Abstract
In our previous study, we found that the antibacterial peptide KLKLLLLLKLK-NH2 (L5) and its d-enantiomer (DL5) activate neutrophils to produce superoxide anions (O2−) and prevent death due to infection by methicillin-resistant Staphylococcus aureus, suggesting that these peptides may elicit in vivo antimicrobial activities through host inflammatory responses mediated by neutrophils. In this study, we investigated the mechanisms behind in vivo antimicrobial prophylaxis by the use of L5 for the treatment of bacterial infection introduced via intra-abdominal implantation. We found that the intraperitoneal treatment with L5 before bacterial infection markedly reduced rates of death due to infection. Treatments with L5 were highly effective in preventing death due to intraperitoneal inoculation of not only S. aureus Smith but also Enterococcus faecalis SR1004 and Escherichia coli EC14. The intra-abdominal administration of L5 induced accumulation of neutrophils, increased levels of reactive oxygen species, and augmented antibacterial activity in the abdominal cavity. In addition, administration of L5 upregulated the expression of the Mig/CXCL9 chemokine gene in thioglycolate-elicited peritoneal macrophages. Our results suggested that the prevention of death by treatment of infected mice with L5 might occur primarily through the activation of a host immune response.
Innate immunity is the universal defense system in all animals. It involves (i) recognition of foreign substances in a nonspecific manner and (ii) responses to pathogens at the early stage of infection. The production of cationic antimicrobial peptides is an evolutionarily conserved response in innate immunity. These peptides possess broad-spectrum antibacterial, antifungal, antiviral, antiprotozoal, and antiseptic properties. The cationic antimicrobial peptides directly kill microorganisms and are also involved in many aspects of host defenses associated with innate and adaptive immune responses (9, 31). For example, mammalian defensins—a family of small cationic antimicrobial peptides containing three pairs of intramolecular disulfide bonds—are capable of enhancing phagocytosis by macrophages (11); inducing activation and degranulation of mast cells, resulting in the release of histamines (2); enhancing cytokine production, e.g., that of interleukin-8 (IL-8), from epithelial cells (28); neutralizing endotoxin responses in macrophages (23); eliciting chemoattraction of monocytes (26), T cells (3), and immature dendritic cells (32); and promoting systemic antigen-specific immune responses when administrated in vivo along with antigens (13, 25).
In invertebrates—which do not possess adaptive immunity—cationic antimicrobial proteins are the main factors providing immunity (10). Their antibacterial activity is comparable to that of various antibiotics. However, the therapeutic application of these proteins may be difficult due to their antigenic properties and toxicity. Structural modifications of these proteins may enable the development of potential clinical therapeutics for the treatment of antibiotic-resistant bacterial infection.
We have previously identified and characterized several inducible antibacterial proteins from Sarcophaga peregrina (14, 16, 17, 20, 29). Among them, sapecin B was found to possess the active core domain of a short amidated α helix motif— RSLCLLHCRLK-NH2 (30). Working with the structure of this peptide, we further optimized the sequences and finally obtained a cationic antimicrobial peptide—KLKLLLLLKLK-NH2 (L5) (1). L5 and its d-enantiomer (DL5) were found to show significant efficacy in prophylactic treatment of methicillin-resistant Staphylococcus aureus (MRSA)-infected mice (15). On the other hand, KLKLLLKLK-NH2 (L3) was found to exhibit strong antibacterial activity in vitro but not the ability to prevent MRSA infection. We also found that L5 and DL5, but not L3, activate human neutrophils (15). Furthermore, we previously reported that L5 binds to calreticulin on the cell surface of human neutrophils and retinoic acid-treated U937 (a human monocyte cell line) (4, 5). Recently, it has been reported that L5 enhances antigen presentation in mice and acts as an effective adjuvant with oligonucleotide-containing deoxyinosine or deoxycytosine (7, 21). We propose that MRSA infection can be prevented by treatment with L5 and DL5 not only through direct antibacterial activity but also through the augmentation of the systemic defense mechanism mediated by neutrophils.
In this study, we found that intraperitoneal injection of L5 and DL5, but not of L3, before bacterial infection remarkably reduced the rate of death for mice. Further, this activity of L5 and DL5 was not dependent on the bacterial type or on direct killing activity. We found that L5 induced an acute inflammatory response in the abdomen of mice (i.e., accumulation of neutrophils), promoted reactive oxygen species (ROS) production, and enhanced antibacterial activity. L5 increased the expression of a chemokine, Mig/CXCL9, in thioglycolate (TGC)-elicited peritoneal macrophages. Our results suggest that L5 prevents death of infected mice through the activation of host innate immunity.
MATERIALS AND METHODS
Peptides.
L5, DL5, and L3 were synthesized by using the solid-phase method and a peptide synthesizer (ABI 433A; Applied Biosystems, Foster City, CA) and purified by high-performance liquid chromatography on a reverse-phase column (Intersil ODS-3; GL Sciences, Tokyo, Japan).
