Abstract
Nikkomycin Z is an antifungal drug that inhibits chitin synthase. This agent is under development as an orphan product for treatment of coccidioidomycosis. Safety and pharmacokinetics of nikkomycin Z were evaluated in healthy male subjects following single, rising oral doses ranging from 250 mg to 2,000 mg. A total of 12 subjects were recruited and divided into two groups. Group 1 (n = 6) received two out of three doses of 250 mg, 1,000 mg, or 1,750 mg and a placebo randomly in place of one of the doses. Group 2 (n = 6) received two out of three doses of 500 mg, 1,500 mg, or 2,000 mg and a placebo in place of one of the doses. Subjects were confined to the study unit overnight prior to dosing, and 12 blood samples were collected over 24 h postdosing while subjects were confined. Subjects returned for additional blood samples and safety evaluations at 48 h and 72 h after each dose. There was a 2-week washout period between doses. Plasma drug concentrations were determined using a validated high-performance liquid chromatography method. Nikkomycin Z was absorbed after oral administration, reaching a maximum concentration in serum of 2.21 μg/ml at 2 h postdose and an area under the concentration-time curve from 0 h to infinity of 11.3 μg·h/ml for the 250-mg dose. Pharmacokinetics appeared linear over the range of 250 to 500 mg; however, relative bioavailability was about 62 to 70% for the 1,000-mg dose and 42 to 47% for doses between 1,500 and 2,000 mg. The mean terminal half-life ranged from 2.1 to 2.5 h and was independent of dose. No serious or dose-related adverse events were observed. This study provides a basis for pharmacokinetic simulations and continued studies of nikkomycin Z administered in multiple doses.
Nikkomycin Z (NikZ) is an antifungal agent derived from Streptomyces tendae. This agent has activity against endemic fungi, particularly Coccidioides spp. NikZ inhibits chitin synthases, which catalyze the synthesis of chitin, an essential structural component of the fungal cell wall (9, 11). This agent has been demonstrated to have activity in mouse models of coccidioidomycosis, histoplasmosis, and blastomycosis after oral gavage administration (2, 5-7). Compared to conventional antifungal agents, including fluconazole and amphotericin B, NikZ resulted in greater killing of Coccidioides spp. and was able to sterilize lung lesions in seven of eight mice dosed with 50 mg/kg/day for 6 days. The conventional agents tested did not sterilize lung lesions in any case (7).
In a variety of preclinical toxicology studies involving rats and dogs, NikZ was well-tolerated with no consistent organ toxicities. The no observed effect level was at least 300 mg/kg of body weight, based on single and chronic dosing studies. In some of these studies the maximum dose of 1,000 mg/kg was given without any detectable toxicity (data on file at the University of Arizona). NikZ is expected to provide selective toxicity against susceptible fungi, since chitin synthases are not present in mammals.
This study represents the first administration of NikZ to humans. NikZ was studied at doses ranging from 250 to 2,000 mg as a single dose given orally in fasting healthy volunteers. The primary goal was to determine the safety, tolerance, and pharmacokinetics.
(Preliminary results for this study were presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, ON, Canada, 28 September to 1 October 1997.)
MATERIALS AND METHODS
The study was performed in 1997 at Leicester Clinical Research Centre Ltd. (Leicester, England) following approval by the Institutional Review Board. Nikkomycin Z was under development at that time by Shaman Pharmaceuticals but development was stopped due to business reasons. Development was resumed in 2007 after the investigational new drug application was transferred to the University of Arizona. Nikkomycin Z was awarded orphan drug status in 2006 (FDA Office of Orphan Products Development). Study procedures were compliant with Good Clinical Practice standards and applicable standard operating procedures. This placebo-controlled study included 12 healthy male volunteers who were divided into two groups. Group 1 received 250, 1,000, and 1,750 mg or corresponding placebo and group 2 received 500, 1,500, and 2,000 mg or corresponding placebo. There was a 2-week washout between each dose and group 2 dosing was initiated 1 week after group 1 dosing. A matching placebo was administered randomly in place of one of the three doses such that each subject received two of the three dose levels and one placebo dose. The primary end points were adverse events, clinical laboratory profiles, and NikZ pharmacokinetics.
