Abstract
Mutation of the staphylococcal accessory regulator (sarA) in Staphylococcus aureus limits but does not abolish the capacity of the organism to form a biofilm. As a first step toward determining whether this limitation is therapeutically relevant, we carried out in vitro studies comparing the relative susceptibility of an S. aureus clinical isolate (UAMS-1) and its isogenic sarA mutant (UAMS-929) in the specific context of a catheter-associated biofilm. The antibiotics tested were daptomycin, linezolid, and vancomycin, all of which were evaluated by using concentrations based on the MIC defined as the breakpoint for a susceptible strain of S. aureus (≤1.0, ≤2.0, and ≤4.0 μg/ml for daptomycin, vancomycin, and linezolid, respectively). Mutation of sarA had no significant impact on the MIC of UAMS-1 for any of the targeted antibiotics, as defined by Etest antimicrobial susceptibility testing. However, mutation of sarA did result in a significant increase in antimicrobial susceptibility to all targeted antibiotics when they were tested in the specific context of a biofilm. Additionally, whether susceptibility was assessed by using UAMS-1 or its sarA mutant, daptomycin was found to be more effective against established S. aureus biofilms than either linezolid or vancomycin.
Staphylococcus aureus is a devastating human pathogen, with the death toll from invasive S. aureus infections recently having passed that from AIDS in the United States (9, 18). These infections range from acute toxemias and septic shock to more chronic infections, including osteomyelitis and infections associated with indwelling medical devices. The latter are characterized by the formation of a biofilm, which has a significant impact not only on the disease process itself but also on the ability of clinicians to effectively treat the infection. This is true because biofilm-associated infections are recalcitrant to antimicrobial therapy, irrespective of the resistance status of the offending strain or the ability to achieve what would otherwise be therapeutic serum levels of antibiotic (10, 21, 30). For this reason, the resolution of biofilm-associated staphylococcal infections often requires surgical debridement to remove infected tissues and/or indwelling devices (5, 20).
An alternative approach to the therapeutic problem of biofilm-associated infection would be the development of methods that specifically prevent or at least limit biofilm formation. This could be done by targeting either the substrate (e.g., by developing novel biomaterials that are less conducive to biofilm formation) or the bacterium (e.g., by developing novel agents capable of limiting biofilm formation). The latter approach requires the identification of those bacterial targets that are most relevant in the specific context of a biofilm. Studies focusing on the staphylococci have led to the identification of many potential targets (24). However, in almost every case, there are conflicting reports about the roles of different genes and gene products in biofilm formation (12, 24, 35).
The single exception is the staphylococcal accessory regulator (sarA), the mutation of which has been shown to limit biofilm formation in both S. aureus and S. epidermidis (2, 7, 15, 25, 28, 31, 32, 33, 34, 37). This is true with respect to both in vitro and in vivo models of biofilm formation (3, 6). However, in no case has mutation of sarA eradicated biofilm formation. This suggests that the therapeutic relevance of any antibiofilm approach directed at sarA would be dependent on the ability to enhance conventional antimicrobial therapy. The studies reported here were aimed at addressing this possibility by determining whether the reduced capacity of an S. aureus sarA mutant to form a biofilm can be correlated with increased susceptibility to specific antibiotics in the context of an established, catheter-associated biofilm.
MATERIALS AND METHODS
Bacterial strains.
S. aureus clinical isolate UAMS-1 and its isogenic sarA mutant (isolate UAMS-929) have been described elsewhere (4). The fact that mutation of sarA limits biofilm formation in UAMS-1 has been confirmed by using a static microtiter plate assay, flow cells, and an in vivo murine model (2, 3). The impact of a mutation in sarA on antibiotic susceptibility under standard growth conditions was assessed by use of an Etest strip (AB Biodisk, Solna, Sweden) with tryptic soy agar as the base medium.
Catheter-based model of biofilm formation.
The relative capacity of UAMS-1 and its sarA mutant to form a biofilm was assessed by using an in vitro method in which 1-cm segments of fluorinated ethylene propylene catheters (14-gauge Introcan Safety catheter; B. Braun, Bethlehem, PA) were first coated with human plasma, as described previously (2). The coated catheters were then placed in the wells of a 12-well microtiter plate containing 2 ml of tryptic soy broth supplemented with glucose and sodium chloride (biofilm medium [BM]) (2, 34). Each well was then inoculated with UAMS-1 or its sarA mutant at an optical density at 600 nm of 0.05 and the plate was incubated at 37°C. Catheters (n = 3) were recovered at daily intervals for 3 days, with the medium being replaced in its entirety each day.
After recovery at each time point, the catheters were rinsed in phosphate-buffered saline (PBS) to remove nonadherent bacteria and then placed into a test tube containing 5 ml of sterile PBS. To remove the adherent bacteria, each catheter was then sonicated for 4 min with a Fisher 550 sonic dismembrator fitted with a microtip probe at 20% capacity (Thermo Fisher Scientific, Waltham, MA). This protocol was empirically determined to provide the level of sonication required to remove all adherent bacteria without a significant loss of viability (6). After sonication, 100-μl aliquots of appropriately diluted samples were plated on tryptic soy agar without antibiotic selection. The total number of bacteria recovered from each catheter was then calculated on the basis of the number of colonies obtained and the corresponding dilution factor.
