Abstract
The aim of this study was to evaluate the antileishmanial effects of topical liposomal paromomycin sulfate (PM) in Leishmania major-infected BALB/c mice. Liposomes containing 10 or 15% PM (Lip-PM-10 and Lip-PM-15, respectively) were prepared by the fusion method and were characterized for their size and encapsulation efficiency. The penetration of PM from the liposomal PM formulations (LPMFs) through and into skin was evaluated in vitro with Franz diffusion cells fitted with mouse skin at 37°C for 8 h. The in vitro permeation data showed that almost 15% of the LPMFs applied penetrated the mouse skin, and the amount retained in the skin was about 60% for both formulations. The 50% effective doses of Lip-PM-10 and Lip-PM-15 against L. major promastigotes in culture were 65.32 and 59.73 μg/ml, respectively, and those against L. major amastigotes in macrophages were 24.64 and 26.44 μg/ml, respectively. Lip-PM-10 or Lip-PM-15 was used topically twice a day for 4 weeks to treat L. major lesions on BALB/c mice, and the results showed a significantly (P < 0.001) smaller lesion size in the mice in the treated groups than in the mice in the control group, which received either empty liposomes or phosphate-buffered saline (PBS). Eight weeks after the beginning of the treatment, every mouse treated with LPMFs was completely cured. The spleen parasite burden was significantly (P < 0.001) lower in mice treated with Lip-PM-10 or Lip-PM-15 than in mice treated with PBS or control liposomes, but no significant difference was seen between the two groups treated with either Lip-PM-10 or Lip-PM-15. The results suggest that topical liposomal PM may be useful for the treatment of cutaneous leishmaniasis.
Leishmaniasis, which has diverse clinical manifestations, is caused by different species of Leishmania and is endemic in many countries (49). Although cutaneous leishmaniasis (CL) is a self-healing disease, healing takes a long time, and healing times of even up to 2 years have been reported (37). Pentavalent antimonials, which are still the first-line treatment for CL, require multiple injections and are painful; as such, they are not tolerated by most of the patients and, moreover, are not always effective. In addition, resistance to pentavalent antimonials has been reported (12, 13, 33).
Paromomycin sulfate (PM) was reported to show anti-Leishmania activity in the 1960s, and since then PM showed promising activity against both CL and visceral leishmaniasis (VL) in clinical trials (13, 31). Recently, the parenteral formulation of PM has been approved for use for the treatment of VL (27). Nevertheless, the systemic use of PM might cause nephrotoxicity. The conventional topical dosage forms of PM have been tested in clinical trials for their activities against CL and showed promising results, but acceptable efficacy was not always seen (2, 3, 5, 13, 18, 23, 28, 43). The formidable barrier nature of the stratum corneum (SC) of the skin does not allow the penetration of drugs with high hydrophilicities and molecular weights, like PM (19). Furthermore, drugs topically applied for the treatment of CL must be able to target the Leishmania parasites within the phagolysosome of the infected macrophages in the deep dermal layer of the skin (13, 34).
Liposomes are colloidal particles and typically consist of phospholipid and cholesterol (24). These lipid molecules form bilayers which entrap water-soluble molecules in their internal water compartment and water-insoluble ones in their lipid bilayers. In the proper formulations and at the appropriate sizes, liposomes deliver drugs to the skin on the basis of the similarity of the bilayer structure of the lipid vesicles to that of the natural membrane and target the macrophages within the dermis (6, 7, 41). Several lipid-based formulations for the treatment of experimental leishmaniasis have been developed (11, 19, 40, 45).
In the study described here, a liposomal formulation of PM was developed, and its delivery to the dermis and the efficacy were checked in vitro and in vivo. Liposomal formulations were prepared by the fusion method and were characterized for their size and encapsulation efficacy. The penetration properties of the formulations across mouse skin were compared by the use of Franz diffusion cells. The anti-Leishmania major activities of the formulations were checked in vitro against promastigotes and amastigotes of L. major and in vivo in L. major-infected susceptible BALB/c mice.
MATERIALS AND METHODS
Animals and parasites.
