Abstract
Ipsat P1A is a recombinant β-lactamase which degrades antibiotic residue in the gastrointestinal tract. In an open-label, single-center controlled trial, 36 healthy subjects were randomized to receive (i) ampicillin (1 g intravenously [i.v.] every 6 h [q6h]), (ii) oral P1A recombinant β-lactamase (8.2 mg q6h), or (iii) ampicillin (1 g i.v. q6h) in combination with oral P1A recombinant β-lactamase (8.2 mg q6h) for 5 days. Fecal samples were collected before treatment, during treatment (days 3 to 5), and at follow-up (day 12). The primary end points were (i) changes in gastrointestinal microflora (determined by temperature gradient gel electrophoresis [TGGE]) and (ii) emergence of bacterial resistance (determined by conventional microbiology and PCR of TEM β-lactamase genes). Thirty-five subjects completed the study. The mean similarity percentages of TGGE profiles between baseline and each treatment day sample were significantly lower for the ampicillin group than for the group receiving ampicillin plus P1A recombinant β-lactamase on days 3, 4, and 5 (P < 0.001). Compared with the ampicillin group, subjects receiving ampicillin plus P1A recombinant β-lactamase had significantly fewer ampicillin-resistant coliforms on days 3, 4, and 5 and at follow-up (P ≤ 0.001) and fewer TEM β-lactamase genes on days 3, 4, and 5 (P < 0.02). P1A recombinant β-lactamase was safe and well tolerated. In healthy subjects, P1A recombinant β-lactamase prevents ampicillin-induced alterations in intestinal microflora, emergence of resistance, and the number of TEM genes.
The health care system has been impacted greatly by the increasing rates of infection with antibiotic-resistant pathogens (14, 20). Many patients in whom infections occur have previously been exposed to antibiotics, either for prophylaxis or as treatment. While antibiotic treatment can reduce the incidence of infections with certain organisms (prophylactic effect), it may not modify others and can even increase the incidence of infections with some organisms. These effects are attributable to direct antimicrobial activity against the causative organism and/or to effects on competing microflora. The effect on intestinal microflora is of particular interest, as it may lead to selection for bacterial strains resistant to antimicrobial agents, a process that may have particularly severe consequences, resulting in increased mortality, morbidity, and costs (5, 8). Antibiotics that are excreted in high concentrations in bile into the intestinal tract can cause profound disruption of the indigenous microflora (9, 29, 30), resulting in an increased incidence of secondary infections due to acquisition and overgrowth of antimicrobial-resistant pathogens, including vancomycin-resistant enterococci, Candida species, and multiresistant gram-negative bacilli, and also in a number of adverse effects observed in Clostridium difficile infections (1, 2, 6, 8).
β-Lactam antibiotics are among the most widely used classes of antimicrobials, and many of these agents are excreted into the intestinal tract in high concentrations. Previous studies have demonstrated that the normal intestinal microflora contains various degrees of antibiotic resistance genes (19, 32, 34) and that healthy individuals may harbor intestinal bacteria that can produce TEM β-lactamases (31, 34). It has further been shown that the resistance patterns of enteric bacteria change in response to increased levels of exposure to antibiotics and that selective pressure from ampicillin treatment can result in increased levels of ampicillin-resistant bacteria (12, 13).
The active ingredient of Ipsat P1A capsule is P1A protein. P1A protein is a recombinant class A β-lactamase with a molecular mass of 29 kDa which is capable of hydrolyzing penicillin, aminopenicillins (e.g., ampicillin), and ureidopenicillins (e.g., piperacillin). P1A protein has structural and functional similarities to naturally occurring β-lactamases in the gastrointestinal (GI) microflora. P1A protein is intended for oral use and is presented in a gastroresistant formulation in P1A pellets designed to protect P1A protein from the influence of the acidic gastric medium in the stomach and to start the release of P1A protein in the intestine when the pH exceeds 5.5. This is achieved by using the pH-dependent polymer Eudragit L 30 D-55.
In dogs, orally administered P1A recombinant β-lactamase was shown to effectively degrade the GI residue of intravenously administered ampicillin, ampicillin-sulbactam, amoxicillin-clavulanate, and piperacillin-tazobactam (10, 11, 24, 33). Studies using mouse models demonstrated that oral administration of P1A recombinant β-lactamase given in conjunction with ampicillin or piperacillin preserved colonization resistance, reduced antibiotic-associated alteration in the indigenous microflora, and prevented overgrowth of vancomycin-resistant enterococci and Clostridium difficile (35, 36, 37).
The aims of this trial with healthy subjects were to evaluate the preventive effect of P1A recombinant β-lactamase on ampicillin-induced changes in GI microflora and the emergence of antimicrobial resistance in intestinal coliforms. The changes in the composition and numbers of selected groups of GI microflora were assessed by culture-based and molecular approaches (temperature gradient gel electrophoresis [TGGE]). Resistance to 10 antimicrobials other than ampicillin was evaluated by determining the susceptibility of coliforms in fecal samples by using the disc diffusion method (CLSI) and quantifying the blaTEM genes in fecal samples by quantitative PCR. In addition, the safety and tolerability of P1A recombinant β-lactamase were studied.
(The results of this study were presented in part at the 15th ECCMID, 2 to 5 April 2005, Copenhagen, Denmark, and at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, 16 to 19 December 2005, Washington, DC.)
MATERIALS AND METHODS
Study design.
This was an open-label, randomized, parallel-group, single-center trial investigating the effects of intravenous ampicillin, given alone or in combination with oral P1A recombinant β-lactamase, on the composition of GI microflora and the development of bacterial resistance to ampicillin in the GI tracts of healthy subjects. It was performed at the Lung Clinic, Tartu University Clinics, Tartu, Estonia, between February and March 2004 in accordance with the International Conference on Harmonisation GCP guidelines (CPMP/ICH/135/95) and the World Medical Association Declaration of Helsinki (1964) and subsequent amendments.