We employed the same the in vitro model to assess relative antimicrobial susceptibility, except that catheters containing established biofilms were removed after the initial overnight incubation, rinsed in PBS, and then transferred to fresh BM without antibiotics or BM containing daptomycin, vancomycin, or linezolid at concentrations corresponding to 5, 10, or 20 times the concentration defined as the breakpoint MIC for a sensitive strain of S. aureus (≤1.0, ≤2.0, and ≤4.0 μg/ml for daptomycin, vancomycin, and linezolid, respectively). In experiments with daptomycin, all BM, including that lacking antibiotic, was further supplemented with 2.5 mM CaCl2. Catheters exposed to each antibiotic at each dose (n = 3) were recovered at daily intervals for 3 days and processed as described above.
Statistical analysis.
The distribution of the bacterial count data was highly skewed and included multiple zero counts, which made it inappropriate to employ traditional parametric analysis (e.g., t test and analysis of variance [ANOVA]). For this reason, these data were analyzed by using nonparametric tests and traditional parametric methods that employed permutation tests to calculate P values (14). Specifically, Wilcoxon rank-sum tests were used to make comparisons between untreated samples, while Kolmogorov-Smirnov tests (8) were used to compare bacterial count distributions for the antibiotic-treated groups. We also performed ANOVA on the logarithmically transformed bacterial count data in order to evaluate the effect of the sarA mutation in the context of both antibiotic concentration and time of exposure. Using these ANOVA models, we were able to assess not only the effect of the sarA mutation but also any interaction between the effect of the mutation and antibiotic dose and/or time of exposure. The significance of the ANOVA test statistics were also calculated by using permutation tests (14). When pairwise testing was appropriate, t tests were used for the logarithmically transformed data, with P values again being calculated by using permutation tests. All statistical analyses were performed by using the R program (version 2.7; The Foundation for Statistical Computing), with P values of ≤0.05 being considered significant.
RESULTS
Previous experiments employing both in vitro and in vivo models of biofilm formation have confirmed that mutation of sarA limits the ability of UAMS-1 to form a biofilm (2, 3). However, we employed a different in vitro model in these experiments because it facilitated our ability to assess antimicrobial susceptibility, and for this reason, it was important that we first assess the impact of mutating sarA on biofilm formation in our catheter-based model of biofilm formation. The results confirmed that mutation of sarA limits but does not eradicate biofilm formation (Fig. 1). Specifically, by comparison to the number of viable bacteria recovered from catheters colonized with the UAMS-1 parent strain, we observed a significant reduction in the number of viable bacteria recovered from catheters colonized with the UAMS-1 sarA mutant at all time points tested (P ≤ 0.001). However, while the level of reduction was statistically significant, it was less than 1 log unit (3.34 × 108 and 8.92 × 107, respectively, on day 3 of treatment). Moreover, the level of colonization remained essentially unchanged for both UAMS-1 and its sarA mutant throughout the 3-day treatment period (Fig. 1).
FIG. 1.
Impact of sarA on biofilm formation. Catheters colonized with UAMS-1 (white bars) or its isogenic sarA mutant (gray bars) were harvested at daily intervals. Biofilm formation was assessed as described in the text. Horizontal lines indicate the median from each group. Each box defines the interquartile range between the 25th and 75th percentiles. Bars extending upward from each box indicate the boundary defined by values 1.5 times higher than the 75th percentile, while those extending downward represent the boundary defined by values 1.5 times lower than the 25th percentile. Open circles represent individual data points outside these two extremes. The numbers are P values obtained in comparisons of the count data for catheters colonized with UAMS-1 or its sarA mutant on each day in the absence of antibiotic exposure.
To assess the clinical relevance of the impact of sarA on biofilm formation, we next assessed whether mutation of sarA increases the susceptibility of S. aureus to antibiotic therapy in the context of an established biofilm. Specifically, we employed our in vitro model of catheter-based biofilm formation to assess the relative susceptibilities of UAMS-1 and its isogenic sarA mutant to daptomycin, linezolid, and vancomycin. As assessed by Etest strip susceptibility testing, mutation of sarA had no impact on the MICs of any of these antibiotics for UAMS-1, with the MICs of daptomycin, linezolid, and vancomycin for both UAMS-1 and its sarA mutant being approximately 0.75, 1.00, and 1.50 μg/ml, respectively (data not shown).