Female BALB/c mice (age, 6 to 8 weeks) were purchased from the Pasteur Institute (Tehran, Iran). The mice were maintained in the Animal House of the Biotechnology Research Center of the Mashhad University of Medical Sciences and were provided with tap water and fed a standard laboratory diet (Khorassan Javane Co., Mashhad, Iran). The animals were housed in a colony room with a 12-h light and 12-h dark cycle at 21°C and had free access to water and food. The animal experiments were carried out according to Ethical Committee Acts of the Mashhad University of Medical Sciences.
The virulence of Leishmania major strain MRHO/IR/75/ER was maintained by passage in BALB/c mice. The amastigotes were isolated from the spleen of an infected mouse and were cultured on NNN (Novy-MacNeal-Nicolle) medium and subcultured in RPMI 1640 (Sigma) containing 10% (vol/vol) heat-inactivated fetal calf serum (FCS), 2 mM glutamine, 100 U/ml of penicillin, and 100 μg/ml of streptomycin sulfate (RPMI-FCS) at 25 ± 1°C.
Chemicals.
Soybean phosphatidylcholine (SPC) and cholesterol were obtained from Avanti Polar Lipids (Alabaster, AL). PM, propylparaben (PP), methylparaben (MP), propylene glycol, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; tissue culture grade), and HEPES were purchased from Sigma; and vitamin E was purchased from Merck (Darmstadt, Germany). 2,4-Dinitro-1-fluorobenzene (DNFB) was purchased from Fluka (Germany).
Preparation of formulations containing PM.
Liposomes containing PM were prepared by the fusion method (20). Briefly, the lipid components consisted of SPC (15%), cholesterol (2%), propylene glycol (7%), vitamin E (0.3%), MP (0.1%), and PP (0.02%); these components were melted at about 75°C (lipid melt). HEPES buffer (10 mM, pH 7.0) and PM (10 or 15%) were heated separately and were added up to 100% to the previously heated melted lipids, and the mixture was vigorously vortexed to allow it to cool to room temperature. The final products were then homogenized with an homogenizer (Ultra-Turrax IKA T10; IKA Werke GmbH & Co. KG, Staufen, Germany) for 5 min at 5,000 rpm. The same procedure used for the preparation of liposomal PM described above was used to prepare control empty liposomes, except that the PM was omitted.
Characterization of liposomes.
The particle diameter of each sample was measured in triplicate by the use of dynamic light scattering (ZetaSizer Nano-ZS; Malvern Instruments Ltd., Worcestershire, United Kingdom).
To determine the efficiency of incorporation (percent encapsulation), certain amounts of liposomal PM were centrifuged (Universal 320 R; Hettich, Germany) at 14,000 × g for 30 min at 4°C and were subsequently washed three times with HEPES buffer. The supernatant and precipitate were then analyzed for PM by adding 1.5 ml DNFB (150 mM in methanol) to 0.5 ml sample, heating the mixture at 80°C for 45 min, making the volume up to 25 ml with chloroform-tetrahydrofuran-water (25:28.2:0.8), and measuring the absorbance at the maximum wavelength (405 nm) after the upper aqueous phase was discarded (32). The encapsulation efficiency of PM in liposomes was then calculated indirectly and directly in triplicate, as follows: for the indirect calculation, the percent encapsulation efficiency was equal to [(total amount of PM added − amount of PM recovered in supernatants)/total amount of PM added] × 100, and for the direct calculation, the percent encapsulation efficiency was equal to (amount of PM recovered in liposome precipitate/total amount of PM added) × 100.
Cell diffusion study.
Jacketed Franz cells with a receiver volume of 25 ml were used, and every experiment was conducted in triplicate at 37°C. Phosphate buffer of pH 7.4 was used as the receiver medium. A suitable size of full-thickness skin of a BALB/c mouse was cut and mounted in the Franz cell, with the SC side facing upward. The mouse was properly shaved with electric clippers on the day before the experiment. The membranes were initially left in the Franz cells for 30 min in order to facilitate hydration. Subsequently, 1 g of the liposomal PM formulation (LPMF) was deposited onto each membrane surface. A 250-μl aliquot was withdrawn from each receiver solution at 1-h intervals and replaced with the same volume of blank PBS solution. Aliquots of the collected samples were analyzed for their PM content, as described above. The derived concentration values were corrected by using the equation Mt(n) = Vr × Cn + Vs × ΣCm, where Mt(n) is the current cumulative mass of drug transported across the skin at time t, n is the number (times) of sampling, Cn is the current concentration in the receiver medium, ΣCm is the summed total of the previously measured concentrations, Vr is the volume of the receiver medium, and Vs corresponds to the volume of the sample removed for analysis.