Organisms.
The bacterial strains used in this study were clinical isolates collected from hospitals in Japan and strains from our laboratory collection.
Assay of protection against bacterial infection.
Five-week-old female ICR mice (CLEA Japan, Inc., Tokyo, Japan) were injected intraperitoneally with 500 μl of Dulbecco's phosphate-buffered saline (PBS; Invitrogen Corp., Carlsbad, CA) containing 2% dimethyl sulfoxide together with L5, DL5, or L3. As the vehicle control, Dulbecco's PBS containing 2% dimethyl sulfoxide was injected. At various intervals after peptide injection as indicated in Table 1, Table 2, and Table 3, a minimum lethal dose of S. aureus Smith (2 × 106 CFU/mouse), Enterococcus faecalis SR1004 (1.7 × 108 CFU/mouse), or Escherichia coli EC14 (2.3 × 104 CFU/mouse) was inoculated into the abdominal cavity. The viability of the mice was examined on day 5 after bacterial infection. We used four or eight mice per group to evaluate the preventive activity of the antimicrobial peptides against death due to infection. All procedures involving animals were approved by the Experimental Animal Committee of Shionogi & Co., Ltd.
TABLE 1.
The prophylaxis activity of treatment with L5, DL5, or L3 at 7 h before abdominal infection with S. aureus Smith, E. faecalis SR1004, and E. coli EC14a
| Species (CFU/mouse) | Peptide (μg/mouse) | Survival of mice
|
|
|---|---|---|---|
| Day 5 (no. of surviving mice/total no. of mice) | %b | ||
| S. aureus Smith (2.0 × 106) | Vehicle | 0/8 | 0 |
| L5 (100) | 3/4 | 75* | |
| L3 (100) | 0/4 | 0 | |
| DL5 (100) | 8/8 | 100*** | |
| E. faecalis SR1004 (1.7 × 108) | Vehicle | 0/4 | 0 |
| L5 (100) | 3/4 | 75 | |
| DL5 (100) | 4/4 | 100* | |
| E. coli EC14 (2.3 × 104) | Vehicle | 0/8 | 0 |
| L5 (100) | 1/4 | 25 | |
| DL5 (100) | 6/8 | 75** | |
The minimum lethal dose of bacteria was inoculated by intraperitoneal injection.
Statistical analysis was carried out by Fisher's exact test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
TABLE 2.
The time course of the interval between treatment and inoculation of S. aureus Smith with respect to the prophylaxis activity of DL5a
| Peptide (μg/mouse) | Interval (h) | Survival of mice
|
|
|---|---|---|---|
| Day 5 (no. of surviving mice/total no. of mice) | %b | ||
| DL5 (50) | −0.5 | 0/4 | 0 |
| 0 | 0/4 | 0 | |
| 1 | 1/4 | 25 | |
| 3 | 5/8 | 62.5* | |
| 7 | 7/8 | 87.5** | |
| 24 | 0/4 | 0 | |
The minimum lethal dose of S. aureus Smith (2 × 106 CFU/mouse) was inoculated by intraperitoneal injection before and after treatment with DL5.
Statistical analysis was carried out by Fisher's exact test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
TABLE 3.
The dose-dependent activity of DL5 for treatment of abdominal infection with S. aureus Smitha
| Peptide (μg/mouse) | Survival of mice
|
|
|---|---|---|
| Day 5 | %b | |
| Vehicle | 0/4 | 0 |
| DL5 (3.13) | 0/4 | 0 |
| DL5 (12.5) | 0/4 | 0 |
| DL5 (25) | 3/4 | 75* |
| DL5 (50) | 7/8 | 87.5** |
| DL5 (100) | 3/4 | 75* |
The minimum lethal dose of S. aureus Smith (2 × 106 CFU/mouse) was inoculated by intraperitoneal injection after treatment with DL5 for 7 h.
Statistical analysis was carried out by Fisher's exact test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Assay of antimicrobial activity in vitro.
The antibacterial activities of the peptides were measured using a previously reported method (15). To determine the MIC, we used the microdilution broth method by following the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS).
Collection of peritoneal cells after challenge with L5 and Cytospin preparation.
The following animal experiments were approved by the Wako Animal Experiment Committee of the Safety Division of RIKEN and were carried out in accordance with the guidelines and the Animal Experiment Handbook of the Wako Institute of RIKEN. Following the intraperitoneal injection of L5 or L3 and an interval of 7 h, the peritoneal cells were obtained by lavage of the abdominal cavity of the mice with 5 ml of cold sterile saline solution. The cells were collected by centrifugation, washed, and resuspended at 1 × 106 cells/ml in 100% heat-inactivated (56°C for 30 min) fetal calf serum. Next, 500 μl of each cell suspension was used for the Cytospin (Thermo Fisher Scientific, Inc., Waltham, MA) preparation. The samples were stained with Diff-Quick (Sysmex International Reagents Co., Ltd., Kobe, Japan) according to the manufacturer's instructions. The numbers of total cells and neutrophils were counted using a microscope.