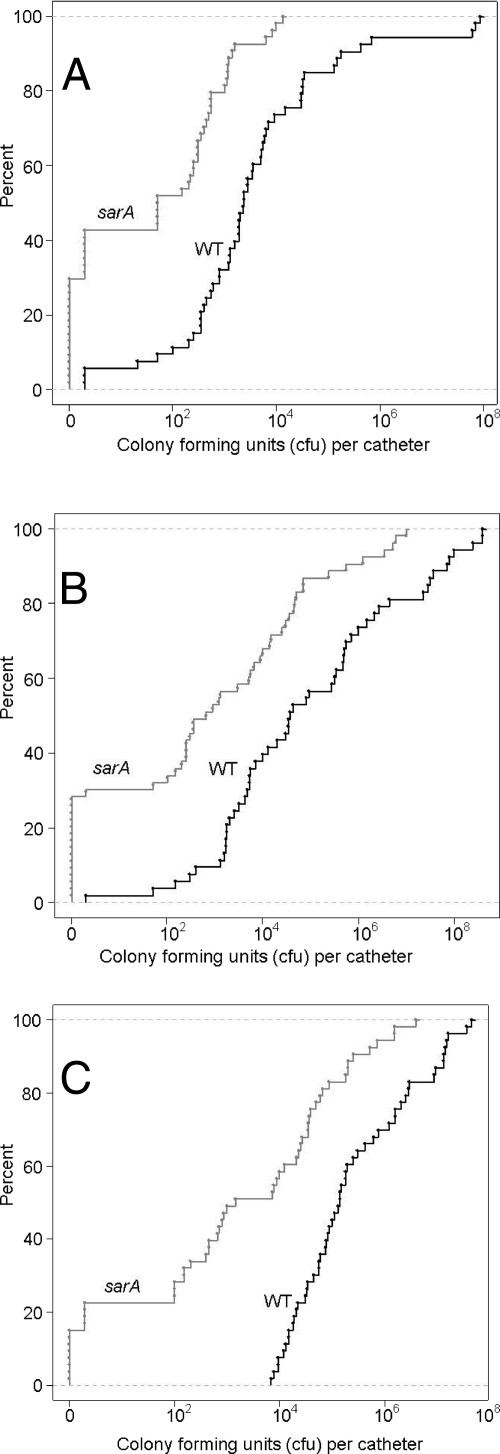
All antibiotics tested in these experiments were evaluated over time at multiple concentrations. However, to assess the overall impact of each antibiotic on the parent strain compared with that on its sarA mutant, we first analyzed the results solely on the basis of the strain rather than the antibiotic concentration or the time of exposure. That analysis confirmed that all three antibiotics had a greater impact on the biofilms formed by the sarA mutant than on the biofilms formed by the UAMS-1 parent strain. For instance, when catheters colonized with the sarA mutant were exposed to daptomycin, over 50% were found to have less than 100 CFU per catheter, while approximately 30% had no detectable bacteria (Fig. 2A). In contrast, when catheters colonized with UAMS-1 were exposed to daptomycin under the same conditions, less than 12% had less than 100 CFU per catheter and all were colonized at a detectable level (Fig. 2A). Statistical analysis confirmed the significance of these differences (P < 0.001) in terms of both the relative number of catheters colonized with <100 CFU and the number cleared of detectable bacteria.
FIG. 2.
Impact of sarA on overall antibiotic susceptibility. Graphs illustrate the overall distribution of count data obtained with individual catheters colonized with UAMS-1 (wild type [WT]) or its sarA mutant and exposed to daptomycin (A), vancomycin (B), or linezolid (C), irrespective of the antibiotic concentration or the time of exposure. The fact that all curves are shifted to the left demonstrates that the sarA mutant was more susceptible to all three antibiotics at all concentrations (5×, 10×, and 20×) and at all time points tested (1, 2, and 3 days).
In the case of vancomycin, 25% of the catheters colonized with the sarA mutant were cleared of detectable bacteria, while ∼35% had less than 100 CFU/catheter (Fig. 2B). In contrast, none of the catheters colonized with UAMS-1 were cleared by exposure to vancomycin, and ∼90% were colonized with >100 CFU/catheter. With linezolid, 14% of the catheters colonized with a sarA mutant were cleared (Fig. 2C), which was the lowest percentage among the three antibiotics tested. However, all of the catheters colonized with UAMS-1 and exposed to linezolid even at the highest concentration (20×) and for the longest period of time tested (3 days) were colonized with at least 104 CFU (Fig. 2C). This presumably reflects the fact that linezolid, unlike daptomycin and vancomycin, is bacteriostatic (23). Nevertheless, these results demonstrate that the greatest impact of the sarA mutation on biofilm-associated susceptibility was with linezolid rather than either of the other antibiotics tested in these experiments.
When the antibiotics were analyzed irrespective of the antibiotic concentration or the time of exposure, all three antibiotics were found to have a greater overall effect on the biofilms formed by the sarA mutant (Fig. 2A to C). However, we did observe both concentration and time-dependent effects in all cases. Not surprisingly, the greatest difference between the susceptibility of UAMS-1 and its sarA mutant with the lower 5× concentrations was observed on day 3, while the greatest difference with the 20× concentration was observed on day 1 (Fig. 3A to C). This was the most evident with daptomycin (Fig. 3A) and vancomycin (Fig. 3B), both of which had a greater impact than linezolid on the clearing of catheter-associated biofilms (Fig. 2A to C). In contrast, the efficacy of linezolid was more dependent on the time of exposure than on the antibiotic concentration (Fig. 3C). However, in all cases, statistical analysis confirmed that the biofilms formed by UAMS-1 were more resistant than those formed by its sarA mutant to daptomycin (P = 0.001), vancomycin (P = 0.003), and linezolid (P = 0.025), even after accounting for the reduced ability of the sarA mutant to form a biofilm.
FIG. 3.
Concentration- and time-dependent impacts of sarA on antibiotic susceptibility. The results illustrate the count data obtained with daptomycin (A), vancomycin (B), and linezolid (C) as a function of the antibiotic concentration and the time of exposure with UAMS-1 (white bars) and its isogenic sarA mutant (gray bars). Each box defines the interquartile range between the 25th and 75th percentiles. Bars extending upward from each box indicate the boundary defined by values 1.5 times higher than the 75th percentile, while those extending downward represent the boundary defined by values 1.5 times lower than the 25th percentile. Open circles represent individual data points outside these two extremes. Statistical analysis confirmed that the sarA mutant was more susceptible than the parent strain to all three antibiotics to an extent that cannot be fully explained by the reduced capacity of the sarA mutant to form a biofilm (see the text). d1, d2, and d3, days 1, 2, and 3, respectively.