For the determination of the amount of liposome retained in the skin, at the end of the experiment, the amount of the formulation remaining on the surface of the membrane was collected and assayed for PM. The amount of PM retained in the skin was then calculated by subtracting the sum of the amount of PM that remained on the surface and the amount of PM that was released (penetrated through the skin) from the whole amount applied (8, 30).
In vitro promastigote assay.
The effects of the formulations on the viability of Leishmania promastigotes were assessed by monitoring MTT metabolism after a 48-h culture period in the presence of the formulations. Parasites were harvested at stationary phase of culture, and 400,000 promastigotes were added to each well of 96-well flat-bottom plates containing different concentrations of the formulations; triplicate wells were used for each concentration. The plates were incubated at 25 ± 1°C for 48 h prior to the addition of MTT (40 μl/well of 5 mg/ml in PBS), and then the plates were incubated in the dark at 37°C for a further 4 h. The formation of formazan was evaluated by adding 50 μl/well 20% sodium dodecyl sulfate and incubating the plates overnight at 37°C, and the relative absorbance was photometrically measured with an enzyme-linked immunosorbent assay reader (Statfax-2100; Awareness Technology) at 545 nm. The relative absorbance was correlated to the number of promastigotes per well by using a standard curve that consisted of the results for different numbers of promastigotes treated with the MTT dye, as explained above. The 50% effective dose (ED50) for each formulation was calculated by the Litchfield-Wilcoxon method with PCS (version 4) software (15, 42).
In vitro amastigote assay.
Cells of the J774 A.1 mouse macrophage cell line (Pasteur Institute) were dispensed at a concentration of 50,000 macrophages/well into eight-well Lab-Tek (Nunc) chamber slides and maintained at 37°C in 5% CO2 for 24 h to allow attachment of the cells. The cells were then infected with L. major promastigotes at a ratio of five promastigotes per macrophage and incubated at 37°C in 5% CO2 for 24 h to allow internalization of the parasites in the cells. The excess amount of promastigotes was removed by gently washing the cells with PBS three times, and the infected cells were incubated for an additional 24 h to allow the establishment of the infection. The cells were then exposed to different concentrations of LPMFs in triplicate for 2 days. The experiment was terminated by methanol fixation of the slides. The slides were then stained with Giemsa and evaluated microscopically to calculate the percentage of infected cells. The ED50 for each formulation was calculated by the Litchfield-Wilcoxon method with PCS (version 4) software (42, 50).
In vivo experiment.
Forty female BALB/c mice (age, 6 to 8 weeks) were inoculated subcutaneously at the base of the tail with 4 × 106 L. major promastigotes harvested at stationary phase. At 4 weeks postinfection, the lesions were measured with calipers in two dimensions, the mean diameters were determined, and the mice were randomly divided into four groups of 10 mice each. No significant differences (P > 0.05) in lesion size were seen among the different groups. The lesions were then treated topically with 50-mg formulations twice a day for 4 weeks. The lesion sizes were measured weekly during treatment and at week 4 after the treatment was stopped (29).
Quantitative parasite burden.
The number of viable L. major parasites in the spleens of the mice was assessed by limiting dilution assay (46). The mice were killed at 8 and 12 weeks after infection; the spleens were aseptically removed and homogenized in 1 ml RPMI-FCS with a sterile syringe piston. The homogenate was diluted with the same medium in eight serial 10-fold dilutions in each well of flat-bottom 96-well microtiter plates containing a solid layer of rabbit blood agar in triplicate, and the plates were incubated at 25 ± 1°C for 7 days. The positive wells (which contained motile parasites) and the negative wells (which did not contain motile parasites) were identified with an inverted microscope. The data are reported as the calculated mean and standard error of the mean of the last positive well multiplied by the dilution factor (29).
Statistical analysis.
The one-way analysis of variance statistical test was used to assess the significance of the differences among the various groups. In the case of a significant F value, the multiple-comparison Tukey test was used to compare the means of the different treatment groups. Results with P values of <0.05 were considered statistically significant.
