Abstract
The octadecyloxyethyl (ODE) and hexadecyloxypropyl (HDP) esters of (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine (HPMPA) are potent inhibitors of orthopoxvirus, herpesvirus, human immunodeficiency virus type 1, and hepatitis B virus replication in vitro. HDP and ODE esters of (S)-HPMPA and (R)-HPMPA were evaluated for their activity in hepatitis C virus (HCV) replicon assays using luciferase (1B and 2A replicons) or RNA (1B) quantification. The ODE ester of (S)-HPMPA [ODE-(S)-HPMPA] was the most active compound, with 50% effective concentrations (EC50s) in the 0.69 to 1.31 μM range. HDP and ODE esters of (R)-HPMPA were severalfold less active, while (S)-HPMPA and (R)-HPMPA were inactive. In genotype 1A and 1B replicons analyzed by HCV RNA analysis, ODE-(S)-HPMPA was the most active compound, with EC50s of 1.8 and 2.1 μM, respectively.
It is estimated that 3 to 4 million persons in the United States are chronically infected with hepatitis C virus (HCV). Additionally, an increasing fraction of the HCV-infected population develops hepatic morbidity over time. Although combination therapy with peginterferon and ribavirin has improved the treatment options for this infection, highly effective and well-tolerated therapy has yet to be realized. The NS5B RNA polymerase of HCV is required for viral replication, and a number of nucleoside and nonnucleoside inhibitors of the enzyme have been described previously (7, 8, 14, 19, 23, 24). Antiviral nucleoside inhibitors that act at the polymerase active site are particularly attractive given that they are active across different HCV genotypes, present a high barrier to resistance (17), and have been successful in other chronic viral infections such as those with human immunodeficiency virus type 1 (HIV-1) and hepatitis B virus (HBV).
Acyclic nucleoside phosphonates, such as cidofovir, adefovir, and tenofovir, have been developed as effective therapy for a number of viral infections, including cytomegalovirus (CMV), HBV, and HIV-1 (6, 9). However, the negative charges associated with the phosphonate moiety limit their oral bioavailability and cellular penetration (5). This has required the development of the currently marketed prodrugs adefovir dipivoxil and tenofovir disoproxil fumarate. (S)-9-[3-Hydroxy-2-(phosphonomethoxy)propyl]adenine [(S)-HPMPA] is active in vitro against herpesviruses (2) and orthopoxviruses (13). We have shown that the hexadecyloxypropyl (HDP) and octadecyloxyethyl (ODE) esters of HPMPA have multiple-log increases in antiviral activity in vitro compared with the unmodified compound against HIV-1, orthopoxviruses, and CMV (3, 10). Esterification with HDP or ODE greatly increases cell uptake and eventual conversion to the acyclic nucleotide diphosphate, the active metabolite of this class of agents (1, 16). This strategy also increases the oral bioavailability of these compounds, making them potentially attractive therapeutics (4, 20, 22).
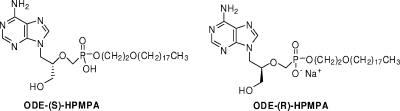
We synthesized the HDP and ODE esters of (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine [HDP-(S)-HPMPA and ODE-(S)-HPMPA, respectively] as previously described (3). The HDP and ODE esters of (R)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine [HDP-(R)-HPMPA and ODE-(R)-HPMPA, respectively] were synthesized as previously described (3) by substituting (R)-trityl glycidyl ether for (S)-trityl glycidyl ether. Structures of the alkoxyalkyl esters ODE-(S)-HPMPA and ODE-(R)-HPMPA are shown in Fig. 1. Compounds were assessed by high-performance liquid chromatography, thin-layer chromatography, proton nuclear magnetic resonance, and liquid chromatography-mass spectrometry and were judged to be >98% pure.
FIG. 1.
Structures of ODE-(S)-HPMPA and ODE-(R)-HPMPA. The structure of the (R) and (S) isomers of ODE-HPMPA is shown. The structure of the (R) and (S) isomers of HDP-HPMPA (not shown) is identical to that of ODE-HPMPA except that the alkoxyalkyl moiety is −(CH2)3O(CH2)15CH3.