Assay of antibacterial activity in the peritoneal cavity.
Following the intraperitoneal injection of 500 μl of L5 solution for 7 h, the minimum lethal dose of S. aureus Cowan I (4 × 105 CFU/mouse) was introduced and incubated for 15 min in the abdominal cavity of the mice. Next, the peritoneal lavage with 5 ml of cold sterile saline solution was collected and serially diluted using PBS. The serial-dilution samples were spread on LB agar plates and incubated for 18 h. The colonies were counted, and the numbers of colonies were compared between the test and control plates (16).
Assay of ROS produced by peritoneal cells.
The collected peritoneal cells were suspended in Hank's balanced salt solution (25 mM HEPES/NaOH [pH 7.4], 137 mM NaCl, 5.4 mM KCl, 0.3 mM Na2HPO4, 0.4 mM KH2PO4, 0.8 mM MgSO4, 1.25 mM CaCl2, and 5.6 mM glucose) at a density of 2 × 105 cells/ml. The cell suspension in the 96-well plate was incubated at 37°C for 5 min, and lucigenin (Sigma, St Louis, MO) was added to produce a final concentration of 0.1 mM. ROS was detected by measuring the lucigenin-dependent chemiluminescence in a Biolumat LB9505 system (EG&G Berthold, Wellesley, MA) at 37°C.
Collection of TGC-elicited peritoneal macrophages, RNA isolation, cDNA synthesis, and PCR.
TGC-elicited peritoneal macrophages were obtained using a previously described method (18). In brief, 3 ml of sterile 3% Bacto Brewer TGC medium (Difco, Detroit, MT) was injected into the abdomen of the mice. After 4 days, peritoneal macrophages were obtained by lavage of the abdominal cavity of the mice with 8 ml of cold sterile PBS. The washed and collected cells were seeded in a 12-well plate at 1 × 106 cells in 1 ml of RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum. The cells were allowed to adhere to the tissue culture plate for 2 h at 37°C prior to gentle rinsing for removal of nonadherent cells. Next, the cells were immediately treated with 25 μg of L5/ml for 2.5, 5, and 7.5 h or with 100 ng of lipopolysaccharide (LPS) from E. coli 0111:B4 (Sigma) for 24 h.
Total RNA was prepared and isolated from L5- or LPS-treated macrophage monolayers by using an RNAspin mini RNA isolation kit (GE Healthcare Bio-Sciences KK, Tokyo, Japan). To generate first-strand cDNA, 400 ng of the total RNA was subjected to reverse transcription (RT) in a 20-μl reaction volume by using a Prime Script RT reagent kit (TaKaRa Bio, Otsu, Japan). PCR was performed using a total volume of 10 μl containing 1 mM MgCl2, a 0.2 mM concentration of each deoxynucleoside triphosphate, a 1 μM concentration of each primer, 0.5 U of Go Taq polymerase, and 2 μl of 10-fold-diluted RT reaction mixture in Green Go Taq reaction buffer (Promega Corporation, Madison, WI). The thermal-cycling parameters consisted of denaturation at 94°C for 15 s, annealing at 56°C for 15 s, and extension at 72°C for 20 s for 25 to 35 cycles. The PCR products were separated on 2% agarose gels in 0.5× Tris-acetate-EDTA and were visualized with ethidium bromide. The oligonucleotides in the primer sets for G3PDH (glyceraldehyde 3-phosphate dehydrogenase) were used as previously reported (18). Real-time PCR was performed using SYBR Premix Ex Taq II (TaKaRa Bio) and a thermal cycler Dice real-time system (TaKaRa Bio). The primer sets and expected sizes of the PCR products for chemokines and G3PDH in real-time PCR are listed in Table 4.
TABLE 4.