RESULTS
Liposome characterization.
The liposomes used in this study were in the submicron size range (Table 1). The zeta average size and the polydispersity of the control liposomes were less than those of the liposomes containing 10 or 15% PM (Lip-PM-10 and Lip-PM-15, respectively); however, no significant difference was seen between Lip-PM-10 and Lip-PM-15.
TABLE 1.
Particle size distribution and polydispersity index of liposomal PM formulations
| Formulation | Avg size (nm) bya:
|
PDIb | |||
|---|---|---|---|---|---|
| Zeta | No. | Vol | Intensity | ||
| Lip-PM-10 | 532.43 ± 164.40 | 89.51 ± 34.68 | 464.13 ± 282.25 | 418.15 ± 84.93 | 0.57 ± 0.05 |
| Lip-PM-15 | 507.97 ± 250.47 | 92.06 ± 8.73 | 629.85 ± 272.11 | 462.71 ± 91.97 | 0.63 ± 0.05 |
| Control liposome | 299.67 ± 46.38 | 66.95 ± 3.08 | 410.70 ± 32.10 | 381.40 ± 17.96 | 0.47 ± 0.07 |
The values are means ± standard deviations (n = 3). There were no statistically significant differences in the particle sizes of the formulations (P > 0.05).
PDI, polydispersity index.
The encapsulation efficiency of PM was determined by both direct and indirect methods. The percent encapsulation of both formulations was about 60%, and there was no significant difference between the percent encapsulation in Lip-PM-10 and Lip-PM-15 (Table 2).
TABLE 2.
Percent encapsulation of PM in LPMFs determined by direct and indirect methods with DNFB reagent
| Formulation | % Encapsulation of formulation bya:
|
|
|---|---|---|
| Direct method | Indirect method | |
| Lip-PM-10 | 60.298 ± 1.187 | 51.676 ± 0.610 |
| Lip-PM-15 | 60.359 ± 1.828 | 57.637 ± 3.747 |
The values are means ± standard deviations (n = 3). There were no statistically significant differences in the encapsulation efficiencies of the formulations (P > 0.05).
Cell diffusion study.
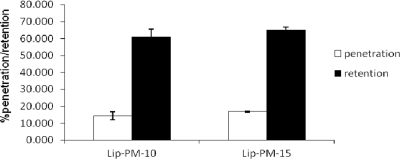
Studies of the in vitro penetration of the formulations across mouse skin were carried out with diffusion cells, and the percentage of PM that penetrated and that was retained in the skin was determined for the formulations for up to 8 h. The proportions of PM in Lip-PM-10 and Lip-PM-15 that penetrated the skin were 14.40% ± 2.42% and 16.92% ± 0.36%, respectively; and the proportions of PM in Lip-PM-10 and Lip-PM-15 that were retained were 61.14% ± 4.22% and 65.25% ± 1.48%, respectively (Fig. 1). There was no significant difference in the percentage of PM that penetrated the skin and the percentage of PM that was retained between the two LPMFs.
FIG. 1.
Percent penetration and retention of PM from Lip-PM-10 and Lip-PM-15 across the mouse skin after 8 h. Franz cell diffusion studies were carried out with jacketed Franz cells contain 25 ml PBS as the receiver medium at 37°C, and samples were drawn at 1-h intervals for up to 8 h. At the end of the experiment, the formulation that remained on the top of mice skin was collected and assayed for detection of the amount of PM. The percent formulation retention was calculated by considering the percentage of the drug released and the remaining useless amount on the skin at the end of the 8-h experiment. Values are means ± standard deviations (n = 3). There were no statistically significant differences in the levels of penetration or retention between the two formulations (P > 0.05).
Effects of LPMFs on L. major promastigotes in vitro.
The ED50s of the Lip-PM-10 and Lip-PM-15 formulations against L. major promastigotes were 65.32 ± 7.57 and 59.73 ± 6.27 μg/ml, respectively. There were no significant differences in the activities of the two liposomal formulations against L. major promastigotes. However, the liposomal PM formulations were three to four times more effective (P < 0.001) than PM in solution (Table 3).
TABLE 3.