The HCV genotype 1B replicon BM4-5 FEO has been previously described (26) and contains a firefly luciferase-neomycin phosphotransferase fusion protein. The SGR-JFH FEO replicon, based on the full-length JFH-1 genotype 2A HCV virus (25), was created by insertion of the PacI-KpnI fragment of the BM4-5 FEO plasmid into the PacI-KpnI-digested SGR-JFH1/Luc plasmid (Mighty Mix; Takara). The SGR-JFH1/Luc plasmid has been previously described (12) and is a bicistronic replicon with the HCV internal ribosome entry site directing translation of the firefly luciferase gene and an encephalomyocarditis virus internal ribosome entry site identical to the one found in the BM4-5 replicon. The sequence of the SGR-JFH FEO plasmid was verified by automated DNA sequencing.
The BM4-5 and SGR-JFH1 FEO plasmids were linearized with ScaI and XbaI, respectively; the SGR-JFH1 FEO plasmid was digested with mung bean nuclease to generate an authentic 3′ end (New England Biolabs). In vitro transcription and generation of cell lines stably expressing the BM4-5 FEO and SGR-JFH-1 FEO replicons were accomplished by electroporation of human hepatoma Huh-7.5.1 cells (a kind gift from Francis Chisari, Scripps Research Institute, La Jolla, CA) and selection with 500 μg/ml of G-418 as we have previously described (26).
Compound activity assays were carried out as previously described using 10,000 (BM4-5- or JFH-1-based) FEO replicon cells per well on 96-well plates (26). Cells and compounds were incubated for 48 to 72 h with all conditions run in triplicate. Additionally, three or four experimental replicates were completed per compound. Luciferase activity was determined using a microplate luminometer (Veritas microplate luminometer; Turner Biosystems) according to the manufacturer's instructions (Bright-Glo; Promega). Relative light units for each condition were used to generate a dose response for each compound by using Prism (version 4; GraphPad Software). The cytotoxicity of each compound was determined using a fluorescent cell viability and death assay (MultiTox-Fluor; Promega). All compounds were tested at concentrations of up to 100 μM (0.3% dimethyl sulfoxide final concentration). In other experiments, anti-HCV activity was determined in genotype 1A and 1B replicons by HCV RNA analysis as previously described (15, 18). Briefly, replicon cell lines were maintained as subconfluent cultures on 96-well plates. Compounds were added daily for 3 days in fresh medium. Twenty-four hours after the last dose of compound, antiviral activity was determined by blot hybridization analysis of intracellular HCV RNA, and cytotoxicity was assessed by neutral red dye uptake. Values for 50% effective concentration (EC50) and for the minimal concentration required to induce 50% cleavage (CC50) were calculated for each test compound by linear regression analysis, using data combined for all treated cultures. Antiviral and toxicity assays utilized triplicate cultures for each drug concentration; 12 untreated cultures were included in each assay. HCV and β-actin RNA quantitation standards were included on each individual hybridization blot (15, 18).
ODE-(S)-HPMPA was the most active compound in the luciferase assays with EC50s of 1.31 and 0.69 μM in 1B and 2A replicons, respectively, while the HDP esters were generally less active (Table 1). In general, the EC50 of the alkoxyalkyl compounds is about fourfold higher in the genotype 2 replicon system (compared to those in genotype 1B) with the exception of ODE-(S)-HPMPA, which shows similar activity levels in both 1B and 2A replicons. (S)-HPMPA and (R)-HPMPA were inactive in both genotype 1 and 2 luciferase replicon systems, consistent with the poor penetration of cells as reported previously for (S)-HPMPA (16). ODE-(R)-HPMPA and HDP-(R)-HPMPA were also active in vitro but were significantly less active than the corresponding (S)-HPMPA enantiomers. However, the (R) enantiomers were less cytotoxic, with CC50 values of >100 μM versus 35.6 and 85.3 μM for ODE-(S)-HPMPA and HDP-(S)-HPMPA, respectively. The selectivity index for ODE-(S)-HPMPA was 27 to 52 (Table 1). The selectivity indices for the current compounds are moderate, in the range of 20 to 40, but encouraging for lead compounds. It should be kept in mind that the cytotoxicity testing was only performed in Huh-7.5.1 cells, a liver cancer cell line, which may be more prone to the antiproliferative effects of nucleosides than are primary hepatocytes or non-tumor-based cell lines (11). In HCV RNA assay results of genotype 1A and 1B replicons, ODE-(S)-HPMPA was active with EC50 values of 1.8 ± 0.3 and 2.1 ± 0.2 μM, similar to those observed in genotypes 1B and 2A with the luciferase assay (Table 1). This finding rules out the possibility that the antiviral activity noted in luciferase assays is artifactual due to effects of the compounds on luciferase activity or ATP pools.