The primer sets and expected sizes of the PCR products for chemokines and GAPDH in real-time PCR
| Gene | Primer sequence
|
Size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| MIP-1α/CCL3 | 5′-ATGAAGGTCTCCACCACTGC-3 | 5′-TCAGGCATTCAGTTCCAGGT-3 | 279 |
| MIP-1β/CCL4 | 5′-ATGAAGCTCTGCGTGTCTGC-3′ | 5′-CACTCATGTACTCAGTGACC-3′ | 262 |
| RANTES/CCL5 | 5′-CTCACCATCATCCTCACTGC-3′ | 5′-ACCCACTTCTTCTCTGGGTT-3′ | 221 |
| TARC/CCL17 | 5′-CATGAGGTCACTTCAGATGC-3′ | 5′-TGCACAGATGAGCTTGCCCT-3′ | 224 |
| MDC/CCL22 | 5′-CTGATGCAGGTCCCTATGGT-3′ | 5′-GGAGTAGCTTCTTCACCCAG-3′ | 201 |
| Eotaxin-2/CCL24 | 5′-TGACCATCCCCTCATCTTGC-3′ | 5′-CTGGACAGCAAACTTGGTTC-3′ | 251 |
| Gro-α/CXCL1 | 5′-AGCCACACTCAAGAATGGTC-3′ | 5′-TCAGAAGCCAGCGTTCACCA-3′ | 153 |
| MIP-2/CXCL2 | 5′-ACTAGCTACATCCCACCCAC-3′ | 5′-ACAGCTGTTCTACTCTCCTC-3′ | 148 |
| IL-8/CXCL8 | 5′-AGCAGAACCAGATTGTAGGG-3′ | 5′-ACTATGCTGGTCTGCTACGGA-3′ | 140 |
| Mig/CXCL9 | 5′-CTGTTCTTTTCCTCTTGGGC-3′ | 5′-GTCCGGATCTAGGCAGGTTT-3′ | 221 |
| IP-10/CXCL10 | 5′-CATTTTCTGCCTCATCCTGC-3′ | 5′-GGATTCAGACATCTCTGCTC-3′ | 210 |
| I-TAC/CXCL11 | 5′-GCTGCTCAAGGCTTCCTTATGT-3′ | 5′-TATGAGGCGAGCTTGCTTGG-3′ | 204 |
| G3PDH | 5′-CCCCATGTTTGTGATGGGTG-3′ | 5′-AGTGATGGCATGGACTGTGG-3′ | 163 |
Statistical analysis.
A statistical analysis of the data was performed using Fisher's exact test and the unpaired Student's t test. The data were first normalized by an arcsin transformation when necessary before the use of an unpaired Student's t test.
RESULTS
Intraperitoneal injection of L5 and DL5 before bacterial infection prevented death in mice.
In our previous study, we found that intravenous injection of L5 and DL5 inhibited death due to MRSA infection in mice (15). In order to examine the effect of L5 and DL5 on host immunity, we performed abdominal inoculation in this study, because systemic immune responses can be analyzed using the lavage in the abdominal cavity. We used S. aureus Smith, E. faecalis SR1004, and E. coli EC14, which are highly toxic and relatively easy to use in experiments. First, the minimum lethal dose of the bacteria in the abdominal cavity (i.e., the dose at which all the mice died within 5 days after bacterial infection) was determined (see the table in the supplemental material). We investigated to determine whether injection of 100 μg of L5, L3, and DL5 into the peritoneal cavity before the bacterial infection prevented death in mice. We found that three of the four mice survived when L5 was injected 7 h before intraperitoneal infection with either S. aureus Smith or E. faecalis SR1004 (Table 1). DL5, the enantiomer of L5, was also effective in preventing death due to infection; however, L3 did not exhibit survival-inducing activity (Table 1). L5 and DL5 injection against E. coli EC14 showed a tendency to be less effective than that against S. aureus Smith and E. faecalis SR1004 (Table 1). The in vitro antibacterial activities of these peptides were measured, and the MICs of L5 and DL5 against S. aureus Smith, E. faecalis SR 1004, and E. coli EC14 were not less than 625 μg/ml (data not shown). This indicated that the in vivo protective activity of L5 and DL5 is not dependent on the direct killing of the bacteria.
The abdominal changes in mice after treatment with L5.
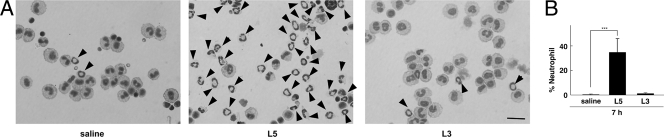
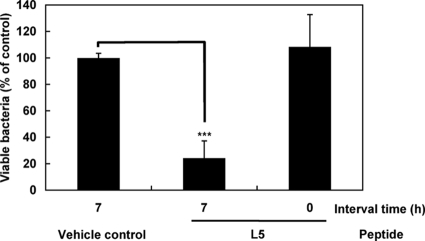
In order to investigate the mode of action of L5, we examined the abdominal cavity for the occurrence of changes after treatment with L5 for 7 h. We collected the peritoneal cells in the lavage of the peritoneal cavity after treatment with L5 for 7 h and prepared Cytospin samples stained with hematoxylin and eosin to examine the changes in the cell population by using a microscope. We found that a large number of neutrophils accumulated in the lavage of the mice treated with L5 (Fig. 1A). Neutrophils did not accumulate in the lavage of vehicle control- and L3-treated mice. After injection of L5 for 7 h, approximately 40% of the cells present were found to be neutrophils, although the number of resident peritoneal neutrophils was small (Fig. 1B). The numbers of total cells in the lavage were almost the same among the saline solution-, L5-, and L3-treated mice (5.6 × 105 cells ± 10% per mouse). Next, we examined the ROS and the antibacterial activity in the abdominal cavity. Using the lucigenin-dependent chemiluminescence method, we measured the ROS that the peritoneal cells had produced after treatment with L5 for 7 h. The cells, possibly the accumulated neutrophils (and partly activated macrophages), apparently produced ROS after treatment with L5 (Fig. 2). ROS was not produced by the cells of the mice treated with L3 or saline solution. Thus, our results strongly suggested that the treatment with L5 would cause the activation of peritoneal cells (neutrophils and macrophages) in vivo as we had observed in vitro. Next, the antibacterial activity in the abdominal cavity was evaluated after treatment with L5 for 7 h. We collected the fluid after infection with S. aureus Cowan I for 15 min and calculated the relative numbers of viable bacteria by counting the CFU in the diluted samples. We observed that the numbers of viable bacteria in the abdominal cavity of the mice treated with L5 for 7 h were less than a quarter of those in the abdominal cavity of the saline-control mice (Fig. 3). This indicated that about 75% of bacteria had been killed or were unable to be harvested by pretreatment with L5. We presumed that L5 enhanced the antibacterial activity in the host abdomen, mainly through the accumulation and activation of the neutrophils that had produced ROS. It also might be possible that L5 activated peritoneal macrophages to efficiently phagocytose and produce ROS, thus increasing bacterium-killing activity. These observations suggested that L5 induced in vivo antimicrobial activity through the activation of host defense systems.