Subjects and study procedures.
Subjects who gave written informed consent were screened for eligibility for 2 weeks before planned entry into the study. For eligibility, subjects had to be healthy adults of either gender who had not taken any antimicrobial drug for at least 4 months before the beginning of the trial, with no known history of hypersensitivity or allergy to any components of ampicillin or β-lactam antibiotics and no history of GI diseases or diarrhea. The use of probiotics or bulk-forming laxatives within 14 days before the study start was prohibited. Confirmation of a negative stool sample for asymptomatic pathogenic bacteria was made 2 days and 1 day before the study start. Eligibility was confirmed, and the pretreatment safety screen was completed, including documentation of concomitant medications, measurement of vital signs, hematology, blood biochemistry and urine profiles, and electrocardiography. Women with a positive pregnancy test or who were not taking adequate contraception were excluded.
At the start of the study, eligible subjects were randomly assigned in equal proportions to one of the following three treatment groups: (i) ampicillin (1 g intravenously [i.v.] every 6 h [q6h]), (ii) P1A recombinant β-lactamase (8.2 mg per os [p.o.] q6h), and (iii) ampicillin (1 g i.v. q6h) plus P1A recombinant β-lactamase (8.2 mg p.o. q6h), for a period of 5 days. Each group consisted of 12 subjects. P1A recombinant β-lactamase was dosed as two 4.1-mg capsules 10 min before the infusion of ampicillin, which was given at a commonly used and approved dose. The dose and treatment duration of P1A recombinant β-lactamase were selected on the basis of preclinical studies (33) and data (unpublished) from previous clinical trials (with 15 healthy subjects and 6 volunteers with ileostomy). The dosing schedule of P1A recombinant β-lactamase was determined by that of ampicillin. Each study subject was scheduled to receive a total of 20 doses of ampicillin and/or P1A recombinant β-lactamase. Half an hour before each infusion, a standardized light snack was provided, and the study subjects fasted for 2 h after each infusion. They remained at the clinical unit from the evening of study day 0 until day 5, when they received the last treatment, followed by fecal sample delivery/collection, and they returned to the clinic for the follow-up visit on day 12. All study subjects completed the study, except for one person in the ampicillin group.
Assessments of efficacy and safety.
Fecal samples were collected before the first administration of ampicillin, P1A recombinant β-lactamase, or ampicillin plus P1A recombinant β-lactamase at baseline (days −1 and 0). Single samples were collected on days 3, 4, and 5 of treatment and at follow-up, on day 12. The samples were collected into sterile plastic tubes and within 30 min of delivery were placed into a freezer at −70°C for storage until analysis.
Safety parameters were reassessed during treatment and at the follow-up visit. Adverse events and concomitant therapy were checked daily during treatment and at follow-up.
Microbiological analysis. (i) Quantification and identification of microbial groups and species.
Counting of anaerobic and aerobic microbial groups and species in fecal samples was performed by standard culture techniques (15, 26). The total counts of Bacteroides fragilis group, Bifidobacterium spp., Lactobacillus spp., Clostridium spp., coliforms, Streptococcus spp., and yeasts were enumerated (detection limit, 102 CFU/g), and isolates were identified by established methods (24, 26). The presence of Clostridium difficile toxins A and B in diarrheal samples was determined with a commercial kit (Premier Toxins A&B; Meridian Diagnostics).
(ii) Antimicrobial susceptibility testing of coliforms.
The total colony counts of coliforms were calculated from both blood and cysteine lactose electrolyte-deficient agar plates of appropriate dilutions. Additionally, 10 colonies of coliforms (or as near to 10 as possible) at each time point, including all visibly different morphotypes, were isolated at random from blood and cysteine lactose electrolyte-deficient agar plates. The isolated coliformic colonies were identified to the species level by established methods (24, 26).
To assess the proportions of ampicillin-resistant, multidrug-resistant, and susceptible coliforms in the total number of coliforms, the susceptibility profiles of the 10 coliform isolates for ampicillin (10 μg), piperacillin (100 μg), gentamicin (10 μg), cephalothin (30 μg), cefotaxime (30 μg), meropenem (10 μg), ciprofloxacin (5 μg), tetracycline (30 μg), trimethoprim (5 μg), trimethoprim-sulfamethoxazole (24 μg), and amoxicillin-clavulanic acid (30 μg) (Oxoid, Hampshire, England) were determined using the disc diffusion method as recommended by the Clinical and Laboratory Standards Institute (28). Escherichia coli ATCC 25922 and E. coli ATCC 35218 were used as control strains (American Type Culture Collection, Manassas, VA). In addition, the susceptibility of obtained Klebsiella isolates was further tested against cefpodoxime, ceftriaxone, and ceftazidime to identify potential extended-spectrum β-lactamase producers.
(iii) Assessment of changes in fecal microflora by TGGE.
A molecular approach based on the sequence variability of the 16S rRNA gene was applied by using TGGE as described previously (27, 40). Bacterial genomic DNA was extracted from 50 mg (wet weight) of homogenized fecal sample by established methods. Primers U968-GC and L1401 were used to amplify the V6-to-V8 regions of the bacterial 16S rRNA gene (40). The TGGE Maxi system (Biometra, Germany) was used for sequence-specific separation of PCR products. The gel was stained with AgNO3 by the technique of Cairns and Murray (4). The fecal samples from the same individual were analyzed simultaneously, and results are given as similarity percentages (Pearson correlation) between the baseline samples (day 0 and day −1) and each treatment day (days 3, 4, and 5) and follow-up (day 12) sample, obtained by using Gelcompar II software (Applied Maths, Belgium).
(iv) Analysis of ampicillin resistance genes (blaTEM) by quantitative PCR.