Subjects underwent screening procedures within 21 days of dosing. They were judged as healthy on the basis of a medical history, physical examination, 12-lead electrocardiogram, and clinical laboratory profiles (hematology and serum chemistry). In addition, a urine drug screen, hepatitis B virus surface antigen, and hepatitis C virus antibody tests were negative. The study involved three treatment periods with identical procedures. The urine drug screen was repeated on admission (day zero), at least 12 h before the planned dose. Subjects fasted overnight and remained fasting until 4 h after the dose. Alcohol was not permitted within 24 h of admission and until after the 72-h blood collection. Smoking, ingestion of beverages and food containing caffeine, or strenuous exercise were not permitted during confinement. Subjects took the study medication on the morning of day 1 with 240 ml of water. NikZ was provided as 250-mg gelatin capsules packed with only nikkomycin Z-HCl. Subjects ingested one to eight capsules to obtain the appropriate dose. Placebo capsules contained anhydrous lactose. Subjects remained in an upright sitting position for at least 15 min after dosing. Subjects were confined to the study unit until 24 h after the dose but then returned at 48 and 72 h for follow-up. Blood for the hematology and clinical chemistry profiles was also collected prior to dosing and at 48 and 72 h. These procedures were repeated for periods 2 and 3 on days 14 to 18 and 28 to 32, respectively. The end-of-study safety assessment was made within 3 to 10 days after the last dose of study medication. This evaluation included an interim medical history, physical examination, 12-lead electrocardiogram, and clinical laboratory profiles.
A peripheral catheter was inserted in a forearm vein and used to collect blood samples for pharmacokinetics determinations. Blood samples (5 ml) were collected in heparin-containing tubes prior to dosing and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 16, and 24 h. Subjects were allowed to leave the clinical unit after the 24-h sample was collected and returned for a 48- and 72-h blood sample collected by direct venipuncture. The blood samples were centrifuged in a refrigerated centrifuge at approximately 1,200 × g for 10 min within 30 min of collection. The plasma was separated and stored at −70°C in polypropylene tubes.
Plasma NikZ concentrations were determined using a validated high-performance liquid chromatography method with UV detection at Phoenix International Life Sciences. The lower limit of quantitation was 48 ng/ml. This analysis was in compliance with Good Laboratory Practice standards and standard operating procedures for the laboratory.
Pharmacokinetic evaluation included noncompartmental analysis using WinNonlin (standard version 2.1; Pharsight Inc., Mountain View, CA). The maximum drug concentration in serum (Cmax) and the time to the Cmax (Tmax) were determined from the observed data. The area under the concentration-time curve from 0 h to the time of the last measured concentration (t*) (AUC0-t*) was determined using the linear trapezoidal method from time zero to the time of the last measured concentration. Plots of the log concentration versus time were evaluated to determine the time range for the terminal linear portion, and this time segment was used to determine the terminal elimination rate constant (λz) and terminal half-life (t1/2). The AUC0-∞ was determined based on the formula AUC0-t* + C*/λZ, where C* is the concentration measured at time t*. Dose proportionality was assessed using the power model implemented with the SAS mixed procedure (Statistical Analysis System v 9.3.1; SAS Institute, Inc., Cary, NC) (12). The power model, PK parameter = C × doseβ1 × eɛ [where PK parameter = AUC or Cmax, C = constant, β1 = dose proportionality exponent, and eɛ = exp(residual error)], provides improved statistical power over the traditional analysis of variance because of simultaneous use of data from all doses. The analysis was performed using Proc Mixed (SAS procedure) with the model ln(PK parameter) = ln(dose) and random interindividual error. A critical range was calculated based on the range of doses being studied (12), and the value for β1 was required to fall within the calculated range in order to conclude dose proportionality.
Population analysis was performed using nonlinear mixed effects modeling (NONMEM V; Globomax, Ellicott City, MD). Various structural models were evaluated on individual subjects and the population. The final model was selected based on goodness of fit (residual analysis), parameter precision, and stability. Presence of absorption lag time and dose-related differences in relative bioavailability were explored. Given the relatively small number of subjects studied and their relative homogeneity (all male, all within narrow weight and age ranges, and all healthy), there were no attempts to incorporate subject covariates into the model. Bootstrapping was performed using Wings for NONMEM with 1,000 runs to estimate parameter confidence intervals. The primary goal of the population modeling was to provide a basis for simulations of different dosing regimens, including multiple dosing.
RESULTS
Informed consent was obtained from all subjects, and all 12 subjects completed the study. The mean age was 29 ± 5.7 years (± standard deviation), and the mean weight was 74.4 ± 10.1 kg. The body mass index averaged 23.1 ± 2.35, and no subject was classified as overweight. Nine subjects were Caucasian, two were black, and one was Asian. Half of the subjects were nonsmokers, while the other half smoked from 5 to 10 cigarettes per day at entry; however, subjects abstained from smoking during confinement.
