × 103 to 21.7 × 103/μl; albumin range, 3.55 to 4.66 g/dl; alanine aminotransferase range, 16.04 to 85.74 U/liter; alkaline phosphatase range, 261 to 2,265 U/liter; aspartate aminotransferase range, 16 to 73 U/liter; blood urea nitrogen range, 12.9 to 31.5 mg/dl; creatinine range, 0.40 to 0.88 mg/dl, and total protein range, 6.51 to 8.36 g/dl (11). The immune response to infection on day 7 in ST-246-treated animals was similar to that seen with vehicle-treated animals, as measured by the levels of poxvirus-specific immunoglobulin M (IgM) and IgG antibodies quantified by enzyme-linked immunosorbent assay (ELISA) (Fig. 3). Since the vehicle-treated animals died after the day 7 measurement prior to induction of the IgG response and no additional data were therefore recorded for those animals at further time points, it could not be determined whether there would be differences in IgG levels in ST-246-treated animals relative to the controls.
FIG. 2.
Disease outcomes for NHP infected with 1 × 108 PFU of VAR and treated with either vehicle alone or ST-246 administered at 300 mg/kg/day starting at day 0 or day 1 postinfection. (A) Kaplan-Meier survival curve. (B) Blood viral DNA mean levels determined as previously described (2) and indicated according to treatment and postinfection day. (C) Total body lesion count mean levels indicated according to treatment and postinfection day. *, all vehicle-treated animals died by day 12, so no further lesion counts were determined for those animals.
TABLE 1.
Mean hematology, temperature, and clinical chemistry values for VAR-infected animalsa
| Animal characteristic | Value (mean ± SD)
|
|||||
|---|---|---|---|---|---|---|
| Placebo
|
Treatment day 0
|
Treatment day 1
|
||||
| 0 dpi | 11 dpi | 0 dpi | 11 dpi | 0 dpi | 11 dpi | |
| HCT (%) | 31.4 ± 1.6 | 27.5 ± 7.5 | 39.2 ± 6.5 | 44.3 ± 11.0 | 35.7 ± 4.0 | 49.6 ± 7.4 |
| PLT (103/μl) | 663 ± 81 | 440 ± 130 | 692 ± 209 | 329 ± 70 | 791 ± 112 | 341 ± 87 |
| MCHC (g/dl) | 33.8 ± 2.7 | 30.5 ± 0.8 | 33.5 ± 1.9 | 31.7 ± 0.2 | 33.9 ± 1.0 | 31.1 ± 1.1 |
| WBC (103/μl) | 15.8 ± 2.0 | 23.9 ± 15.1 | 8.7 ± 3.3 | 8.2 ± 2.1 | 8.2 ± 2.8 | 7.5 ± 2.2 |
| Temp (°C) | 38.4 ± 1.1 | 35.7 ± 1.6 | 37.2 ± 1.1 | 38.0 ± 0.6 | 37.0 ± 1.3 | 37.3 ± 0.9 |
| ALB (g/dl) | 3.35 ± 0.35 | 1.75 ± 0.49 | 3.07 ± 0.15 | 2.47 ± 0.12 | 2.77 ± 0.06 | 2.90 ± 0.10 |
| ALT (U/liter) | 70.00 ± 19.80 | 30.50 ± 7.80 | 31.00 ± 1.00 | 49.70 ± 13.50 | 41.30 ± 14.00 | 60.30 ± 28.40 |
| ALP (U/liter) | 220 ± 130 | 578 ± 129 | 322 ± 193 | 277 ± 145 | 158 ± 105 | 141 ± 56 |
| AST (U/liter) | 65 ± 23 | 45 ± 13 | 57 ± 7 | 55 ± 15 | 74 ± 29 | 58 ± 11 |
| BUN (mg/dl) | 23.0 ± 2.8 | 22.0 ± 2.8 | 18.7 ± 6.4 | 19.0 ± 3.0 | 17.0 ± 3.1 | 18.7 ± 1.5 |
| CRE (mg/dl) | 0.85 ± 0.07 | 0.60 ± 0.14 | 0.87 ± 0.12 | 0.73 ± 0.12 | 0.77 ± 0.0 | 0.70 ± 0.1 |
| TP (g/dl) | 6.85 ± 0.3 | 6.10 ± 0.71 | 6.40 ± 0.40 | 6.37 ± 0.45 | 6.27 ± 0.12 | 6.43 ± 0.42 |
HCT, hematocrit; PLT, platelet count; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell count; ALB, albumin; ALT, alanine amino transferase; ALP, alkaline phosphatase; AST, aspartate amino transferase; BUN, blood urea nitrogen; CRE, creatinine; TP, total protein; dpi, days postinfection.
TABLE 2.