The emergence of ampicillin resistance was evaluated by performing quantitative PCR of TEM β-lactamase genes (blaTEM) existing in the fecal samples of the study subjects (3). Bacterial genomic DNA was extracted from 50-mg (wet weight) fecal samples by use of a FASTDNA spin kit (for soil) according to the manufacturer's instructions (QBiogene, Carlsbad, CA). The blaTEM genes in the fecal samples were quantified using primers TemH (forward; AGGAAGAGTATGAGTAT) and TemE (reverse; TCGTCGTTTGGTATGGC) (21, 22). The P16S907F (AAA-CTY-AAA-KGA-ATT-GAC-GG) and P16S1100R (GGG-TTG-CGC-TCG-TTG) primers were used to amplify the V6 region of the bacterial 16S rRNA gene in order to quantify the total bacterial genomic DNA in fecal samples (18). A Taq DNA polymerase kit from Eurogentec (Belgium) was used for the PCRs, and PCR product formation was measured with Sybr green (Molecular Probes, The Netherlands) on a Smartcycler (Cepheid) real-time PCR machine. The number of blaTEM genes was expressed as a percentage of total bacterial genomic DNA.
Statistical analysis.
All analyses were performed by intention-to-treat analysis according to the randomization schedule. All subjects with at least one dose of study treatment were included in the analyses.
All statistical tests were performed as two-sided tests. P values of <0.05 were considered statistically significant.
The mean of day −1 (screening) and day 0 (entry) values was used as the baseline value for all statistical analyses, except for the similarity index (%), where analyses were performed in comparison to day −1 and to day 0 separately. Because the results from day −1 and day 0 were highly similar, only the similarity index (%) results from samples compared to day 0 are presented.
Continuous variables were summarized by treatment group and visit day, using the following statistics: number of subjects (n), mean, standard deviation, standard error of the mean (SEM), minimum, median, and maximum. The geometric mean was calculated for responses of total counts of different bacterial groups or species and yeasts (CFU/g). The summary figures for the similarity index were based on the mean and SEM. The figure for the number (%) of resistant coliforms was based on median values, since nonparametric methods were used in the statistical analyses.
The Pearson correlation coefficient between similarity index (%) and the number of ampicillin-resistant coliforms (CFU/g on a logarithmic scale [base 10]) was calculated by treatment group. Correlation coefficients were calculated in relation to study day 0 by use of the similarity index (%).
All statistical analyses were done using SAS System for Windows, version 8.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient characteristics.
Thirty-six healthy Caucasian subjects between 18 and 39 years of age were entered into the study. Baseline demographic characteristics and body mass indexes were similar for the three studied groups. Of the 36 subjects enrolled, 35 subjects completed the trial. One subject, in the ampicillin treatment group, was withdrawn from study treatment on day 2, after receiving eight doses of ampicillin because of an adverse event (diarrhea), but remained in the study for all subsequent assessments.
Effect of P1A recombinant β-lactamase on ampicillin-induced changes in the composition of GI microflora.
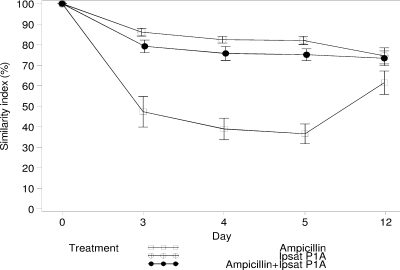
Ampicillin treatment was associated with a marked change in the composition of the intestinal microflora. The mean decrease in the similarity indices of TGGE profiles of the fecal samples during treatment for the ampicillin group was 59%, compared to 17% and 23% for the groups receiving P1A recombinant β-lactamase and ampicillin plus P1A recombinant β-lactamase, respectively (Fig. 1). The mean similarity percentages of TGGE profiles of fecal samples for the ampicillin group during treatment were 47.3%, 38.9%, and 36.6% (days 3, 4, and 5, respectively), and those for the group receiving ampicillin plus P1A recombinant β-lactamase were 79.2%, 75.8%, and 75.1% (days 3, 4, and 5, respectively). The difference between groups was statistically significant during treatment (P < 0.0001 at days 3, 4, and 5) but not at 7 days posttreatment (P = 0.07; day 12). The mean similarity percentages of TGGE profiles of fecal samples in the P1A recombinant β-lactamase group were 86.1%, 82.4%, and 82.0% during treatment (days 3, 4, and 5, respectively), and statistical analysis showed no significant differences between the groups receiving P1A recombinant β-lactamase and ampicillin plus P1A recombinant β-lactamase during the study days (P = 0.31, P = 0.19, P = 0.15, and P = 0.85 on days 3, 4, 5, and 12, respectively).
FIG. 1.
Changes in similarity index (%) in healthy subjects receiving ampicillin, P1A recombinant β-lactamase (Ipsat P1A), and ampicillin plus P1A recombinant β-lactamase. The values are presented as mean percent changes from the baseline (day 0). Each value is the mean ± SEM for 12 study subjects, except for the ampicillin group, for which the values on study days 4 and 5 are from 11 study subjects.
The plate count results for aerobic and anaerobic microbial species showed a strong suppression in baseline-related counts of Bifidobacterium and Streptococcus species in the ampicillin group during treatment (P < 0.0001 for both) (Table 1). No changes were observed in the groups receiving ampicillin plus P1A recombinant β-lactamase and P1A recombinant β-lactamase. For both bacterial species, the difference between the groups receiving ampicillin and ampicillin plus P1A recombinant β-lactamase during treatment was significant (P < 0.001 and P < 0.002, respectively).
TABLE 1.