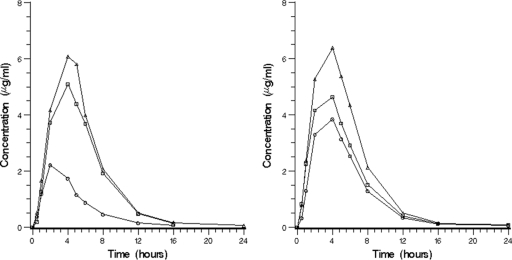
Nikkomycin Z was absorbed following oral administration, with a median Tmax ranging from 2 to 4 h. Although the Tmax tended to be later for the higher doses, there was considerable overlap. All subjects had detectable NikZ concentrations and these remained detectable for at least 12 h. Table 1 provides mean pharmacokinetic parameter values according to dose level. The mean plasma concentration-time profiles are shown in Fig. 1. Bioavailability was dose dependent and was similar for the 250- and 500-mg dose levels. There were two groups of six subjects, each receiving two of three doses. The relative bioavailability based on the AUC0-∞ was 70% for 1,000 mg and 47% for 1,750 mg for group 1, using 250 mg as the reference dose. For group 2, relative bioavailability was 41% for both the 1,500-mg and 2,000-mg doses, using 500 mg as the reference dose. Using the power model, the 90% confidence interval for the exponent (regression slope) was 0.377 to 0.579 for Cmax and 0.415 to 0.648 for the AUC0-∞. Since these ranges fall below the calculated critical range (0.893 to 1.11; see Materials and Methods), we concluded that the Cmax and AUC0-∞ do not increase proportional to dose (Fig. 2). The rho values were 1.43 and 1.46, respectively, indicating that dose proportionality is expected at doses up to 357 mg. Although 500 mg appeared to have excellent relative bioavailability compared to 250 mg, different subjects received these two doses.
TABLE 1.
NikZ pharmacokinetic parameters by dose level
| Dose (mg) | Subject group | Mean value (% relative SD)
|
||||
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Tmaxa (h) | AUC0-t* (μg·h/ml) | AUC0-∞ (μg·h/ml) | t1/2 (h) | ||
| 250 | 1 | 2.21 (13.6) | 2 (2) | 11.3 (18.0) | 11.6 (16.7) | 2.53 (10.7) |
| 500 | 2 | 3.99 (13.6) | 3 (2-4) | 24.3 (12.1) | 24.6 (11.9) | 2.22 (2.64) |
| 1,000 | 1 | 5.14 (24.8) | 4 (2-5) | 32.1 (22.6) | 32.5 (22.7) | 2.06 (4.88) |
| 1,500 | 2 | 4.85 (28.2) | 2 (2-4) | 30.3 (33.1) | 30.6 (33.0) | 2.21 (9.5) |
| 1,750 | 1 | 6.44 (28.0) | 4 (4-5) | 37.4 (29.5) | 37.9 (29.5) | 2.12 (3.68) |
| 2,000 | 2 | 6.42 (7.03) | 4 (2-5) | 40.2 (3.00) | 40.7 (2.58) | 2.09 (8.81) |
For Tmax data, values are medians (ranges in parentheses).
FIG. 1.
Mean concentration (in micrograms per milliliter) of nikkomycin versus time for the two subject groups (n = 4 per dose level). The left panel includes data for subjects who received doses of 250 mg (○), 1,000 mg (□), and 1,750 mg (Δ) (group 1). The right panel includes data for doses of 500 mg (○), 1,500 mg (□), and 2,000 mg (Δ) (group 2).
FIG. 2.
Dose proportionality of nikkomycin Z based on the Cmax (left) and AUC0-∞ (right). Crossbars represent mean values for each dose level.
A one-compartment model with first-order absorption, including absorption lag time, and with first-order elimination was selected. Use of a two-compartment model resulted in overparameterization and instability and did not substantially improve the fit when successful runs were obtained. Final model parameters and 95% confidence intervals obtained from the bootstrap analysis are provided in Table 2. Initially, a term for the fraction absorbed was added for all doses except 250 mg. The F for the 500-mg dose was only slightly above 1. Doses between 1,500 and 2,000 mg exhibited similar F values without a directional trend; thus, these were assumed to be the same. In the final analysis, the relative bioavailability for 250 to 500 mg was fixed at 1; the relatively bioavailability of the 1,000-mg dose was 61.6%; the relatively bioavailability for doses of 1,500 to 2,000 mg was 43.9%. Interindividual variability for oral clearance was 3.55%, but there was also an interoccasion variability of 16.4%. The interindividual variabilities for volume and the first-order absorption rate constant (Ka) were 21.1% and 12.7%, respectively. We were unable to model interoccasion variability for volume and Ka.