TABLE 1.
Effect of HDP-(S)-HPMPA and ODE-(S)-HPMPA on HCV replication in vitro
| Compounda | EC50 (μM) | CC50 (μM) | Selectivity (CC50/EC50) |
|---|---|---|---|
| BM4-5 FEO, genotype 1B | |||
| (S)-HPMPA | >100 | >100 | |
| (R)-HPMPA | >100 | >100 | |
| ODE-(S)-HPMPA (4) | 1.31 ± 0.66 | 35.6 ± 6.8 | 27 |
| ODE-(R)-HPMPA (4) | 7.0 ± 8.21 | >100 | >14 |
| HDP-(S)-HPMPA (4) | 2.02 ± 0.95 | 85.3 ± 6.8 | 42 |
| HDP-(R)-HPMPA (4) | 6.71 ± 2.95 | >100 | >15 |
| SGR-JFH-1 FEO, genotype 2A | |||
| (S)-HPMPA | >100 | >100 | |
| (R)-HPMPA | >100 | >100 | |
| ODE-(S)-HPMPA (4) | 0.69 ± 0.71 | 35.6 ± 6.8 | 52 |
| ODE-(R)-HPMPA (4) | 26.7 ± 14.3 | >100 | >4 |
| HDP-(S)-HPMPA (4) | 6.5 ± 12.5 | 85.3 ± 20.8 | 13 |
| HDP-(R)-HPMPA (4) | 41.0 ± 3.61 | >100 | >2 |
Data are the means ± standard deviations. The number in parentheses is the number of replicate determinations.
There are currently few anti-HCV nucleosides in clinical studies; the development of NM283 (2′-C-methylcytidine prodrug) and R1626 (4′-azidocytidine prodrug) was recently discontinued. Additionally, most compounds currently or recently under clinical investigation select for one of two polymerase resistance mutations—the S282T mutation for 2′-C-methyl compounds (19) or the S96T mutation for 4′-azidocytidine (17). Thus, new anti-HCV nucleoside candidates are needed, particularly if they possess unique resistance profiles.
Taken together the alkoxyalkyl derivatives of HPMPA offer low micromolar potency with reasonable toxicity profiles and are therefore potential lead compounds which can be modified in attempts to enhance potency and/or decrease toxicity. HDP-(S)-HPMPA and ODE-(S)-HPMPA are orally bioavailable and are active in lethal animal models of orthopoxvirus disease (21) and murine and human cytomegalovirus (22). HDP-(S)-HPMPA provides excellent drug exposure in the liver when given orally and is orally active in transgenic HBV mice (J. D. Morrey, B. E. Korba, J. R. Beadle, D. L. Wyles, and K. Y. Hostetler, submitted for publication). Studies are currently under way to isolate and characterize drug-resistant mutants and to evaluate whether replicon cells can be cured by longer drug exposures. We are also evaluating the activity of acyclic nucleoside phosphonates containing other nucleobases and other lipid ester side chains to better define the spectrum of antiviral activity, oral pharmacokinetics, and mechanism of action and to determine resistance profiles.
Acknowledgments
This work was funded in part by grants AI-071803 and AI-074057 (K.Y.H.), AI-076558 (R.T.S.), and AI-069989 (D.L.W.) from the National Institute of Allergy and Infectious Disease.
K.Y.H. has an equity interest in and serves as a consultant to Chimerix Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies.
Footnotes
Published ahead of print on 16 March 2009.