Changes in number (CFU/g) for microbial groups and species in subject groups during treatment and at follow-upb
| Microorganism | Change in numbera
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Ampicillin group
|
Ampicillin + P1A recombinant β-lactamase group
|
|||||||
| Day 3 | Day 4 | Day 5 | Day 12 | Day 3 | Day 4 | Day 5 | Day 12 | |
| B. fragilis group | — | — | — | — | — | — | — | — |
| Bifidobacterium spp. | ↓↓↓ | ↓↓↓ | ↓↓↓ | — | —** | —*** | —** | — |
| Lactobacillus spp. | ↓ | ↓ | ↓ | — | — | — | — | — |
| Clostridium spp | — | ↓ | ↓ | — | — | — | ↓ | — |
| Coliforms | — | ↑ | — | — | — | — | — | — |
| Streptococcus spp. | ↓↓↓ | ↓↓↓ | ↓↓↓ | — | —** | —*** | —* | — |
| Yeasts | — | ↑↑↑ | ↑↑ | — | — | — | — | — |
↓↓↓, strong suppression (P < 0.0001); ↓, mild suppression (P < 0.05); ↑↑↑, strong induction (P < 0.0001); ↑↑, moderate induction (P < 0.01); —, no significant change. Statistically significant differences between the groups receiving ampicillin and ampicillin plus P1A recombinant β-lactamase are indicated as follows: *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
There was no change in number for any microorganisms for the group receiving P1A recombinant β-lactamase only.
Compared to the baseline level, there was a slight but significant suppression in counts of Lactobacillus and Clostridium species in the ampicillin group (P < 0.05), whereas the B. fragilis group remained unchanged. For the ampicillin group, antibiotic administration produced a mild inductive effect on coliforms (P < 0.05) and a strong induction of counts of yeast during treatment (P < 0.0001). No changes among these microbes were detected in the groups receiving ampicillin plus P1A recombinant β-lactamase and P1A recombinant β-lactamase compared to the baseline level. There was a significant difference between the groups receiving ampicillin and ampicillin plus P1A recombinant β-lactamase on day 4 (P < 0.05).
Effect of P1A recombinant β-lactamase on the emergence of ampicillin- and multidrug-resistant coliforms.
A total of 2,101 coliform isolates were collected (on average, 10 isolates per sample), among which 92% were Escherichia coli, 6% were Klebsiella spp., and 1.4% were Enterobacter spp. (Table 2). Only a few isolates were members of Citrobacter and Morganella species (not included in the table). Compared to baseline, for the ampicillin group the proportion of ampicillin-resistant Klebsiella-positive fecal samples increased during and after treatment, by 85% (23/27 samples; P < 0.001) and 50% (5/10 samples; P < 0.01), respectively. No such changes were observed in the group receiving ampicillin plus P1A recombinant β-lactamase during or after treatment. None of the resistant Klebsiella isolates were extended-spectrum β-lactamase producers.
TABLE 2.
Resistance rates (%) for coliform isolates in subject groupsa
| Antibiotic | Resistance rate (%) for isolates
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin
|
Ampicillin + PIA
|
PIA
|
|||||||||||||||||||||||||
|
E. coli
|
Klebsiella
|
Enterobacter
|
E. coli
|
Klebsiella
|
Enterobacter
|
E. coli
|
Klebsiella
|
Enterobacter
|
|||||||||||||||||||
| Day 0 (n = 236) | Days 3 to 5 (n = 220) | Day 12 (n = 113) | Day 0 (n = 1) | Days 3 to 5 (n = 111) | Day 12 (n = 4) | Day 0 (n = 4) | Days 3 to 5 (n = 8) | Day 12 (n = 2) | Day 0 (n = 212) | Days 3 to 5 (n = 338) | Day 12 (n = 107) | Day 0 (n = 4) | Days 3 to 5 (n = 5) | Day 12 (n = 0) | Day 0 (n = 6) | Days 3 to 5 (n = 4) | Day 12 (n = 3) | Day 0 (n = 234) | Days 3 to 5 (n = 350) | Day 12 (n = 120) | Day 0 (n = 0) | Days 3 to 5 (n = 2) | Day 12 (n = 0) | Day 0 (n = 2) | Days 3 to 5 (n = 1) | Day 12 (n = 0) | |
| Ampicillin | <1 | 50 | 17 | 100 | 98 | 100 | 50 | 75 | 100 | 6 | 15 | 22 | 100 | 80 | 0 | 83 | 100 | 0 | 2 | 1 | 4 | 0 | 100 | 0 | 100 | 100 | 0 |
| Piperacillin | 0 | 23 | 2 | 0 | 30 | 25 | 0 | 63 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 100 | 100 | 0 | <1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Amox/clav | <1 | 1 | 0 | 0 | <1 | 0 | 50 | 63 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 83 | 100 | 67 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 0 |
| Cephalothin | <1 | 8 | 0 | 0 | 0 | 0 | 100 | 38 | 100 | <1 | 6 | 8 | 0 | 0 | 0 | 100 | 100 | 67 | 2 | 0 | 2 | 0 | 0 | 0 | 100 | 100 | 0 |
| Cefotaxime | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | <1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Meropenem | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | <1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tetracycline | 0 | 47 | 17 | 0 | 33 | 25 | 0 | 75 | 100 | 15 | 42 | 33 | 0 | 0 | 0 | 0 | 25 | 0 | 21 | 25 | 34 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gentamicin | 0 | 10 | <1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ciprofloxacin | 0 | <1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trim+Trim/Sulfa | <1 | 13 | 3 | 0 | 4 | 0 | 0 | 0 | 0 | 9 | 12 | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 7 | 17 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fully susceptible | 98 | 48 | 82 | 0 | <1 | 0 | 0 | 0 | 0 | 84 | 55 | 60 | 0 | 20 | 0 | 0 | 0 | 0 | 78 | 73 | 60 | 0 | 0 | 0 | 0 | 0 | 0 |
For day 0, the number of isolates is the sum of isolates on day −1 and day 0. Amox/clav, amoxicillin-clavulanic acid; Trim+Trim/Sulfa = trimethoprim plus trimethoprim-sulfamethoxazole (combined results).