TABLE 2.
Parameter estimates for the final population modela
| Parameter | Value (95% CI) | Variability (%) |
|---|---|---|
| Oral clearance (liters/h) | 22.0 (19.8-24.4) | IIV, 3.55; BOV, 16.4 |
| Vol of distribution (liters) | 41.5 (34.0-50.6) | IIV, 21.2 |
| Ka (h−1) | 0.407 (0.363-0.428) | IIV, 12.73 |
| Tlag (h) | 0.695 (0.349-0.754) | |
| Relative bioavailability for: | ||
| 250-500 mg | 1.0 | |
| 1,000 mg | 0.616 (0.465-0.722) | |
| 1,500-2,000 mg | 0.439 (0.353-0.510) |
Abbreviations: CI, confidence interval; Tlag, absorption time lag; IIV, intraindividual variability; BOV, between-occasion variability (expressed as coefficient of variation).
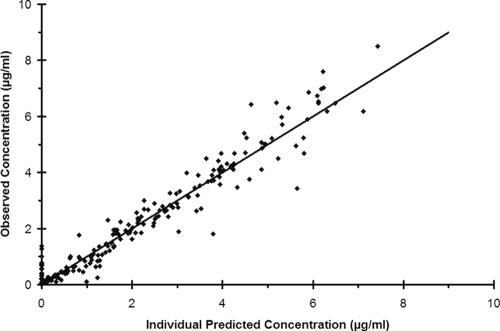
Figure 3 shows the goodness of fit for the selected model. There was a slight bias for some subjects when the predicted concentration was 0 at the 0.5-h sampling time. The concentration was detectable in many of the subjects; however, this time was during the absorption lag time. The improvement in overall fit justified this bias at the earliest time point. A posterior predictive check showed that simulated concentrations at the dose levels studied were representative of the observed data.
FIG. 3.
Goodness of fit for the selected population pharmacokinetic model. Observed plasma drug concentrations are shown versus the predicted concentrations. The solid line represents the line of identity.
This is the first experience with administration of nikkomycin Z to humans. There were 21 adverse events reported among nine subjects. One case each of headache and dizziness were reported at the 250-mg dose level and were considered possibly related to nikkomycin Z by the clinical investigator. Other adverse events were considered unrelated to nikkomycin Z by the clinical investigator and included headache, migraine, toothache, earache, vasodilation, hematoma, and upper respiratory infection. Headaches, toothache, mild diarrhea, and upper respiratory infection were also reported in association with placebo administration. There was no apparent dose-related toxicity or events that were considered probably or definitely caused by nikkomycin Z. One subject had elevated bilirubin (7× upper limit of normal [ULN]), alkaline phosphatase (3× ULN), and aspartate transaminase (1.7× ULN) levels at the 72-h interval, following his second dose (1,000 mg). All of these tests were normal at the 48-h time point and except for a slightly elevated bilirubin level (1.5× ULN) were within normal limits at 7 days postdosing.
DISCUSSION
NikZ represents a novel class of antifungal drugs that inhibit chitin synthase. Chitin is a structural component of fungal cell walls that is present in all fungi in various amounts. Activity against various fungi, however, depends on the binding affinity for the different chitin synthases, the number of different chitin synthases present, and the importance of chitin in relation to other components, such as glucans (4, 9). This agent has potent in vitro activity against Coccidioides spp. and is active against other endemic fungal pathogens, including Histoplasma capsulatum and Blastomyces dermatidis. In vivo activity has been demonstrated in mouse models of coccidiodomycosis, blastomycosis, and histoplasmosis (2, 5-7).
Nikkomycin Z is currently being developed as an orphan drug for treatment of human coccidioidomycosis. A number of antifungal agents are useful for treatment of complicated coccidioidomycis, including amphotericin B, lipid formulations of amphotericin B, fluconazole, and itraconazole (3). Newer azoles, including voriconazole and posaconazole, may be useful in refractory cases (1, 8, 10); however, clinical experience with these agents is limited. None of these drugs has been shown to improve outcomes of primary coccidioidomycosis or to prevent complications. They are used for complicated pulmonary and extrapulmonary infections and basically serve to suppress progression of infection while the immune system contains and eventually cures the infection. Treatment duration ranges from a minimum of 6 months to several years. The more severe forms of coccidioidomycosis, including meningitis and disseminated infection in immunocompromised patients, generally require treatment for life (3).