In vitro activities of formulations against L. major promastigotes and amastigotes
| Formulation | ED50 (μg/ml) for L. majora:
|
|
|---|---|---|
| Promastigotes | Amastigotes | |
| Lip-PM-10 | 65.32 ± 7.57b | 24.64 ± 1.51b |
| Lip-PM-15 | 59.73 ± 6.27b | 26.44 ± 1.89b |
| PM in solution | 205.70 ± 10.05 | 83.31 ± 16.65 |
| Control empty liposome | Inactive | Inactive |
The values are means ± standard deviations (n = 3).
There were statistically significant differences (P < 0.001) between the results for LPMFs and those for PM in solution.
Effects of LPMFs on L. major amastigotes in vitro.
The ED50s of the Lip-PM-10 and Lip-PM-15 formulations against intracellular amastigotes were 24.64 ± 1.51 and 26.44 ± 1.89 μg/ml, respectively. There were no significant differences in the activities of the two liposomal formulations against L. major amastigotes. However, the liposomal PM formulations were three to four times more effective (P < 0.001) than PM in solution (Table 3).
Effects of topical LPMFs on the sizes of ulcers induced in BALB/c mice infected with L. major.
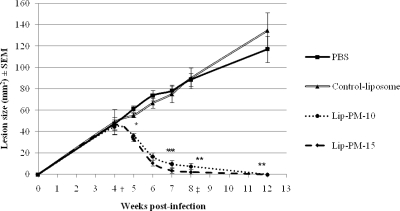
There were no significant differences (P > 0.05) in the lesion sizes among the different groups before the initiation of the treatment (Fig. 2, week 4 postinfection). The topical application of both LPMFs caused significant reductions in the lesion sizes. The effect was statistically significant after the week first of treatment (P < 0.05) and onward (P < 0.001). At week 3 after treatment initiation, two mice receiving Lip-PM-15 and one mouse receiving Lip-PM-10 were completely cured. At week 4 after treatment initiation, six mice receiving Lip-PM-15 and two mice receiving Lip-PM-10 were completely cured. At week 4 after the treatment was stopped, every mouse in the group that received either Lip-PM-10 or Lip-PM-15 was completely cured, and no relapse was observed in the animals treated with either one of the LPMFs. No statistically significant differences were seen between the lesion sizes of the animals treated with the two experimental LPMFs, and no statistically significant differences were seen between the two control groups that received PBS or control empty liposomes.
FIG. 2.
Effect of topical liposomal PM on the course of disease in a BALB/c mouse model of CL caused by L. major. Female BALB/c mice (age, 6 to 8 weeks) were infected by the subcutaneous injection of 4 × 106 stationary-phase L. major promastigotes at the base of the tail. At 4 weeks postinfection, the lesions were measured with calipers in two dimensions, the mean diameters were determined, and the mice were randomly housed in groups of 10 mice each. The lesions were treated topically with 50-mg formulations twice a day for 4 weeks. During treatment, the lesion sizes were determined weekly and were monitored for 12 weeks. †, beginning of treatment; values are the means ± standard errors of the means (n = 10), and there were no significant (P > 0.05) differences among the groups; *, values are the means ± standard errors of the means (n = 10), and there were significant (P < 0.05) differences between the LPMF-treated and the control groups; **, values are the means ± standard errors of the means (n = 10), and there were significant (P < 0.001) differences between the LPMF-treated and the control groups; ‡, end of treatment; values are the means ± standard errors of the means (n = 6), and there were significant (P < 0.001) differences between the LPMF-treated and the control groups.
Effects of topical LPMFs on splenic parasite burden.
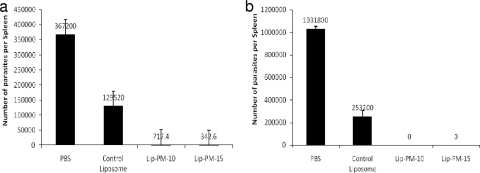
At week 8 postinfection, the mice treated with either of the LPMFs showed a significantly (P < 0.001) lower parasite burden than the mice in the control groups that received either PBS or empty liposomes (Fig. 3a), but no significant differences were seen between the two groups treated with either Lip-PM-10 or Lip-PM-15, and no significant differences were seen between the two control groups that received either PBS or empty liposome. However, the number of parasites in the group that received empty liposomes was less than that in the group that received PBS. At week 12, no parasite was detected in the spleens of mice treated with either Lip-PM-10 or Lip-PM-15, but the number of parasites in the control groups that received either PBS or empty liposome was found to be higher (Fig. 3b).