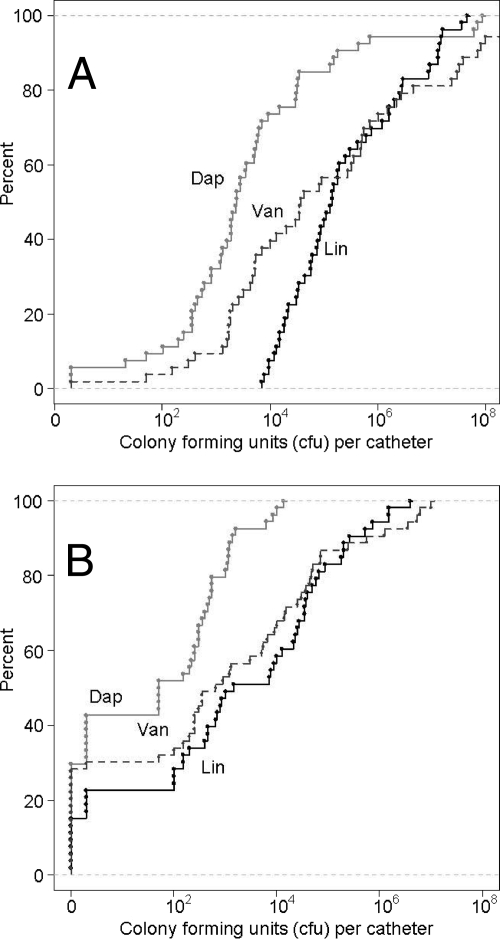
The results discussed above demonstrate that the mutation of sarA in S. aureus clinical isolate UAMS-1 limits biofilm formation and increases the susceptibilities of the bacteria within an established biofilm to daptomycin, vancomycin, and linezolid. To determine whether each antibiotic was equivalent in this regard, we carried out a comparison in which relative efficacy was evaluated not by comparison of the results for treated to those for untreated catheters but, rather, by comparison of the results obtained between antibiotics. Statistical analysis confirmed that daptomycin was more effective than vancomycin (P ≤ 0.002) and linezolid (P ≤ 0.001), irrespective of whether the comparison was based on UAMS-1 (Fig. 4A) or its sarA mutant (Fig. 4B). Vancomycin was also found to be more effective than linezolid, although in this case the effect was apparent with the wild-type strain (P = 0.002) but not the sarA mutant (P = 0.582). Given the results discussed above, this presumably reflects the greater impact of the sarA mutation on linezolid susceptibility rather than the absence of a correlation between the mutation of sarA and increased vancomycin susceptibility.
FIG. 4.
Relative overall efficacy of individual antibiotics. Graphs illustrate the overall distribution of the count data obtained with individual catheters colonized with UAMS-1 (A) or its sarA mutant (B) and exposed to daptomycin (Dap), vancomycin (Van), or linezolid (Lin), irrespective of the antibiotic concentration or the time of exposure. The fact that curves obtained with daptomycin are shifted to the left by comparison to the curves obtained with both vancomycin and linezolid is indicative of the greater efficacy of daptomycin in the context of biofilms formed by both UAMS-1 and its sarA mutant.
To further evaluate the relative efficacy of each antibiotic, we carried out additional analyses based on both the antibiotic concentration and the time of exposure. This was done independently for UAMS-1 and its isogenic sarA mutant. With respect to UAMS-1, we did not observe a significant difference with the 5× antibiotic concentrations at any time point (Fig. 5). However, after 1 day of exposure, daptomycin was found to be more effective than either vancomycin or linezolid at both the 10× concentrations (P = 0.0032) and the 20× concentrations (P = 0.0033). At the 10× concentrations, daptomycin was also found to be more effective than either linezolid or vancomycin on both day 2 (P = 0.0027) and day 3 (P = 0.0027). The same was true with the 20× concentration on day 2 (P = 0.0051); however, on day 3, the efficacy of vancomycin was comparable to that of daptomycin, with both yielding colony counts significantly lower than those observed with linezolid (P = 0.0039). The increased efficacy of daptomycin by comparison to the efficacies of both linezolid and vancomycin was most evident when comparisons of the results obtained with different concentrations of antibiotics were made. Specifically, on both day 1 and day 2, treatment with daptomycin resulted in significantly lower colony counts with a UAMS-1 biofilm even when the comparison was made between 10× daptomycin and each of the other antibiotics at the 20× concentration (Fig. 6).
FIG. 5.
Time- and concentration-dependent effects with the wild-type strain. The results illustrate the count data obtained when catheters colonized with UAMS-1 were exposed to each antibiotic at each concentration over time. Each box defines the interquartile range between the 25th and 75th percentiles. Bars extending upward from each box indicate the boundary defined by values 1.5 times higher than the 75th percentile, while those extending downward represent the boundary defined by values 1.5 times lower than the 25th percentile. Open circles represent individual data points outside these two extremes. The numbers in each box are P values based on pairwise comparisons between different antibiotics at each concentration and each time point. Dap, daptomycin; Van, vancomycin; Lin, linezolid.
FIG. 6.