Mean hematology, temperature, and clinical chemistry values for MPX-infected animalsa
| Animal characteristic | Value (mean ± SD)
|
|||||
|---|---|---|---|---|---|---|
| Placebo
|
Treatment day 1
|
Treatment day 3
|
||||
| 0 dpi | 9 dpi | 0 dpi | 9 dpi | 0 dpi | 9 dpi | |
| HCT (%) | 37.6 ± 0.3 | 34.0 ± 6.8 | 38.6 ± 0.8 | 39.5 ± 1.6 | 39.1 ± 1.9 | 35.9 ± 0.9 |
| PLT (103/μl) | 365 ± 56 | 555 ± 356 | 339 ± 67 | 350 ± 109 | 313 ± 36 | 406 ± 86 |
| MCHC (g/dl) | 32.8 ± 1.0 | 32.7 ± 2.2 | 32.4 ± 0.4 | 30.9 ± 0.6 | 32.6 ± 0.2 | 31.7 ± 0.3 |
| WBC (103/μl) | 7.2 ± 3.3 | 25.1 ± 17.5 | 6.0 ± 1.3 | 12.6 ± 5.5 | 9.1 ± 2.8 | 12.3 ± 3.6 |
| Temp (°C) | 37.1 ± 1.4 | 33.9 ± 4.5 | 37.0 ± 0.7 | 37.3 ± 1.2 | 38.0 ± 0.4 | 37.1 ± 0.4 |
| ALB (g/dl) | 3.20 ± 0.99 | 2.00 ± 0.71 | 3.27 ± 0.06 | 3.33 ± 0.40 | 3.23 ± 0.06 | 2.93 ± 0.06 |
| ALT (U/liter) | 33.00 ± 2.83 | 174.00 ± 199.40 | 49.67 ± 13.65 | 107.67 ± 41.36 | 39.00 ± 14.00 | 44.33 ± 24.01 |
| ALP (U/liter) | 394 ± 193 | 907 ± 964 | 330 ± 51 | 318 ± 70 | 334 ± 164 | 268 ± 177 |
| AST (U/liter) | 54 ± 12 | 524 ± 663 | 56 ± 2 | 71 ± 21 | 58 ± 4 | 46 ± 5 |
| BUN (mg/dl) | 15.0 ± 4.2 | 46.5 ± 36.1 | 12.7 ± 2.5 | 21.3 ± 1.5 | 13.7 ± 2.1 | 10.7 ± 1.2 |
| CRE (mg/dl) | 0.90 ± 0.00 | 2.10 ± 1.84 | 1.00 ± 0.17 | 0.90 ± 0.10 | 1.10 ± 0.10 | 0.87 ± 0.21 |
| TP (g/dl) | 7.30 ± 1.27 | 6.25 ± 0.07 | 7.57 ± 0.25 | 8.10 ± 0.17 | 7.47 ± 0.12 | 7.57 ± 0.93 |
HCT, hematocrit; PLT, platelet count; MCHC, mean corpuscular hemoglobin concentration; WBC, white blood cell count; ALB, albumin; ALT, alanine amino transferase; ALP, alkaline phosphatase; AST, aspartate amino transferase; BUN, blood urea nitrogen; CRE, creatinine; TP, total protein; dpi, days postinfection.
FIG. 3.
Antiorthopoxvirus immune response in NHP infected with 1 × 108 PFU of VAR and treated with either vehicle alone or ST-246 administered at 300 mg/kg/day (data represent averages of results determined for three vehicle-treated or six ST-246-treated animals). (A) ELISA IgM data for ST-246-treated NHP measured on days 0, 7, 15, and 21. (B) ELISA IgM data for vehicle-treated NHP measured on days 0 and 7. (C) ELISA IgG data for ST-246-treated NHP measured on days 0, 7, 15, and 21. (D) ELISA IgG data for vehicle-treated NHP measures on days 0 and 7. Five negative human serum samples were used to determine a cutoff value (COV) equal to the average optical density (OD) seen with the negative serum samples plus 3 standard deviations. The OD-COV observed for some animals at day 0 approached 0.07. This observation parallels our experience with human studies in that the results represented by this low range of values may be equivocal and as such represent the low end of the assay. The antigen in the ELISA is whole vaccinia virus purified via the use of two sucrose cushions (10). d, day.