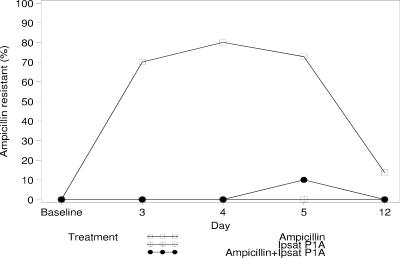
No differences in the number of ampicillin-resistant coliforms were observed in pairwise comparisons between the groups at baseline. The median percentage of ampicillin-resistant coliforms in the ampicillin group was significantly increased, from a negligible level (5/241 isolates) at baseline to 70.0% (71/113 isolates; P = 0.002), 80.0% (76/112 isolates; P = 0.004), and 72.7% (79/114 isolates; P = 0.002) during ampicillin administration (days 3, 4, and 5, respectively) and 13.6% (25/119 isolates; P = 0.02) at follow-up (day 12) (Fig. 2). In contrast, the rates of resistance for the groups receiving ampicillin plus P1A recombinant β-lactamase and P1A recombinant β-lactamase remained below 10% at all times. There were significant differences between the groups receiving ampicillin and ampicillin plus P1A recombinant β-lactamase in the changes in resistance rate from baseline to days 3, 4, and 5 (P < 0.001, P = 0.003, and P = 0.002, respectively). No significant differences were observed between the groups receiving P1A recombinant β-lactamase and ampicillin plus P1A recombinant β-lactamase (P > 0.3 for all days).
FIG. 2.
Changes in number of ampicillin-resistant coliforms in healthy subjects receiving ampicillin, P1A recombinant β-lactamase (Ipsat P1A), and ampicillin plus P1A recombinant β-lactamase. Median values are presented.
In comparison with the baseline values, a significant increase in the median number of tetracycline-resistant coliforms was observed in the ampicillin group on days 3 (36%; 45/113 isolates), 4 (50%; 48/112 isolates), and 5 (58%; 54/114 isolates) (P = 0.016 for all). Similar increases were observed in the group receiving ampicillin plus P1A recombinant β-lactamase, as follows: for day 3, 20% (38/116 isolates; P = 0.03); for day 4, 35% (52/118 isolates; P = 0.004); and for day 5, 41% (54/113 isolates; P = 0.008). No significant changes in the number of tetracycline-resistant coliforms were found in the P1A recombinant β-lactamase group. There were no differences in the number of tetracycline-resistant coliforms between the groups receiving ampicillin and ampicillin plus P1A recombinant β-lactamase.
A significant increase in resistance to β-lactams other than ampicillin was found in the ampicillin group on day 3 (20%; P = 0.04). There was a significant difference in this variable on day 3 between the groups receiving ampicillin and ampicillin plus P1A recombinant β-lactamase (P < 0.03). Resistance to other antibiotics, such as ciprofloxacin, gentamicin, trimethoprim, and trimethoprim-sulfamethoxazole, was found in a small number of isolates.
Correlation between number of ampicillin-resistant coliforms and similarity index (%).
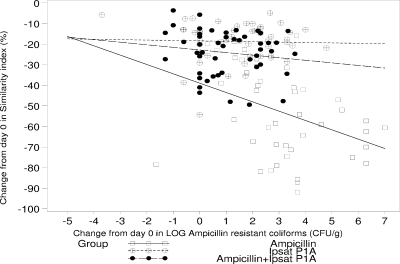
Figure 3 shows the correlation between the similarity index (%) and the number of ampicillin-resistant coliforms (CFU/g). There was a statistically significant negative correlation (−0.39; P = 0.008) in the similarity index (%) related to day 0 for the ampicillin group. The other two groups, those receiving ampicillin plus P1A recombinant β-lactamase and P1A recombinant β-lactamase, also had negative but not statistically significant correlations (−0.03 [P = 0.826] and −0.15 [P = 0.317], respectively).
FIG. 3.
Correlations between similarity index (%) and number of ampicillin-resistant coliforms for the ampicillin group (−0.389; P = 0.008), P1A recombinant β-lactamase (Ipsat P1A) group (−0.033; P = 0.826), and group receiving ampicillin plus P1A recombinant β-lactamase (−0.147; P = 0.317).
Number of blaTEM ampicillin resistance genes.
A total of seven subjects (one in the ampicillin group and three each in the groups receiving P1A recombinant β-lactamase and ampicillin plus P1A recombinant β-lactamase) had measurable amounts of blaTEM genes in one of the two baseline samples. As shown in Table 3, ampicillin treatment was associated with a statistically significant increase in the number of blaTEM genes on days 3 and 5 (P = 0.008 and P = 0.03, respectively) and at follow-up (P = 0.03). No statistically significant increases in the number of blaTEM genes were observed in the groups receiving P1A recombinant β-lactamase and ampicillin plus P1A recombinant β-lactamase. Comparing the changes in the ampicillin group to those for the group receiving ampicillin plus P1A recombinant β-lactamase, statistically significant differences were observed on treatment days 3, 4, and 5 (P = 0.02, P = 0.01, and P = 0.02, respectively).
TABLE 3.