Comparisons across antibiotic concentrations with the wild-type strain. The count data obtained on days 1 and 2 for catheters colonized with UAMS-1 were exposed to daptomycin at a 10× concentration (Dap10) were compared with those for catheters exposed to linezolid or vancomycin at a 20× concentration (Lin20 and Van20, respectively). Each box defines the interquartile range between the 25th and 75th percentiles. Bars extending upward from each box indicate the boundary defined by values 1.5 times higher than the 75th percentile, while those extending downward represent the boundary defined by values 1.5 times lower than the 25th percentile. Open circles represent individual data points outside these two extremes. The numbers above each bar are P values obtained for comparisons made between daptomycin and each of the other antibiotics.
The increased efficacy of daptomycin was also evident when the same analysis was done by focusing on biofilms formed by the UAMS-1 sarA mutant. In fact, statistically significant differences were observed even on day 1 with all antibiotic concentrations tested (Fig. 7). However, on day 2, the only significant difference was observed with 20× antibiotic concentrations (P = 0.0072), and no significant differences were observed on day 3 at any concentration. The fact that very low colony counts were observed with all three antibiotics at all concentrations on day 3 (Fig. 7) indicates that the lack of an antibiotic-dependent difference further reflects the increased susceptibility of biofilms formed by a sarA mutant rather than the relative efficacy of the individual antibiotics. Further support for this hypothesis comes from the observation that daptomycin was found to a greater impact on day 1 at the 5× concentration even by comparison to the impact of linezolid or vancomycin at 20× concentrations (Fig. 8).
FIG. 7.
Time- and concentration-dependent effects with the sarA mutant. The results illustrate the count data obtained when catheters colonized with the UAMS-1 sarA mutant were exposed to each antibiotic at each concentration over time. Each box defines the interquartile range between the 25th and 75th percentiles. Bars extending upward from each box indicate the boundary defined by values 1.5 times higher than the 75th percentile, while those extending downward represent the boundary defined by values 1.5 times lower than the 25th percentile. Open circles represent individual data points outside these two extremes. The numbers in each box are P values obtained from pairwise comparisons between different antibiotics at each concentration and each time point. Dap, daptomycin; Van, vancomycin; Lin, linezolid.
FIG. 8.
Comparisons across antibiotic concentrations with the sarA mutant. The count data obtained on day 1 when catheters colonized with the UAMS-1 sarA mutant were exposed to daptomycin at a 5× concentration (Dap5) were compared with those for catheters exposed to linezolid at a 10× or a 20× concentration (Lin10 and Lin20, respectively) or vancomycin at a 10× or a 20× concentration (Van10 and Van20, respectively). Each box defines the interquartile range between the 25th and 75th percentiles. Bars extending upward from each box indicate the boundary defined by values 1.5 times higher than the 75th percentile, while those extending downward represent the boundary defined by values 1.5 times lower than the 25th percentile. Open circles represent individual data points outside these two extremes. The numbers above each bar are P values for comparisons made between daptomycin and each of the other antibiotics.
DISCUSSION
The presence of a biofilm compromises antimicrobial therapy for the treatment of S. aureus infections, irrespective of issues related to acquired antibiotic resistance. As a first step toward determining whether inhibitors of sarA would offer a therapeutic advantage in this regard, we used an in vitro model to examine whether mutation of sarA in an S. aureus clinical isolate (UAMS-1) enhances the efficacy of antimicrobial therapy in the specific context of a biofilm. On the basis of the presumption that any effects that we observed might be dependent on the antibiotic under study, we examined this question using three different antimicrobial agents (daptomycin, linezolid, and vancomycin), all of which were chosen because they are primary antistaphylococcal agents that are active even against methicillin-resistant S. aureus (MRSA) strains, including community-acquired MRSA strains (1, 23). Each antibiotic also has a different mode of action, with daptomycin being a membrane-active cyclic lipopeptide, linezolid being an inhibitor of protein synthesis, and vancomycin being an inhibitor of cell wall biosynthesis.
Although our choice of antibiotics was based in part on their activity against MRSA, we employed methicillin-sensitive strain UAMS-1 in these experiments on the basis of four considerations. First, it is a clinical isolate obtained by surgical biopsy directly from the bone from a patient with osteomyelitis (29). This is relevant, in that osteomyelitis is one of the forms of S. aureus infection that is most definitively associated with the formation of a biofilm (5). Second, numerous studies have confirmed that UAMS-1 forms a robust biofilm both in vitro and in vivo (2, 3, 4, 6). Third, UAMS-1 belongs to clonal complex 30 (CC30) (6), and independent studies have confirmed that strains belonging to CC30 are among the most prominent clinical isolates of S. aureus and may even have an increased tendency to cause complicated hematogenous infections, including osteomyelitis (13). Fourth, mutation of sarA has been shown to limit the capacity of UAMS-1 to form a biofilm in a manner comparable to that of other S. aureus clinical isolates, including MRSA (2). On the basis of these considerations, we are confident that the results that we report here represent clinically relevant observations both in the general context of S. aureus clinical isolates and in the specific context of an established S. aureus biofilm.