FIG. 3.
Splenic parasite burdens in BALB/c mice treated with topical liposomal PM. The number of viable L. major parasites in the spleens of different groups of mice was quantified by the limiting dilution assay at 8 weeks (a) and 12 weeks (b) after infection with L. major promastigotes. The spleens were aseptically removed, homogenized in RPMI-FCS, and diluted with the same medium in eight serial 10-fold dilutions in each well of flat-bottom 96-well microtiter plates containing a solid layer of blood agar. The plates were kept at 25°C for 1 week and were then read to score the number of positive wells (positive wells contained one or more promastigotes). The final titer was the last dilution for which the well contained at least one parasite. (a) Values are the means ± standard errors of the means (n = 4), and there were statistically significant (P < 0.001) differences between the LPMF-treated and the control groups; (b) values are the means ± standard errors of the means (n = 6), and there were statistically significant (P < 0.001) differences between the LPMF-treated and the control groups.
DISCUSSION
Although CL, which is the most common form of leishmaniasis, is a self-healing lesion, it is difficult to treat, especially when it is induced by particular species, such as L. tropica (48, 49). The treatment of CL solely depends on the use of antimonials, which requires multiple injections; when a patient has a limited number of lesions, the intralesional injection of antimonials is usually done (37, 38). Although intralesional injection is an effective method, it needs special medical services which are not available in most areas where CL is endemic, and multiple injections around the lesion are very painful and not every patient is able to tolerate the treatment (13). Furthermore, resistance to the pentavalent antimonials has now been reported (12, 13). Therefore, the search for new treatments for CL has been ongoing for decades, but no topical treatment is yet available.
PM, like all the aminoglycoside antibiotics, inhibits protein biosynthesis in sensitive organisms (1). PM ointments were shown to be effective in an experimental model of murine leishmaniasis (17, 38, 39). The results of most clinical trials showed an acceptable level of efficacy of PM for the treatment of CL, especially lesions caused by L. major (5, 43). The low level of penetration of PM into the skin is a major drawback and results in a low level of efficacy of PM for the treatment of some lesions, especially if the lesions are not ulcerated. The formidable barrier nature of the SC does not allow the penetration of drugs with high hydrophilicities and molecular masses, like PM (molecular mass, 713.71) (19, 26, 31). Drugs need to be able to reach the Leishmania parasites, which reside in phagolysosomes of infected macrophages deep in the dermal layer of the skin (13, 34).
The current study was designed to prepare and characterize LPMFs and then determine the effects of the LPMFs in vitro against L. major promastigotes and L. major growth in cells of the J774 A.1 mouse macrophage cell line and in vivo against ulcers induced by L. major infection in susceptible BALB/c mice.
Interest in PM as a treatment for leishmaniasis started in the 1980s, when topical formulations for the treatment of CL and a parenteral formulation for the treatment of VL were developed (13). The topical treatment of CL with PM has been shown to be effective in some cases (2, 3, 5, 13, 18, 23, 28, 43).
In 1984, El-On and colleagues (17) described the activity of 15% PM plus 12% methylbenzethonium chloride in soft paraffin and it was marketed in Israel, but clinical studies of this formulation reported that it induced local toxicity and was not well tolerated (2, 13).
Other topical formulations have been investigated, including one containing 15% PM and 10% urea, which was shown to be effective and tolerated (9); but in a clinical trial in Iran, Faghihi and Tavakoli-kia (18) found this formulation to be much less effective than intralesional antimony, with cure frequencies of 16.6% and 41.7%, respectively. Asilian et al. (5) also found that even with 4 weeks of treatment, about one-third of all patients remained uncured. In 2005, Iraji and Sadeghinia conducted a double-blind study in Iran and showed that this formulation was of no use for the treatment of human CL (28). Grogl et al. (25) introduced a new topical formulation of PM which consisted of a complex cream containing 15% PM and 0.5% gentamicin. In clinical trials, there were no significant differences in the cure rates achieved with this formulation and those achieved with placebo (44).