Numbers of ampicillin resistance genes (blaTEM), expressed as percentages of the respective genomic DNA of the sample
| Day | Ampicillin group
|
P1A recombinant β-lactamase group
|
Ampicillin plus P1A recombinant β-lactamase group
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SEM | Min | Max | Prevalence | n | Mean | SEM | Min | Max | Prevalence | n | Mean | SEM | Min | Max | Prevalence | |
| Baseline | 12 | 0 | 0 | 0 | 0.0006 | 1/12 | 12 | 0.0006 | 0.0006 | 0 | 0.0068 | 3/12 | 12 | 0.001 | 0.0007 | 0 | 0.0086 | 3/12 |
| Day 3 | 12 | 11.642a,b | 8.8986 | 0 | 107.49 | 8/12 | 12 | 0 | 0 | 0 | 0.0004 | 1/12 | 12 | 0.0014b | 0.0005 | 0 | 0.0047 | 5/12 |
| Day 4 | 11 | 7.8243b | 6.5669 | 0 | 74.085 | 5/11 | 12 | 0 | 0 | 0 | 0 | 0/12 | 12 | 0.0005b | 0.0004 | 0 | 0.0043 | 2/12 |
| Day 5 | 11 | 6.2559a,b | 5.5761 | 0 | 61.888 | 6/11 | 12 | 0 | 0 | 0 | 0.0004 | 1/12 | 12 | 0.0081b | 0.0066 | 0 | 0.0795 | 2/12 |
| Day 12 | 12 | 0.2392a | 0.2273 | 0 | 2.7377 | 6/12 | 12 | 0.0007 | 0.0006 | 0 | 0.0074 | 2/12 | 12 | 0.0084 | 0.0052 | 0 | 0.0569 | 3/12 |
There were statistically significant increases on day 3 (P < 0.008), day 5 (P < 0.03), and day 12 (P < 0.03).
There were statistically significant differences between the groups receiving ampicillin and ampicillin plus P1A recombinant β-lactamase on day 3 (P < 0.02), day 4 (P < 0.01), and day 5 (P < 0.01).
Correlation between similarity index (%) and number of blaTEM ampicillin resistance genes.
There was a statistically significant negative correlation (−0.31; P = 0.04) in the similarity index (%) related to day 0 for the ampicillin group. There were no statistically significant correlations in the other study groups.
Safety.
No serious adverse events were reported. The only adverse event related to study treatment (and the only GI adverse event reported) was severe diarrhea in a subject in the ampicillin group, which led to withdrawal from study medication on day 3. No loose stool or change in the consistency or color of the stool was detected in the fecal sample for that day, but diarrhea was observed in the day 4 and day 5 samples. Testing on these days for Clostridium difficile toxins A and B was negative. The unrelated adverse events reported for the groups receiving P1A recombinant β-lactamase alone and ampicillin plus P1A recombinant β-lactamase were hypotension and headache, respectively. There were no clinically significant changes in hematology or blood biochemistry reported during treatment and at follow-up for any group.
DISCUSSION
The major finding of this study is the demonstration of the preventive effect of P1A recombinant β-lactamase on ampicillin-induced changes in the normal intestinal microflora and on the emergence of antibiotic resistance in healthy subjects.
Changes in intestinal microflora during the administration of antibiotics are known to cause an overgrowth of resistant, potentially pathogenic microorganisms, leading to an increased incidence of secondary infections with high mortality rates, especially in severely ill and/or immunocompromised patients and patients treated in intensive care units and long-term care facilities (6, 8). When antibiotics are administered parenterally, part of the antibiotic dose reaches the intestine via the enterohepatic cycle. Antibiotics that are excreted in large amounts into the GI tract have been shown to produce marked changes in indigenous intestinal bacteria, leading to acquisition and colonization with resistant pathogens. It is known that ampicillin is excreted in the bile in unchanged form, and its levels in gallbladder bile were found to be equal to or higher than those in serum (16, 25). Studies demonstrated that administration of ampicillin to healthy subjects was associated with a decrease in the number of enterococci, streptococci, and corynebacteria, while the number of Enterobacteriaceae and yeasts increased (38).
To evaluate the protective effect of P1A recombinant β-lactamase on the normal intestinal microflora during ampicillin administration, we calculated the similarity percentages of TGGE profiles of fecal samples and the changes in the numbers of specific bacterial species and yeasts in fecal samples by culturing and evaluated the development of resistance.
Changes in similarity index (%) reflect the fluctuations in mainly anaerobic bacteria. The TGGE method used for similarity index (%) evaluation differentiates PCR-amplified 16S rRNA gene fragments, producing a molecular profile of the bacterial species in the mixed bacterial population. Intravenous ampicillin produced marked changes in similarity percentages: in comparison with the baseline, significant decreases (average, 60%) were observed during all dosing days. P1A recombinant β-lactamase effectively prevented these changes: the decrease in similarity percentages for the group receiving ampicillin plus P1A recombinant β-lactamase was approximately 20% and was statistically significantly different from that for the ampicillin group. Importantly, the magnitude of changes in similarity percentages for the group receiving ampicillin plus P1A recombinant β-lactamase did not differ from that for the P1A recombinant β-lactamase group both during the dosing days and at follow-up.
This study also demonstrated that for the ampicillin group, administration of antibiotic changed the counts of cultured anaerobic bacterial populations, as observed by decreases in counts of Bifidobacterium, Lactobacillus, and Clostridium organisms. For the aerobic microflora, a strong suppression in counts of streptococci was detected, in contrast to an inductive effect on coliforms. These changes were not observed in the groups receiving ampicillin plus P1A recombinant β-lactamase and P1A recombinant β-lactamase alone, demonstrating that P1A recombinant β-lactamase prevents alterations in normal intestinal microflora after ampicillin administration to healthy subjects.
Next, we assessed the effect of P1A recombinant β-lactamase on the emergence of antibiotic resistance during ampicillin dosing. It is known that exposure to penicillin antibiotics as a class and exposure to ampicillin and ampicillin-sulbactam individually are the significant independent risk factors associated with the isolation of ampicillin-sulbactam-resistant E. coli (17). The emergence of resistance to Enterobacteriaceae during treatment with ampicillin-sulbactam is associated with a risk for superinfections, which was observed in 5% of the treated patients in one study (5).
The results of our study are in a good agreement with these data. In the ampicillin group, all study subjects had statistically significant increases in the number of ampicillin-resistant coliforms in the fecal samples collected during treatment, as did 7 of 12 subjects in the follow-up sample. The median values for ampicillin-resistant coliforms increased >70% during treatment compared to baseline. In parallel with these changes, ampicillin dosing also significantly increased the number of blaTEM genes during treatment and at follow-up.