Excluding the observation that mutation of sarA limits the ability of UAMS-1 to form a biofilm, which has been well established by using various other models of biofilm formation (2, 3, 6), there are two primary conclusions from the results that we report. The first is that mutation of sarA results in increased antibiotic susceptibility in the specific context of an established biofilm. This was true of all antibiotics tested. More importantly, it was true even after the reduced capacity of the sarA mutant to form a biofilm was taken into account. The reasons for this are unclear, but one possible explanation is that biofilms formed by the sarA mutant are simply thinner or less dense. On the basis of the presumption that antibiotic penetration into the biofilm is one factor that contributes to its intrinsic resistance, the increased susceptibility of a sarA mutant could reflect the greater penetration of antibiotic into the bulk of bacterial cells within the biofilm rather than some inherent increase in susceptibility associated with mutation of sarA. This hypothesis is consistent with the observation that when susceptibility was measured by Etest, no change in the MIC of any of the antibiotics tested for the sarA mutant was observed. It is also consistent with a report indicating that the penetration of vancomycin into the deeper regions of an S. aureus biofilm is delayed, perhaps to the point that the bacteria in these regions have the opportunity to adapt in a manner that promotes some degree of intrinsic resistance (16). Indeed, Szomolay et al. (31) proposed a model in which the ultimate outcome of exposure to an antibiotic is a race between the lethal effects of the antibiotic and the bacterium's ability to adapt, with the biofilm essentially slowing penetration to the point where this race is won by a larger proportion of the bacteria. In this context, mutation of sarA could in effect limit this delay and thereby provide S. aureus with less of an opportunity to initiate an effective adaptive response.
There are several possible explanations for why a biofilm formed by a sarA mutant might differ from biofilms formed by wild-type strains. For instance, mutation of sarA has been shown to result in reduced amounts of the polysaccharide intercellular adhesion (3) and increased amounts of extracellular enzymes, including nuclease (36), with the latter being relevant, in that DNA was recently shown to contribute to the extracellular matrix of a UAMS-1 biofilm (27). Mutation of sarA also results in the increased production of several extracellular proteases (6, 36). Increased protease production has been associated with a decreased capacity to bind to fibronectin (4, 17), and to the extent that fibronectin binding could potentially contribute to both the attachment and the accumulation phases of biofilm formation, this could also result in biofilms that are thinner and/or less structured and thus more susceptible to antibiotic penetration.
An alternative explanation for our results is that mutation of sarA limits the adaptive response of S. aureus, irrespective of whether it has any impact on antibiotic penetration. This is consistent with the observation that mutation of sarA results in global changes in gene expression, many of which involve genes that are also differentially expressed in a biofilm (3, 6). These include numerous genes involved in central metabolic processes. Hence, sarA mutants may simply be less fit and therefore less able to withstand the insult of an antibiotic in a manner that is apparent in a biofilm but not in more conventional growth settings, including those employed in our susceptibility tests with Etest strips. Resolution of these issues will require further investigation, including the analysis of additional mutants. The more important point in the context of this report is that, irrespective of the mechanism involved, mutation of sarA increases antibiotic susceptibility in the specific context of an established biofilm.
The second important conclusion from our results is that daptomycin is a more effective antibiotic than either linezolid or vancomycin in the context of an established S. aureus biofilm. We base this conclusion on the observation that the greatest likelihood of clearing a biofilm, whether it was formed by UAMS-1 or its sarA mutant, was when the biofilm was exposed to daptomycin. This was evident when the analysis was done independently of the antibiotic concentration or the time of exposure and when it was done by taking both of these factors into account. More directly, analysis of the results obtained with both UAMS-1 and its sarA mutant demonstrated that daptomycin is more effective than either linezolid or vancomycin both at lower concentrations and at earlier time points, both of which are important clinical considerations. Our decision to test each antibiotic at concentrations corresponding to 5×, 10×, and 20× the breakpoint MIC defined for a susceptible strain of S. aureus rather than the specific MIC for the strains under study is also relevant in this regard. This decision was based on two considerations. The first was the findings of preliminary experiments that demonstrated that concentrations less than 5× had little effect in the context of an established biofilm (data not shown). This confirms the inherent recalcitrance of biofilm-associated bacteria to antimicrobial therapy, even in the absence of issues related to a reduced capacity to deliver antibiotics to the site of infection. The second was that use of the breakpoint MIC rather than the specific MIC for the targeted strains makes it more likely that our results will be applicable to S. aureus strains other than UAMS-1. However, equivalent amounts based on the breakpoint MIC do not necessarily translate to equivalent amounts based on the actual MIC, and in the context of relative antibiotic efficacy, the latter may well be more important than the former.
Specifically, because daptomycin has the lowest breakpoint MIC (≤1.0 μg/ml) and linezolid has the highest (≤4.0 μg/ml), the lowest absolute concentrations of any antibiotic employed in these experiments were those employed with daptomycin (5.0, 10.0, and 20.0 μg/ml at 5×, 10×, and 20× concentrations, respectively), with those employed for linezolid being the highest (20.0, 40.0, and 80.0 μg/ml at 5×, 10×, and 20× concentrations, respectively). The breakpoint MIC for vancomycin (≤2.0 μg/ml) is twice that for daptomycin (≤1.0 μg/ml), and the absolute concentrations of vancomycin used here were adjusted accordingly. However, the vancomycin MIC of UAMS-1 and its sarA mutant (1.5 μg/ml) was also twice as high as the daptomycin MIC (0.75 μg/ml). Thus, the amounts of daptomycin and vancomycin were equivalent with respect to the specific strains targeted in these experiments (6.67×, 13.3×, and 26.6× at 5×, 10×, and 20× concentrations, respectively, on the basis of the actual MICs for UAMS-1 and its sarA mutant). At the same time, the breakpoint MIC for linezolid is considerably higher (≤4.0 μg/ml) than the actual MIC for UAMS-1 or its sarA mutant (1.0 μg/ml), which means that the concentration of linezolid relative to the specific strains targeted in these experiments was considerably higher (20×, 40×, and 80× at 5×, 10×, and 20× concentrations, respectively) than that employed for daptomycin or vancomycin. Taking all of these considerations into account, we conclude that daptomycin is in fact more effective in clearing an established S. aureus biofilm than either vancomycin or linezolid. Although few studies have made a direct examination of this issue, our results are consistent with the fact that membrane-active agents like daptomycin are more active against slowly or even nongrowing bacteria like those characteristically observed within a biofilm (22). They are also consistent with the findings described in the reports of a few other studies that used alternative biofilm models to make similar comparisons (19, 26).