FIG. 1.
Neutrophil recruitment in the lavage of abdominal cavity after treatment with L5 for 7 h. (A) Microscopic observations of peritoneal cells. Mice were treated with intraperitoneal injections of L5, L3, or saline solution, and abdominal lavage was collected after 7 h. Samples were prepared using a Cytospin system, fixed, and stained with Diff-Quick. Neutrophils are indicated by arrowheads. Bar, 100 μm. (B) The change in cell populations after treatment with L5 for 7 h. Cell populations were counted using a microscope. The bars indicate the average percentages of neutrophils in the five fields + standard deviations. Statistical analysis was carried out by an unpaired Student's t test after the data were normalized by an arcsin transformation (***, P < 0.001).
FIG. 2.
Detection of ROS produced by peritoneal cells. Peritoneal cells were collected after treatment with L5, L3, or saline solution for 7 h. The collected peritoneal cells were washed, suspended in Hank's balanced salt solution, and put into 96-well plates. Lucigenin was added to the well, and the chemiluminescence was detected using Biolumat. This experiment was repeated, and the graph shows the representative data points. RLU, relative light units.
FIG. 3.
Antibacterial activity in the abdominal cavity after treatment with L5 for 7 h. At 7 h after intraperitoneal injection of L5, a lethal dose of S. aureus Cowan I (4 × 105 CFU/mouse) was introduced and incubated for 15 min in the abdominal cavity of the mice. The peritoneal lavage was collected and serially diluted with PBS. The serial dilution samples were spread on LB agar plates, and colonies were counted after incubation for 18 h. Statistical analysis of the numbers of colonies was carried out by an unpaired Student's t test (***, P < 0.001). The bars indicate the percentages of viable bacteria relative to a saline control + standard deviations (n = 3).
L5 stimulated the gene expression of the chemokine Mig/CXCL9 in TGC-elicited peritoneal macrophages.
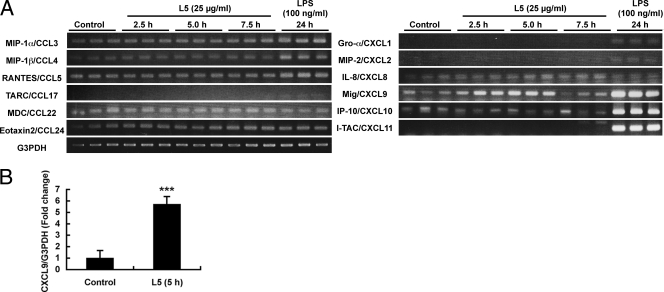
In order to determine the effects of L5 on other immune cells, we examined the changes in chemokine gene expression in the peritoneal macrophages stimulated by L5 in vitro. We used TGC-elicited peritoneal macrophages, because they are very useful in such experiments and are generally accepted as an alternative to resident cells. We examined the levels of mRNA expression in the 12 chemokines. The effect of L5 treatment for 2.5, 5.0, and 7.5 h on chemokine gene expression in the peritoneal macrophages was examined. As the positive control, LPS (100 ng/ml) from E. coli was used. For the first screening, we employed the conventional method of RT-PCR using agarose gel analysis, with ethidium bromide staining. A notable increase in Mig/CXCL9 mRNA expression was detected in the peritoneal macrophages after treatment with L5 for 2.5 to 5.0 h (Fig. 4A). The mRNA level in Mig/CXCL9 returned to normal after treatment with L5 for 7.5 h. Thus, the effect of L5 on Mig/CXCL9 expression was temporary. As compared to those seen with LPS, the effects of L5 on chemokine expression in the macrophages were specific and limited. To determine the effects of L5 on chemokine expression in TGC-elicited macrophages, we performed quantitative real-time RT-PCR. We chose MIP-1α/CCL3, Gro-α/CXCL1, MIP-2/CXCL2, IL-8/CXCL8, and Mig/CXCL9 as candidates for real-time PCR. Among them, only Mig/CXCL9 expression significantly increased in the macrophages treated with L5 compared to those treated with the control at 5.0 h (Fig. 4B).