In the experimental mouse model of coccidioidomycosis, NikZ was effective in sterilizing lungs of 7/8 mice treated with NikZ at 50 mg/kg twice daily for 6 days. This fungicidal effect was not observed with fluconazole or amphotericin B, raising the possibility that NikZ may be more effective than existing therapies (7). If this fungicidal effect is found in the management of natural infections, NikZ has the potential to shorten the course of illness, to prevent complications from progressive infection if treatment is initiated early, and to better manage and possibly cure chronic complicated forms of coccidioidomycosis. However, the clinical potential of NikZ must be evaluated in clinical trials. The current study represents one step toward establishing its safety and providing a pharmacokinetic basis for dose regimen design.
Nikkomycin Z was well-tolerated when given as a single oral dose to healthy subjects, and the oral bioavailability appears to be sufficient to warrant continued development. There were no dose-related adverse events noted with doses up to 2,000 mg; however, the relative bioavailability is lower with doses of 1,000 mg or higher. The terminal elimination half-life was 2 to 2.5 h, suggesting that either dosing every 8 or 12 h will be necessary.
Acknowledgments
This work was funded in part by the Office of Orphan Product Development, Food and Drug Administration, grant number 1R01FD003347-01, NIH-NIAID 1 R34 AI072320-01, the U.S. Department of Veterans Affairs, and the J. T. Tai & Company Foundation.
Data were generated while nikkomycin Z was under license at Shaman Pharmaceuticals. R.R.S. and R.F.H. were employed at Shaman and involved in study oversight during conduct of the clinical study. In 2005, the designated investigational new drug application for nikkomycin Z was transferred to the University of Arizona. Valley Fever Solutions Inc. was formed in 2007 to assist in commercialization of nikkomycin Z. J.N.G. is Chief Medical Officer and a major stockholder.
Footnotes
Published ahead of print on 6 April 2009.
REFERENCES
- 1.Antony, S. J., P. Jurczyk, and L. Brumble. 2006. Successful use of combination antifungal therapy in the treatment of coccidioides meningitis. J. Nat. Med. Assoc. 98:940-942. [PMC free article] [PubMed] [Google Scholar]
- 2.Clemons, K. V., and D. A. Stevens. 1997. Efficacy of nikkomycin Z against experimental pulmonary blastomycosis. Antimicrob. Agents Chemother. 41:2026-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galgiani, J. N., N. M. Ampel, J. E. Blair, A. Catanzaro, R. H. Johnson, D. A. Stevens, and P. L. Williams. 2005. Coccidioidomycosis. Clin. Infect. Dis. 41:1217-1223. [DOI] [PubMed] [Google Scholar]
- 4.Gaughran, J. P., M. H. Lai, D. R. Kirsch, and S. Silverman. 1994. Nikkomycin Z is a specific inhibitor of Saccharomyces cerevisiae chitin synthase isozyme Chs3 in vitro and in vivo. J. Bacteriol. 176:5857-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg, J., P. Connolly, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, E. Brizendine, R. Hector, and J. Wheat. 2000. Comparison of nikkomycin Z with amphotericin B and itraconazole for treatment of histoplasmosis in a murine model. Antimicrob. Agents Chemother. 44:1624-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graybill, J. R., L. K. Najvar, R. Bocanegra, R. F. Hector, and M. F. Luther. 1998. Efficacy of nikkomycin Z in the treatment of murine histoplasmosis. Antimicrob. Agents Chemother. 42:2371-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hector, R. F., B. L. Zimmer, and D. Pappagianis. 1990. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob. Agents Chemother. 34:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrin, K. V., A. Miranda, and D. Loebenberg. 2005. Posaconazole therapy for systemic coccidioidomycosis in a chimpanzee (Pan troglodytes): a case report. Mycoses 48:447-452. [DOI] [PubMed] [Google Scholar]
- 9.Obi, K., J. Uda, K. Iwase, O. Sugimoto, H. Ebisu, and A. Matsuda. 2000. Novel nikkomycin analogues: inhibitors of the fungal cell wall biosynthesis enzyme chitin synthase. Bioorg. Med. Chem. Lett. 10:1451-1454. [DOI] [PubMed] [Google Scholar]
- 10.Ramani, R., and V. Chaturvedi. 2007. Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non-endemic areas. Mycopathalogia 163:315-319. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Herrera, J., and G. San-Blas. 2003. Chitin synthesis as a target for antifungal drugs. Curr. Drug Targets Infect. Disord. 3:77-91. [DOI] [PubMed] [Google Scholar]
- 12.Smith, B. P., F. R. Vandenhende, K. A. DeSante, N. A. Farid, P. A. Welch, P. T. Calloghan, and S. T. Forgue. 2000. Confidence interval criteria for assessment of dose proportionality. Pharm. Res. 17:1278-1283. [DOI] [PubMed] [Google Scholar]