Finally, it is important to emphasize that, even with daptomycin, the clearance of bacteria from catheter-associated biofilms required concentrations significantly higher than the CSLI-defined breakpoint MIC. In the case of daptomycin, the concentrations employed in our experiments (5.0, 10,0 and 20.0 μg/ml) roughly reflect the concentration range observed in human plasma, with the lower value being comparable to the concentrations observed in patients receiving once-daily dosing with 4 mg/kg of body weight daptomycin (6.37 μg/ml) and the higher value reflecting the plasma concentrations observed in patients receiving 8 mg/kg (15.3 μg/ml) (11). Although the highest concentrations of vancomycin and linezolid used in our experiments (40 and 80 μg/ml, respectively) exceed the levels typically observed in the plasma of patients undergoing systemic therapy (38, 39), the same is generally true for both of these antibiotics. This suggests that the impact of mutating sarA on antibiotic efficacy, which was apparent to various degrees with all three antibiotics at all concentrations tested in our experiments, would in fact be therapeutically relevant. On the basis of this finding, we believe that inhibitors of sarA-mediated regulation, whether they are directed at transcription of the gene itself, the functional activity of SarA, or those downstream targets in the sarA regulon that are most relevant in the specific context of biofilm formation, would have the capacity to enhance the efficacy of antimicrobial therapy in the important context of an S. aureus biofilm-associated infection.
Acknowledgments
This research was supported by funding from Cubist Pharmaceuticals (to M.S.S.) and grant AI069087 from the National Institute of Allergy and Infectious Disease (to M.S.S.).
Footnotes
Published ahead of print on 16 March 2009.
REFERENCES
- 1.Avdic, E., and S. E. Cosgrove. 2008. Management and control strategies for community-associated methicillin-resistant Staphylococcus aureus. Expert Opin. Pharmacother. 9:1463-1479. [DOI] [PubMed] [Google Scholar]
- 2.Beenken, K. E., J. S. Blevins, and M. S. Smeltzer. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 71:4206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beenken, K. E., P. M. Dunman, F. McAleese, F. D. Macapagal, E. Murphy, S. J. Projan, J. S. Blevins, and M. S. Smeltzer. 2004. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 186:4665-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blevins, J. S., K. E. Beenken, M. O. Elasri, B. K. Hurlburt, and M. S. Smeltzer. 2002. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect. Immun. 70:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady, R. A., J. G. Leid, J. H. Calhoun, J. W. Costerton, and M. E. Shirtliff. 2008. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 52:13-22. [DOI] [PubMed] [Google Scholar]
- 6.Cassat, J. E., P. M. Dunman, E. Murphy, S. J. Projan, K. E. Beenken, K. J. Palm, S. J. Yang, K. C. Rice, K. W. Bayles, and M. S. Smeltzer. 2006. Transcriptional profiling of a Staphylococcus aureus clinical isolate and its isogenic agr and sarAA mutants reveals global differences in comparison to the laboratory strain RN6390. Microbiology 152:3075-3090. [DOI] [PubMed] [Google Scholar]
- 7.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2004. Inactivation of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J. Bacteriol. 186:6208-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conover, W. J. 1971. Practical nonparametric statistics. John Wiley & Sons, Inc., New York, NY.
- 9.Davis, S. L., M. B. Perri, S. M. Donabedian, C. Manierski, A. Singh, D. Vager, N. Z. Haque, K. Speirs, R. R. Muder, B. Robinson-Dunn, M. K. Hayden, and M. J. Zervos. 2007. Epidemiology and outcomes of community-associated methicillin-resistant Staphylococcus aureus infection. J. Clin. Microbiol. 45:1705-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Pozo, J. L., and R. Patel. 2007. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 82:204-209. [DOI] [PubMed] [Google Scholar]
- 11.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration. Antimicrob. Agents. Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick, F., H. Humphreys, and J. P. O'Gara. 2005. The genetics of staphylococcal biofilm formation—will a greater understanding of pathogenesis lead to better management of device-related infection? Clin. Microbiol. Infect. 11:967-973. [DOI] [PubMed] [Google Scholar]
- 13.Fowler, V. G., Jr., C. L. Nelson, L. M. McIntyre, B. N. Kreiswirth, A. Monk, G. L. Archer, J. Federspiel, S. Naidich, B. Remortel, T. Rude, P. Brown, L. B. Reller, G. R. Corey, and S. R. Gill. 2007. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J. Infect. Dis. 196:738-747. [DOI] [PubMed] [Google Scholar]
- 14.Good, P. I. 2005. Permutation, parametric and bootstrap tests of hypotheses, 3rd ed. Springer, New York, NY.