Administration of P1A recombinant β-lactamase in conjunction with ampicillin significantly reduced the number of ampicillin-resistant coliforms and decreased the number of TEM β-lactamase genes. Moreover, P1A recombinant β-lactamase reduced the number of fecal samples which were positive for ampicillin-resistant Klebsiella. These data indicate that P1A recombinant β-lactamase effectively prevents the development of ampicillin-induced resistance in the GI microflora.
It is known that intestinal bacteria such as Enterobacteriaceae and Enterobacter spp. can produce extracellular β-lactamases (7, 23, 39). These β-lactamases are expected to be hydrolyzed rapidly by bacillar extracellular proteases and are not present in the GI tract in amounts which can effectively degrade the intestinal antibiotic residue. This assumption is supported by the data from the current study, which showed a significant decrease in similarity index for the ampicillin group. In contrast, P1A recombinant β-lactamase was shown in vitro in human ileal chyme to have a half-life of 2 h (unpublished data), which results in its presence in the GI tract at a concentration which can hydrolyze the antibiotic. Assuming that microbially produced intestinal β-lactamases are not present in the GI tract in substantial amounts, it is unlikely that there will be a competition between them and P1A recombinant β-lactamase.
The results of this study demonstrate for the first time the effectiveness of a novel approach to combat antibiotic resistance and to protect the intestinal microflora during antibiotic administration to healthy subjects by oral administration of recombinant β-lactamase. The data are in good agreement with the hypothesis that antibiotics promote the overgrowth of resistant microorganisms in the intestinal tract, primarily through the inhibition of anaerobes, and that enzymatic inactivation of the portion of antibiotic that is excreted into the intestinal tract results in preservation of the indigenous intestinal microflora and prevention of emergence of antibiotic resistance (8, 35).
In this study, we demonstrated that oral administration of P1A recombinant β-lactamase to healthy subjects prevented ampicillin-induced disturbances in the normal intestinal microflora and reduced the emergence of antibiotic resistance and the number of TEM β-lactamase genes. Further studies are needed to demonstrate the effectiveness of P1A recombinant β-lactamase in patients.
Acknowledgments
We thank personnel in Ipsat Therapies Oy/Ltd. and Tartu University Hospital, Lung Clinic, Tartu, Estonia, for their technical assistance, Ulla Airaksinen and Pertti Koski for discussions, and Marion Carson for her valuable contribution.
This work was supported by Ipsat Therapies, Helsinki, Finland.
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Bartlett, J. G. 2002. Antibiotic-associated diarrhea. N. Engl. J. Med. 346:334-339. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. G. 2006. Narrative review: the epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 145:758-764. [DOI] [PubMed] [Google Scholar]
- 3.Bartosch, S., A. Fite, G. T. Macfarlane, and M. E. T. McMurdo. 2004. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 70:3575-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairns, M. J., and V. Murray. 1994. Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. BioTechniques 17:915-919. [PubMed] [Google Scholar]
- 5.Carmeli, Y., J. Castro, G. M. Eliopoulos, and M. H. Samore. 2001. Clinical isolation and resistance patterns of and superinfection with 10 nosocomial pathogens after treatment with ceftriaxone versus ampicillin-sulbactam. Antimicrob. Agents Chemother. 45:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiaens, G., Y. Ciccarell, P. Damas, M.-P. Hayette, P. Melin, M. Nys, and P. De Mol. 2006. Prospective survey of digestive tract colonization with Enterobacteriaceae that produce extended-spectrum β-lactamases in intensive care units. J. Hosp. Infect. 62:386-388. [DOI] [PubMed] [Google Scholar]
- 7.Coque, T. M., A. Oliver, J. C. Perez-Diaz, F. Baquero, and R. Canton. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum β-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 46:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donskey, C. J. 2004. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39:219-226. [DOI] [PubMed] [Google Scholar]
- 9.Endlund, C., and C. E. Nord. 2000. Effect on the human normal microflora of oral antibiotics for treatment of urinary tract infections. J. Antimicrob. Chemother. 46(Suppl. S1):41-48. [PubMed] [Google Scholar]
- 10.Harmoinen, J., K. Vaali, P. Koski, K. Syrjänen, O. Laitinen, K. Lindevall, and E. Westermarck. 2003. Enzymic degradation of β-lactam antibiotic, ampicillin, in the gut: a novel treatment modality. J. Antimicrob. Chemother. 51:361-365. [DOI] [PubMed] [Google Scholar]
- 11.Harmoinen, J., S. Mentula, M. Heikkilä, M. van der Rest, P. J. Rajala-Schultz, C. J. Donskey, R. Frias, P. Koski, N. Wickstrand, H. Jousimies-Somer, E. Westermarck, and K. Lindevall. 2004. Orally administered targeted recombinant beta-lactamase prevents ampicillin-induced selective pressure on the gut microbiota: a novel approach to reducing antimicrobial resistance. Antimicrob. Agents Chemother. 48:75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heritage, J., B. Ransome, P. A. Chambers, and M. H. Wilcox. 2001. A comparison of culture and PCR to determine the prevalence of ampicillin-resistant bacteria in the fecal flora of general practice patients. J. Antimicrob. Chemother. 48:287-292. [DOI] [PubMed] [Google Scholar]
- 13.Houndt, T., and H. Ochman. 2000. Long-term shifts in patterns of antibiotic resistance in enteric bacteria. Appl. Environ. Microbiol. 66:5406-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, R. N., and M. A. Pfaller. 1998. Bacterial resistance: a worldwide problem. Diagn. Microbiol. Infect. Dis. 31:379-388. [DOI] [PubMed] [Google Scholar]
- 15.Jousimies-Somer, H., P. Summanen, D. M. Citron, E. J. Baron, H. M. Wexler, and S. M. Finegold. 2002. Wadsworth-KTL anaerobic bacteriology manual, 6th ed. Star Publishing, Belmont, CA.