REFERENCES
- 1.Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-(14)C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini, J., A. Holy, J. Jindrich, L. Naesens, R. Snoeck, D. Schols, and E. De Clercq. 1993. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 37:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beadle, J. R., W. B. Wan, S. L. Ciesla, K. A. Keith, C. Hartline, E. R. Kern, and K. Y. Hostetler. 2006. Synthesis and antiviral evaluation of alkoxyalkyl derivatives of 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine against cytomegalovirus and orthopoxviruses. J. Med. Chem. 49:2010-2015. [DOI] [PubMed] [Google Scholar]
- 4.Ciesla, S. L., J. Trahan, W. B. Wan, J. R. Beadle, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 5.Connelly, M. C., B. L. Robbins, and A. Fridland. 1993. Mechanism of uptake of the phosphonate analog (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in Vero cells. Biochem. Pharmacol. 46:1053-1057. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq, E., and A. Holý. 2005. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4:928-940. [DOI] [PubMed] [Google Scholar]
- 7.Dhanak, D., K. J. Duffy, V. K. Johnston, J. Lin-Goerke, M. Darcy, A. N. Shaw, B. Gu, C. Silverman, A. T. Gates, M. R. Nonnemacher, D. L. Earnshaw, D. J. Casper, A. Kaura, A. Baker, C. Greenwood, L. L. Gutshall, D. Maley, A. Del Vecchio, R. Macarron, G. A. Hofmann, Z. Alnoah, H. Y. Cheng, G. Chan, S. Khandekar, R. M. Keenan, and R. T. Sarisky. 2002. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:38322-38327. [DOI] [PubMed] [Google Scholar]
- 8.Gopalsamy, A., A. Aplasca, G. Ciszewski, K. Park, J. W. Ellingboe, M. Orlowski, B. Feld, and A. Y. Howe. 2006. Design and synthesis of 3,4-dihydro-1H-[1]-benzothieno[2,3-c]pyran and 3,4-dihydro-1H-pyrano[3,4-b]benzofuran derivatives as non-nucleoside inhibitors of HCV NS5B RNA dependent RNA polymerase. Bioorg. Med. Chem. Lett. 16:457-460. [DOI] [PubMed] [Google Scholar]
- 9.Holy, A. 2003. Phosphonomethoxyalkyl analogs of nucleotides. Curr. Pharm. Des. 9:2567-2592. [DOI] [PubMed] [Google Scholar]
- 10.Hostetler, K. Y., K. A. Aldern, W. B. Wan, S. L. Ciesla, and J. R. Beadle. 2006. Alkoxyalkyl esters of (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]adenine are potent inhibitors of the replication of wild-type and drug-resistant human immunodeficiency virus type 1 in vitro. Antimicrob. Agents Chemother. 50:2857-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hostetler, K. Y., S. Rought, K. A. Aldern, J. Trahan, J. R. Beadle, and J. Corbeil. 2006. Enhanced antiproliferative effects of alkoxyalkyl esters of cidofovir in human cervical cancer cells in vitro. Mol. Cancer Ther. 5:156-159. [DOI] [PubMed] [Google Scholar]
- 12.Kato, T., T. Date, M. Miyamoto, M. Sugiyama, Y. Tanaka, E. Orito, T. Ohno, K. Sugihara, I. Hasegawa, K. Fujiwara, K. Ito, A. Ozasa, M. Mizokami, and T. Wakita. 2005. Detection of anti-hepatitis C virus effects of interferon and ribavirin by a sensitive replicon system. J. Clin. Microbiol. 43:5679-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keith, K. A., M. J. Hitchcock, W. A. Lee, A. Holý, and E. R. Kern. 2003. Evaluation of nucleoside phosphonates and their analogs and prodrugs for inhibition of orthopoxvirus replication. Antimicrob. Agents Chemother. 47:2193-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klumpp, K., G. Kalayanov, H. Ma, P. S. Le, V. Leveque, W. R. Jiang, N. Inocencio, A. De Witte, S. Rajyaguru, E. Tai, S. Chanda, M. R. Irwin, C. Sund, A. Winqist, T. Maltseva, S. Eriksson, E. Usova, M. Smith, A. Alker, I. Najera, N. Cammack, J. A. Martin, N. G. Johansson, and D. B. Smith. 2008. 2′-Deoxy-4′-azido nucleoside analogs are highly potent inhibitors of hepatitis C virus replication despite the lack of 2′-alpha-hydroxyl groups. J. Biol. Chem. 283:2167-2175. [DOI] [PubMed] [Google Scholar]