Based on these results, a MPX challenge study was conducted at the U.S. Army Medical Research Institute for Infectious Disease under conditions of biosafety level 3 containment. The study was designed to determine whether administration of ST-246 could protect NHP from a uniformly lethal infection with MPX. Cynomolgus monkeys (male, with a mean age of 6.2 ± 0.7 years and a mean weight of 6.2 ± 1.3 kg) were infected via IV injection with the MPX Zaire '79 strain, and ST-246 was administered orally at 300 mg/kg of body weight once per day for a period of 14 days starting at 1 or 3 days postinfection and followed by 5 ml/kg of a 30% suspension of hydrated homogenized monkey biscuits. Based on previous work with this model system, by 3 days postexposure the monkeys were expected to be fully viremic and exhibiting symptoms of monkeypox disease, including fever, with one-third showing lesions and the rest on the verge of exhibiting elaborating lesional disease.
Eight monkeys were infected with 5 × 107 PFU of the Zaire '79 strain of MPX and randomly assigned into two cohorts of four monkeys each. Within each cohort, three monkeys were randomly chosen to receive ST-246 and one monkey to receive vehicle. Monkeys in cohort 1 received either ST-246 at 300 mg/kg per day or vehicle for 14 days starting on day 1 postinfection, and monkeys in cohort 2 received drug or vehicle starting on day 3 postinfection. Following MPX exposure, the infected animals were observed at least twice each day for up to 33 days for signs of illness. Blood samples were collected from the infected animals for virological, hematological, immunological, and chemical analyses. For those that died during the study, a full necropsy was performed to collect tissues for pathological examination. At the end of the study, surviving animals were anesthetized, exsanguinated, and euthanized.
As shown in Fig. 4, ST-246, when administered at 24 h or 72 h after infection, protected animals from MPX disease and death, whereas both control animals exhibited significant disease and were dead by day 13 (one vehicle control animal died on day 9 and the other on day 13). Peak viral loads greater than 108 genomes per ml were observed in the control animals between days 5 and 8. In the presence of ST-246, viral load was reduced by almost 5 logs regardless of when drug treatment was initiated (Fig. 4B). With regard to skin disease, control animals developed approximately 1,500 lesions prior to death whereas no lesions were observed in either drug-treated group (Fig. 4C).
FIG. 4.
Disease outcome for NHP infected with 5 × 107 PFU of MPX and treated with either vehicle alone or ST-246 administered at 300 mg/kg/day starting at day 1 or day 3 postinfection. (A) Kaplan-Meier survival curve. (B) Blood viral DNA mean by treatment and postinfection day. (C) Total body lesion count mean according to treatment and postinfection day. *, all vehicle-treated animals died by day 13, so no further lesion counts were determined for those animals.
Previous work with ST-246 in a number of different small-animal models of orthopoxvirus-induced disease has demonstrated it to be safe and effective. We have extended this work to NHP challenged with VAR or MPX, the two orthopoxvirus pathogens which pose the greatest threat to the human population. The results demonstrate that ST-246, even when administered at a time postinfection when disease symptoms are evident, protects NHP from disease or death, supporting the potential use of ST-246 for prophylactic or therapeutic intervention in VAR or MPX disease. This conclusion is further supported by the recent successful use of ST-246 for treatment of a patient suffering from acute eczema vaccinatum (12, 21). The NHP model is likely to be predictive of human disease outcome in that VAR- or MPX-induced disease closely resembles the human condition and the characteristics of metabolism of ST-246 in NHP and humans appear to be similar (unpublished data). Thus, this proof-of-concept experiment will lay the foundation for additional experiments to establish the pharmacokinetic and pharmacodynamic parameters necessary to establish an effective human dose.
Acknowledgments
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
Footnotes
Published ahead of print on 6 April 2009.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2003. Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb. Mortal. Wkly. Rep. 52:642-646. [PubMed] [Google Scholar]
- 2.Earl, P. L., J. L. Americo, L. S. Wyatt, L. A. Eller, J. C. Whitbeck, G. H. Cohen, R. J. Eisenberg, C. J. Hartmann, D. L. Jackson, D. A. Kulesh, M. J. Martinez, D. M. Miller, E. M. Mucker, J. D. Shamblin, S. H. Zwiers, J. W. Huggins, P. B. Jahrling, and B. Moss. 2004. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature 428:182-185. [DOI] [PubMed] [Google Scholar]
- 3.Grosenbach, D. W., R. Jordan, D. S. King, A. Berhanu, T. K. Warren, D. L. Kirkwood-Watts, S. Tyavanagimatt, Y. Tan, R. L. Wilson, K. F. Jones, and D. E. Hruby. 2008. Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination. Vaccine 26:933-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson, D. A., T. V. Inglesby, J. G. Bartlett, M. S. Ascher, E. Eitzen, P. B. Jahrling, J. Hauer, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. Perl, P. K. Russell, K. Tonat, and the Working Group on Civilian Biodefense. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127-2137. [DOI] [PubMed] [Google Scholar]
- 5.Huhn, G. D., A. M. Bauer, K. Yorita, M. B. Graham, J. Sejvar, A. Likos, I. K. Damon, M. G. Reynolds, and M. J. Kuehnert. 2005. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin. Infect. Dis. 41:1742-1751. [DOI] [PubMed] [Google Scholar]
- 6.Hutin, Y. J., R. J. Williams, P. Malfait, R. Pebody, V. N. Loparev, S. L. Ropp, M. Rodriguez, J. C. Knight, F. K. Tshioko, A. S. Khan, M. V. Szczeniowski, and J. J. Esposito. 2001. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 7:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahrling, P. B., L. E. Hensley, M. J. Martinez, J. W. Leduc, K. H. Rubins, D. A. Relman, and J. W. Huggins. 2004. Exploring the potential of variola virus infection of cynomolgus macaques as a model for human smallpox. Proc. Natl. Acad. Sci. USA 101:15196-15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahrling, P. B., John W. Huggins, M. Sofi Ibrahim, James V. Lawler, and James W. Martin. 2007. Smallpox and related orthopoxviruses, p. 215-240. In Z. F. Dembek (ed.), Medical aspects of biological warfare. TMM Publications, Office of The Surgeon General, Washington, DC.