- 15.Handke, L. D., S. R. Slater, K. M. Conlon, S. T. O'Donnell, M. E. Olson, K. A. Bryant, M. E. Rupp, J. P. O'Gara, and P. D. Fey. 2007. SigmaB and SarA independently regulate polysaccharide intercellular adhesin production in Staphylococcus epidermidis. Can. J. Microbiol. 53:82-91. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson, K. K., D. A. Goldmann, and G. B. Pier. 2005. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 49:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson, A., P. Saravia-Otten, K. Tegmark, E. Morfeldt, and S. Arvidson. 2001. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to up-regulation of extracellular proteases. Infect. Immun. 69:4742-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klevens, R. M., M. A. Morrison, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, A. S. Craig, E. R. Zell, G. E. Fosheim, L. K. McDougal, R. B. Carey, and S. K. Fridkin. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763-1771. [DOI] [PubMed] [Google Scholar]
- 19.LaPlante, K. L., and L. A. Mermel. 2007. In vitro activity of daptomycin and vancomycin lock solutions on Staphylococcus aureus biofilms in a central venous catheter model. Nephrol. Dial. Transplant. 22:2239-2246. [DOI] [PubMed] [Google Scholar]
- 20.Lew, D. P., and F. A. Waldvogel. 2004. Osteomyelitis. Lancet 364:369-379. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, K. 2008. Multidrug tolerance of biofilms and persister cells. Curr. Top. Microbiol. Immunol. 322:107-131. [DOI] [PubMed] [Google Scholar]
- 22.Mascio, C. T. M., J. D. Alder, and J. A. Silverman. 2007. Bactericidal action of daptomycin against stationary-phase and non-dividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51:4255-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreillon, P. 2008. New and emerging treatment of Staphylococcus aureus infections in the hospital setting. Clin. Microbiol. Infect. 3:32-41. [DOI] [PubMed] [Google Scholar]
- 24.O'Gara, J. P. 2007. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 270:179-188. [DOI] [PubMed] [Google Scholar]
- 25.O'Neill, E., C. Pozzi, P. Houston, D. Smyth, H. Humphreys, D. A. Robinson, and J. P. O'Gara. 2007. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J. Clin. Microbiol. 45:1379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raad, I., H. Hanna, Y. Jiang, T. Dvorak, R. Reitzel, G. Chaiban, R. Sherertz, and R. Hachem. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-associated methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice, K. C., E. E. Mann, J. L. Endres, E. C. Weiss, J. E. Cassat, M. S. Smeltzer, and K. W. Bayles. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 104:8113-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanks, R. M., M. A. Meehl, K. M. Brothers, R. M. Martinez, N. P. Donegan, M. L. Graber, A. L. Cheung, and G. A. O'Toole. 2008. Genetic evidence for an alternative citrate-dependent biofilm formation pathway in Staphylococcus aureus that is dependent on fibronectin binding proteins and the GraRS two-component regulatory system. Infect. Immun. 76:2469-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeltzer, M. S., J. R. Thomas, S. G. Hickmon, R. A. Skinner, C. L. Nelson, D. Griffith, T. R. Parr, Jr., and R. P. Evans. 1997. Characterization of a rabbit model of staphylococcal osteomyelitis. J. Orthop. Res. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 30.Stewart, P. S. 2002. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292:107-113. [DOI] [PubMed] [Google Scholar]
- 31.Szomolay, B., I. Klapper, J. Dockery, and P. S. Stewart. 2005. Adaptive responses to antimicrobial agents in biofilms. Environ. Microbiol. 7:1186-1191. [DOI] [PubMed] [Google Scholar]
- 32.Tao, J. H., C. S. Fan, S. E. Gao, H. J. Wang, G. X. Liang, and Q. Zhang. 2006. Depression of biofilm formation and antibiotic resistance by sarA disruption in Staphylococcus epidermidis. World J. Gastroenterol. 12:4009-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tormo, M. A., M. Martí, J. Valle, A. C. Manna, A. L. Cheung, I. Lasa, and J. R. Penadés. 2005. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J. Bacteriol. 187:2348-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trotonda, M. P., A. C. Manna, A. L. Cheung, I. Lasa, and J. R. Penadés. 2005. SarA positively controls bap-dependent biofilm formation in Staphylococcus aureus. J. Bacteriol. 187:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang, L. H., S. T. Daily, E. C. Weiss, and M. S. Smeltzer. 2007. Mutation of traP in Staphylococcus aureus has no impact on expression of agr or biofilm formation. Infect. Immun. 75:4528-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsang, L. H., J. E. Cassat, L. N. Shaw, K. E. Beenken, and M. S. Smeltzer. 2008. Factors contributing to the biofilm-deficient phenotype of Staphylococcus aureus sarA mutants. PLoS ONE 3:e3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48:1075-1087. [DOI] [PubMed] [Google Scholar]
- 38.Vardakas, K. Z., I. Kiourmis, and M. E. Falagas. 2009. Association of pharmacokinetic and pharmacodynamic aspects of linezolid with infection outcome. Curr. Drug Metab. 10:2-12. [DOI] [PubMed] [Google Scholar]
- 39.Yang, Y.-H., W.-Y. Wu, H.-H. Yeh, and S.-H. Chen. 2007. Simultaneous determination of cefepime and vancomycin in plasma and cerebrospinal fluid by MEKC with direct sample injection and application for bacterial meningitis. Electrophoresis 28:1788-1797. [DOI] [PubMed] [Google Scholar]