FIG. 4.
Induction of Mig/CXCL9 expression by L5 in TGC-elicited peritoneal macrophages. (A) Effect of L5 on chemokine expression in TGC-elicited peritoneal macrophages. The cDNA samples from the macrophages treated with 25 μg of L5/ml for 2.5, 5.0, or 7.5 h or with 100 ng of LPS/ml for 24 h were applied to PCR (n = 3). The PCR products were separated on 2% agarose gels in 0.5× Tris-acetate-EDTA and visualized using ethidium bromide. (B) mRNA expression in TGC-elicited peritoneal macrophages measured by quantitative real-time RT-PCR. Expression levels were normalized by the use of G3PDH. Error bars indicate + standard deviations (n = 4 per group). CXCL9 mRNA expression was increased by stimulation with L5. Statistical analysis was carried out by an unpaired Student's t test after the data were normalized by arcsin transformation (***, P < 0.001).
The time course of interval and dose dependency with regard to DL5.
The d-enantiomer of L5 was expected to be resistant to tryptic digestion and remain in the body for a longer duration. Thus, DL5 could be beneficial for clinical use. For a future application, we tested the time course of an interval for treatment with DL5 prior to infection (Table 2). There was no therapeutic effect demonstrated by abdominal treatment with 50 μg of DL5 after S. aureus Smith inoculation at 30 min or at the time of infection in the same abdominal cavity. However, significant survival activity was observed due to treatment with DL5 at 3 or 7 h before infection. Prevention of death due to infection was not observed at 24 h after treatment. Thus, we demonstrated that at least 3 h of the interval time was needed for the significant activity of DL5 and that the activity persisted until at least 7 h (Table 2). These results indicated that the activity of DL5 required some interval time for the induction of host immune responses. Next, the dose-dependent activity of DL5 was observed. The minimum effective dose of DL5 was 25 μg/mouse, and a significant effect of DL5 was observed at doses between 25 and 100 μg/mouse (Table 3).
DISCUSSION
In our previous study, (15), MRSA was introduced intravenously into each mouse and that systemic infection was efficiently treated by the intravenous administration of L5. The aim of the present study was to determine whether L5 prevents death due to infection through the activity of host immune system by activating the neutrophils to produce ROS. We subjected the abdomen of mice to administration of both bacteria and L5 and analyzed the changes in the lavage fluid. We obtained convincing evidence that the intraperitoneal treatment with L5 elicited inflammatory responses in the abdominal cavity, thereby protecting the mice from death due to infection. After injection of L5 and an interval of 7 h, many neutrophils migrated, ROS were produced, and antibacterial activity in the abdomen was elevated almost threefold. These changes were indicative of the in vivo prevention of death due to infection. L5 was found to prevent death due to infection when it was injected 7 h before the bacterial infection. Some time is required after the injection of L5 for the induction of host immune responses. Further, treatment with L3 failed to accumulate neutrophils, produce ROS, and prevent death due to infection. In addition, L5 showed a protective effect in vivo with regard to infection due to several types of bacteria whose growth in vitro was not inhibited by L5. This verified that the elevated antibacterial activity in the abdominal cavity after treatment with L5 for 7 h was not due to the direct killing of the bacteria but was due to the accumulated neutrophils and elevated ROS.
One of our notable findings was that L5 upregulated expression of the Mig/CXCL9 chemokine gene in the TGC-elicited peritoneal macrophages. We expect that resident macrophages would also respond to L5 in the manner in which the TGC-elicited ones did in vivo, at least with respect to expression of Mig/CXCL9, although we need to confirm this. Induction of Mig/CXCL9 expression by L5 was observed in the TGC-elicited macrophages 2.5 to 5 h after stimulation in vitro, i.e., prior to in vivo accumulation of the neutrophils. These results indicated that treatment with L5 might indirectly induce migration of the leukocytes and antibacterial activity through Mig/CXCL9. Mig/CXCL9 is a chemokine that attracts monocytes. However, it has been reported that elevated Mig/CXCL9 and IP-10/CXCL10 expression resulted in neutrophil accumulation and that that expression was suppressed by anti-Mig/CXCL9 and anti-IP-10/CXCL10 antibodies (22). Thus, such expression could partly help in neutrophil recruitment in the abdomen after treatment with L5. In addition, since Mig/CXCL9 is known to possess antibacterial activity (6), it is possible that Mig/CXCL9 contributed to the increase in antibacterial activity.