- 9.Jordan, R., D. Tien, T. C. Bolken, K. F. Jones, S. R. Tyavanagimatt, J. Strasser, A. Frimm, M. L. Corrado, P. G. Strome, and D. E. Hruby. 2008. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob. Agents Chemother. 52:1721-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karem, K. L., M. Reynolds, Z. Braden, G. Lou, N. Bernard, J. Patton, and I. K. Damon. 2005. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin. Diagn. Lab. Immunol. 12:867-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga, T., K. Kanefuji, and K. Nakama. 2005. Individual reference intervals of hematological and serum biochemical parameters in cynomolgus monkeys. Int. J. Toxicol. 24:377-385. [DOI] [PubMed] [Google Scholar]
- 12.Marris, E. 2007. Dramatic rescue relieves rare case of smallpox infection. Nat. Med. 13:517. [DOI] [PubMed] [Google Scholar]
- 13.Meyer, H., M. Perrichot, M. Stemmler, P. Emmerich, H. Schmitz, F. Varaine, R. Shungu, F. Tshioko, and P. Formenty. 2002. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 40:2919-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore, Z. S., J. F. Seward, and J. M. Lane. 2006. Smallpox. Lancet 367:425-435. [DOI] [PubMed] [Google Scholar]
- 15.Nalca, A., J. M. Hatkin, N. L. Garza, D. K. Nichols, S. W. Norris, D. E. Hruby, and R. Jordan. 2008. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antivir. Res. 79:121-127. [DOI] [PubMed] [Google Scholar]
- 16.Parker, S., A. Nuara, R. M. Buller, and D. A. Schultz. 2007. Human monkeypox: an emerging zoonotic disease. Future Microbiol. 2:17-34. [DOI] [PubMed] [Google Scholar]
- 17.Quenelle, D. C., R. M. Buller, S. Parker, K. A. Keith, D. E. Hruby, R. Jordan, and E. R. Kern. 2007. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 51:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quenelle, D. C., M. N. Prichard, K. A. Keith, D. E. Hruby, R. Jordan, G. R. Painter, A. Robertson, and E. R. Kern. 2007. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob. Agents Chemother. 51:4118-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sbrana, E., R. Jordan, D. E. Hruby, R. I. Mateo, S. Y. Xiao, M. Siirin, P. C. Newman, A. P. A. Travassos da Rosa, and R. B. Tesh. 2007. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 76:768-773. [PubMed] [Google Scholar]
- 20.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 21.Vora, S., I. Damon, V. Fulginiti, S. G. Weber, M. Kahana, S. L. Stein, S. I. Gerber, S. Garcia-Houchins, E. Lederman, D. Hruby, L. Collins, D. Scott, K. Thompson, J. V. Barson, R. Regnery, C. Hughes, R. S. Daum, Y. Li, H. Zhao, S. Smith, Z. Braden, K. Karem, V. Olson, W. Davidson, G. Trindade, T. Bolken, R. Jordan, D. Tien, and J. Marcinak. 2008. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 46:1555-1561. [DOI] [PubMed] [Google Scholar]
- 22.Whitley, R. J. 2003. Smallpox: a potential agent of bioterrorism. Antivir. Res. 57:7-12. [DOI] [PubMed] [Google Scholar]
- 23.Yang, G., D. C. Pevear, M. H. Davies, M. S. Collett, T. Bailey, S. Rippen, L. Barone, C. Burns, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, R. L. Buller, E. Touchette, K. Waller, J. Schriewer, J. Neyts, E. DeClercq, K. Jones, D. Hruby, and R. Jordan. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79:13139-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]