- 16.Karachalios, G., and K. Charalabopoulos. 2002. Biliary excretion of antimicrobial drugs. Chemotherapy 48:280-297. [DOI] [PubMed] [Google Scholar]
- 17.Kaye, K. S., A. D. Harris, H. Gold, and Y. Carmeli. 2000. Risk factors of ampicillin-sulbactam-resistant Escherichia coli in hospitalized patients. Antimicrob. Agents Chemother. 44:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 19.Levy, S. B., B. Marshall, S. Schluederberg, D. Rowse, and J. Gavis. 1988. High frequency of antimicrobial resistance in human fecal flora. Antimicrob. Agents Chemother. 32:1801-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy, S. B., and B. Marshall. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10(Suppl.):S122-S129. [DOI] [PubMed] [Google Scholar]
- 21.Mabilat, C., and P. Courvalin. 1990. Development of “oligotyping” for characterization and molecular epidemiology of TEM beta-lactamases in members of the family Enterobacteriaceae. Antimicrob. Agents Chemother. 34:2210-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mabilat, C., and S. Goussard. 1993. PCR detection and identification of genes for extended-spectrum-lactamases, p. 553-563. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, DC.
- 23.Machado, E., T. M. Coque, R. Canton, A. Novais, J. C. Sousa, F. Baquero, and L. Peixe on behalf of The Portugese Resistance Study Group. 2007. High diversity of extended-spectrum β-lactamases among clinical isolates of Enterobacteriaceae from Portugal. J. Antimicrob. Chemother. 60:1370-1374. [DOI] [PubMed] [Google Scholar]
- 24.Mentula, S., J. Harmoinen, M. Heikkila, E. Westermarck, M. Rautio, P. Huovinen, and E. Kononen. 2005. Comparison between cultured small-intestinal and fecal microbiotas in beagle dogs. Appl. Environ. Microbiol. 71:4169-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris, D. L., C. S. Ubhi, C. S. Robertson, and K. W. Brammer. 1986. Biliary pharmacokinetics of sulbactam plus ampicillin in humans. Rev. Infect. Dis. 8(Suppl. 5):S589-S592. [DOI] [PubMed] [Google Scholar]
- 26.Murray, P. R., E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.). 1999. Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 27.Muyzer, G., E. C. de Waal, and G. A. Uitterlinden. 1993. Profiling of complex populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests; approved standard, 7th ed. NCCLS document M2-A7. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 29.Nord, C. E., L. Kager, and A. Heimdahl. 1984. Impact of antimicrobial agents on the gastrointestinal microflora and the risk of infections. Am. J. Med. 15:99-106. [DOI] [PubMed] [Google Scholar]
- 30.Nord, C. E., and A. Heimdahl. 1986. Impact of orally administered antimicrobial agents on human oropharyngeal and colonic microflora. J. Antimicrob. Chemother. 18:159-164. [DOI] [PubMed] [Google Scholar]
- 31.Nord, C. E., and M. Hedberg. 1990. Resistance to β-lactam antibiotics in anaerobic bacteria. Rev. Infect. Dis. 12:S231-S234. [DOI] [PubMed] [Google Scholar]
- 32.Osterblad, M., A. Hakanen, R. Manninen, T. Leistevuo, R. Peltonen, O. Meurman, P. Huovinen, and P. Kotilainen. 2000. A between-species comparison of antimicrobial resistance in enterobacteria in fecal flora. Antimicrob. Agents Chemother. 44:1479-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raatesalmi, K., and T. Korkolainen. 2007. The effect of Ipsat P1A, a novel drug for prevention of antibiotic-induced intestinal pathogenic colonization, on the gut antibiotics levels after intravenous administration of beta-lactam antibiotic/beta-lactamase inhibitors in the dog, abstr. P1792. Abstr. 17th Eur. Cong. Clin. Microbiol. Infect. Dis., Munich, Germany.
- 34.Stark, C. A., C. Edlund, S. Sjöstedt, G. Kristensen, and C. E. Nord. 1993. Antimicrobial resistance in human oral and intestinal anaerobic microflora. Antimicrob. Agents Chemother. 37:1665-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stiefel, U., N. J. Pultz, J. Harmoinen, P. Koski, K. Lindevall, M. S. Helfand, and C. J. Donskey. 2003. Oral administration of β-lactamase preserves colonization resistance of piperacillin treated mice. J. Infect. Dis. 188:1605-1609. [DOI] [PubMed] [Google Scholar]
- 36.Stiefel, U., J. Harmoinen, P. Koski, S. Kaariainen, N. Wickstrand, K. Lindevall, N. J. Pultz, R. A. Bonomo, M. S. Helfand, and C. J. Donskey. 2005. Orally administered recombinant metallo-β-lactamase preserves colonization resistance of piperacillin-tazobactam-treated mice. Antimicrob. Agents Chemother. 49:5190-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stiefel, U., M. Riggs, T. Heinonen, P. Koski, and C. J. Donskey. 2007. Orally administered β-lactamase enzymes represent a novel strategy to prevent colonization by Clostridium difficile, abstr. K-607, p. 337. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL. [DOI] [PubMed]
- 38.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 39.Valverde, A., T. M. Coque, L. G.-S. Miguel, F. Baquero, and R. Canton. 2008. Complex molecular epidemiology of extended-spectrum β-lactamases in Klebsiella pneumoniae: a long-term perspective from a single institution in Madrid. J. Antimicrob. Chemother. 61:64-72. [DOI] [PubMed] [Google Scholar]
- 40.Zoetendal, E. G., A. D. L. Akkermans, and W. M. de Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]