Neutrophils, which are normally resident in the bloodstream, migrate toward the site of inflammation during the acute phase of infection. L5 induced the migration and activation of the neutrophils; however, the initial trigger and the main factor responsible for these responses to L5 have not yet been clarified. In our previous study, we found that L5 binds to the cell surface calreticulin of human neutrophils and activates to produce O2− and that this effect is inhibited by adding an antibody to calreticulin (4). Therefore, we believe that L5 may directly bind to the calreticulin of the migrated neutrophils, thereby resulting in the production of ROS and the elevation of the antibacterial activity in the abdomen of the mice. Some cationic antimicrobial peptides demonstrate both a direct chemotactic activity for neutrophils and an indirect induction of chemokine production (9, 24, 31). Thus, it is possible that L5 possesses chemotactic activity or indirectly causes the neutrophils to migrate through chemokine production (e.g., that of Mig/CXCL9).
We did not study the relationship between the activity of L5 binding to calreticulin and the prophylaxis activity of L5. Calreticulin is a calcium binding protein (60 kDa) and is found in the endoplasmic reticulum and nucleus and on the cell surface. Recently, it has been reported that the calreticulin on the cell surface plays a role in the neutrophil function together with CD59 (8), phagocytosis of macrophages together with CD91 (19, 27), and autocrine regulation of T-cell motility and migration together with thrombospondin-1 (12). These functions may modulate the innate and/or adaptive immune responses. Binding of L5 to the immune cell surface of calreticulin may be a trigger of host inflammatory response.
We found that DL5 was as potent as L5 in preventing death due to bacterial infection in vivo. The d-enantiomer of L5 was expected to be resistant to tryptic digestion and remain in the body for a longer period. Thus, treatment with DL5 could be beneficial for clinical use. In our previous study, we showed that DL5 activated the neutrophils to produce O2− (15). However, we need to explore the mechanisms behind the action by using DL5, e.g., determining whether it binds to the cell surface calreticulin.
In conclusion, we found that L5 induced the activation of the host immune responses and protected the mice from death due to infection. We propose a novel therapeutic intervention that activates the host immunity in infectious diseases and has an advantage in treatment of antibiotic-resistant bacterial infection.
Supplementary Material
Acknowledgments
We thank Jan-Hyun Cho, Yuki Nakajima, and Hajime Okuyama for helpful discussions and Noemi Koh for technical assistance.
This work was partly supported by a grant for the “Chemical Biology Research Program” from RIKEN. N.A. is a Research Fellow of the Japan Society for the Promotion of Science, and this work was also supported in part by the “Grant-in-Aid for JSPS Fellows” from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
Published ahead of print on 16 March 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Alvarez-Bravo, J., S. Kurata, and S. Natori. 1994. Novel synthetic antimicrobial peptides effective against methicillin-resistant Staphylococcus aureus. Biochem. J. 302:535-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Befus, A. D., C. Mowat, M. Gilchrist, J. Hu, S. Solomon, and A. Bateman. 1999. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J. Immunol. 163:947-953. [PubMed] [Google Scholar]
- 3.Chertov, O., D. F. Michiel, L. Xu, J. M. Wang, K. Tani, W. J. Murphy, D. L. Longo, D. D. Taub, and J. J. Oppenheim. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271:2935-2940. [DOI] [PubMed] [Google Scholar]
- 4.Cho, J.-H., K. J. Homma, S. Kanegasaki, and S. Natori. 1999. Activation of human neutrophils by a synthetic anti-microbial peptide, KLKLLLLLKLK-NH2, via cell surface calreticulin. Eur. J. Biochem. 266:878-885. [DOI] [PubMed] [Google Scholar]
- 5.Cho, J.-H., K. J. Homma, S. Kanegasaki, and S. Natori. 2001. Activation of human monocyte cell line U937 via cell surface calreticulin. Cell Stress Chaperones 6:148-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, A. M., T. Ganz, A. M. Liese, M. D. Burdick, L. Liu, and R. M. Strieter. 2001. IFN-inducible ELR− CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 167:623-627. [DOI] [PubMed] [Google Scholar]
- 7.Fritz, J. H., S. Brunner, M. L. Birnstiel, M. Buschle, A. von Gabain, F. Mattner, and W. Zauner. 2004. The artificial antimicrobial peptide KLKLLLLLKLK induces predominantly a Th2-type immune response to co-injected antigens. Vaccine 22:3274-3284. [DOI] [PubMed] [Google Scholar]
- 8.Ghiran, I., L. B. Klickstein, and A. Nicholson-Weller. 2003. Calreticulin is at the surface of circulating neutrophils and uses CD59 as an adaptor molecules. J. Biol. Chem. 278:21024-21031. [DOI] [PubMed] [Google Scholar]
- 9.Hancock, R. E. W., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann, J. A. 2003. The immune response of Drosophila. Nature 426:33-38. [DOI] [PubMed] [Google Scholar]
- 11.Ichinose, M., M. Asai, K. Imai, and M. Sawada. 1996. Enhancement of phagocytosis by corticostatin I (CSI) in cultured mouse peritoneal macrophages. Immunopharmacology 35:103-109. [DOI] [PubMed] [Google Scholar]
- 12.Li, S. S., A. Forslow, and K.-G. Sundqvist. 2005. Autocrine regulation of T cell motility by calreticulin-thrombospondin-1 interaction. J. Immunol. 174:654-661. [DOI] [PubMed] [Google Scholar]
- 13.Lillard, J. W., P. N. Boyaka, O. Chertov, J. J. Oppenheim, and J. R. McGhee. 1999. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. USA 96:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuyama, K., and S. Natori. 1988. Molecular cloning of cDNA for sapecin and unique expression of the sapecin gene during the development of Sarcophaga peregrina. J. Biol. Chem. 263:17112-17116. [PubMed] [Google Scholar]
- 15.Nakajima, Y., J. Alvarez-Bravo, J.-H. Cho, K.J. Homma, S. Kanegasaki, and S. Natori. 1997. Chemotherapeutic activity of synthetic antimicrobial peptides: correlation between chemotherapeutic activity and neutrophil-activating activity. FEBS Lett. 415:64-66. [DOI] [PubMed] [Google Scholar]
- 16.Natori, S. 1977. Bactericidal substance induced in the haemolymph of Sarcophaga peregrina larvae. J. Insect Physiol. 23:1169-1173. [Google Scholar]
- 17.Natori, S. 1994. Function of anti-microbial proteins in insects, p. 123-134. In H.G. Boman (ed.), Antimicrobial peptides. John Wiley, New York, NY.