- 15.Korba, B. E., A. B. Montero, K. Farrar, K. Gaye, S. Mukerjee, M. S. Ayers, and J. F. Rossignol. 2008. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antivir. Res. 77:56-63. [DOI] [PubMed] [Google Scholar]
- 16.Magee, W. C., K. A. Aldern, K. Y. Hostetler, and D. H. Evans. 2008. Cidofovir and (S)-9-[3-hydroxy-(2-phosphonomethoxy)propyl]adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. Antimicrob. Agents Chemother. 52:586-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCown, M. F., S. Rajyaguru, P. S. Le, S. Ali, W. R. Jiang, H. Kang, J. Symons, N. Cammack, and I. Najera. 2008. The hepatitis C virus replicon presents a higher barrier to resistance to nucleoside analogs than to nonnucleoside polymerase or protease inhibitors. Antimicrob. Agents Chemother. 52:1604-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuse, C., J. A. Rinaudo, K. Farrar, F. Wells, and B. E. Korba. 2005. Enhancement of antiviral activity against hepatitis C virus in vitro by interferon combination therapy. Antivir. Res. 65:23-34. [DOI] [PubMed] [Google Scholar]
- 19.Olsen, D. B., A. B. Eldrup, L. Bartholomew, B. Bhat, M. R. Bosserman, A. Ceccacci, L. F. Colwell, J. F. Fay, O. A. Flores, K. L. Getty, J. A. Grobler, R. L. Lafemina, E. J. Markel, G. Migliaccio, M. Prhavc, M. W. Stahlhut, J. E. Tomassini, M. MacCoss, D. J. Hazuda, and S. S. Carroll. 2004. A 7-deaza-adenosine analog is a potent and selective inhibitor of hepatitis C virus replication with excellent pharmacokinetic properties. Antimicrob. Agents Chemother. 48:3944-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Painter, G. R., M. R. Almond, L. C. Trost, B. M. Lampert, J. Neyts, E. De Clercq, B. E. Korba, K. A. Aldern, J. R. Beadle, and K. Y. Hostetler. 2007. Evaluation of hexadecyloxypropyl-9-R-[2-(phosphonomethoxy)-propyl]-adenine, CMX157, as a potential treatment for human immunodeficiency virus type 1 and hepatitis B virus infections. Antimicrob. Agents Chemother. 51:3505-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quenelle, D. C., D. J. Collins, B. P. Herrod, K. A. Keith, J. Trahan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2007. Effect of oral treatment with hexadecyloxypropyl-[(S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)-ad- enine] [(S)-HPMPA] or octadecyloxyethyl-(S)-HPMPA on cowpox or vaccinia virus infections in mice. Antimicrob. Agents Chemother. 51:3940-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quenelle, D. C., D. J. Collins, L. R. Pettway, C. B. Hartline, J. R. Beadle, W. B. Wan, K. Y. Hostetler, and E. R. Kern. 2008. Effect of oral treatment with (S)-HPMPA, HDP-(S)-HPMPA or ODE-(S)-HPMPA on replication of murine cytomegalovirus (MCMV) or human cytomegalovirus (HCMV) in animal models. Antivir. Res. 79:133-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomei, L., S. Altamura, L. Bartholomew, A. Biroccio, A. Ceccacci, L. Pacini, F. Narjes, N. Gennari, M. Bisbocci, I. Incitti, L. Orsatti, S. Harper, I. Stansfield, M. Rowley, R. De Francisco, and G. Migliaccio. 2003. Mechanism of action and antiviral activity of benzimidazole-based allosteric inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Virol. 77:13225-13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomei, L., S. Altamura, L. Bartholomew, M. Bisbocci, C. Bailey, M. Bosserman, A. Cellucci, E. Forte, I. Incitti, L. Orsatti, U. Koch, R. De Francisco, D. B. Olsen, S. S. Carroll, and G. Migliaccio. 2004. Characterization of the inhibition of hepatitis C virus RNA replication by nonnucleosides. J. Virol. 78:938-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyles, D. L., K. A. Kaihara, F. Vaida, and R. T. Schooley. 2007. Synergy of small molecular inhibitors of hepatitis C virus replication directed at multiple viral targets. J. Virol. 81:3005-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]