- 18.Ogawa, K., M. Funaba, L. S. Mathews, and T. Mizutani. 2000. Activin A stimulates typeIV collagenase (matrix metalloprotease-2) production in mouse peritoneal macrophages. J. Immunol. 165:2997-3003. [DOI] [PubMed] [Google Scholar]
- 19.Ogden, C. A., A. deCathelineau, P. R. Hoffmann, D. Bratton, B. Ghebrehiwet, V. A. Fadok, and P. M. Henson. 2001. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194:781-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada, M., and S. Natori. 1983. Purification of an antibacterial protein from haemolymph of Sarcophaga peregrina (flesh fly) larvae. Biochem. J. 211:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schellack, C., K. Prinz, A. Egyed, J. H. Fritz, B. Wittmann, M. Ginzler, G. Swatosch, W. Zauner, C. Kast, S. Akira, A. V. Gabain, M. Buschle, and K. Lingnau. 2006. IC31, a novel adjuvant signaling via TLR9, induces potent cellular and humoral immune responses. Vaccine 24:5461-5472. [DOI] [PubMed] [Google Scholar]
- 22.Schuh, J. M., K. Blease, and C. M. Hogaboam. 2002. CXCR2 is necessary for the development and persistence of chronic fungal asthma in mice. J. Immunol. 168:1447-1456. [DOI] [PubMed] [Google Scholar]
- 23.Scott, M. G., A. C. E. Vreugdenhil, W. A. Buurman, R. E. W. Hancock, and M. R. Gold. 2000. Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 164:549-553. [DOI] [PubMed] [Google Scholar]
- 24.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. W. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169:3883-3891. [DOI] [PubMed] [Google Scholar]
- 25.Tani, K., W. J. Murphy, O. Chertov, R. Salcedo, C. Y. Koh, I. Utsunomiya, S. Funakoshi, O. Asai, S. H. Herrmann, J. M. Wang, L. W. Kwak, and J. J. Oppenheim. 2000. Defensins act as potent adjuvants that promote cellular and humoral immune responses in mice to a lymphoma idiotype and carrier antigens. Int. Immunol. 12:691-700. [DOI] [PubMed] [Google Scholar]
- 26.Territo, M. C., T. Ganz, M. E. Selsted, and R. Lehrer. 1989. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Investig. 84:2017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandivier, R. W., C. A. Ogden, V. A. Fadok, P. R. Hoffmann, K. K. Brown, M. Botto, M. J. Walport, J. H. Fisher, P. M. Henson, and K. E. Greene. 2002. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 169:3978-3986. [DOI] [PubMed] [Google Scholar]
- 28.Van Wetering, S., S. P. Mannesse-Lazeroms, M. A. Van Sterkenburg, M. R. Daha, J. H. Dijkman, and P. S. Hiemstra. 1997. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am. J. Physiol. 272:L888-L896. [DOI] [PubMed] [Google Scholar]
- 29.Yamada, K., and S. Natori. 1993. Purification, sequence and antibacterial activity of two novel sapecin homologues from Sarcophaga embryonic cells: similarity of sapecin B to charybdotoxin. Biochem. J. 291:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada, K., and S. Natori. 1994. Characterization of the antimicrobial peptide derived from sapecin B, an antibacterial protein of Sarcophaga peregrina (flesh fly). Biochem. J. 298:623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 32.Yang, D., Q. Chen, O. Chertov, and J. J. Oppenheim. 2000. Human neutrophil defensins selectively chemoattract naïve T and immature dendritic cells. J. Leukoc. Biol. 68:9-14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.