Topical treatment of CL is required because Leishmania amastigotes lie deep in the dermal layer, making penetration difficult (13, 34). PM formulated in conventional ointment and cream bases is not as effective due to its relatively large molecular size, high degree of solubility in water, and oligosaccharide nature, which make it difficult to penetrate the SC of the skin. Agents topically applied for the treatment of CL must be able to pass through the SC of the skin so that they are active against parasites inside macrophages. Therefore, the candidate drugs not only need to target the macrophages in the dermis but also need to cross the phagolysosomal vacuole membrane and parasite membrane to reach to the cytosole of the parasite (34).
In the present study, topical LPMFs were evaluated for their effectiveness for the treatment of ulcers caused by L. major in BALB/c mice. Liposomes in the proper formulations and sizes have been shown to be able to pass through the SC, reach the epidermis and deep dermis, and also target the macrophages within the dermis (7, 22, 35, 36, 41). The presence of intact liposomes in the epidermis and dermis has also been shown by electron microscopy studies (21). The level of penetration of liposomes into the epidermis and dermis depends upon the kind of phospholipid, its concentration, the sizes of the liposomes, and the penetration enhancer used (20, 22, 47). Phospholipids with choline head groups interact with and hydrate the skin more efficiently and have stronger penetration than other types of phospholipids (7, 22, 47); therefore, SPC was used to prepare the LPMFs. To transfer liposomal formulations to the epidermis and dermis, the phosphatidylcholine concentration needs to be more than 3 to 6 mg/cm2 of skin (22), and therefore, an SPC concentration of 15% was selected for use in this study. Studies showed that liposomes with smaller sizes penetrate the SC more efficiently and reach the epidermis and dermis (47). In this study, topical PM liposomes prepared by the fusion method plus homogenization provided liposomes of submicron sizes (Table 1). Analysis of the particle size distribution showed that the average size of most of the population of LPMFs was less than 100 nm (according to the average size by number; Table 1), even though the zeta average size of the particles was about 500 nm, which might have been due to the high concentration of lipids in the formulations and the presence of some unhomogenized liposomes. Furthermore, the results of the Franz diffusion cell studies across mouse skin showed high percentages of penetration and retention in the skin when the formulations were used (Fig. 1), which proves that these vesicles possess a high penetration ability. Cholesterol was included in the topical PM liposome formulation to stabilize the lipid bilayers and decrease the leakage of encapsulated PM and vesicle aggregation; vitamin E was used to prevent SPC oxidation, PP and MP were used as microbial preservatives, and HEPES was to control the pH of the liposomal formulations to achieve maximum stability (24).
The fusion method was used to prepare the topical PM liposomes. The fusion method is one of the more suitable methods for the preparation of topical liposomes, as it provides homogeneous liposomes (20). The fusion method is simple, efficient, and reproducible; is devoid of organic solvents like chloroform; and yields homogeneous liposomes with high encapsulation efficiencies. The encapsulation efficiencies for Lip-PM-10 and Lip-PM-15 were about 60% (Table 2), and no crystallization of either formulation was observed during storage. Furthermore, liposomes prepared by this method showed enough viscosity that they could be applied directly on the skin without the need for the liposomal formulation to be mixed with other bases.
The leishmanicidal activities of the formulations were tested against both the extracellular promastigote and the intracellular amastigote forms of the parasite. The ED50s of the formulations against promastigotes and amastigotes were determined (Table 3) and showed that the ED50 of PM-containing liposomes against promastigotes was about twice that against amastigotes. A similar conclusion was drawn on the basis of the finding that intramacrophage amastigotes are more susceptible to PM than promastigotes (10, 12). The assays with promastigotes and amastigotes also showed that the processes used for the preparation of LPMFs by the fusion method do not affect the activity of the PM.
The results of the in vivo study with BALB/c mice infected with L. major showed that the topical LPMFs induced the complete cure of the lesions induced by L. major infection in susceptible BALB/c mice (Fig. 2), and the mice had significantly lower parasite burdens in the spleen than the control mice (Fig. 3). It is speculated that after the topical application of LPMFs, at least some of the vesicles, especially those smaller than 100 nm, pass through the SC of intact skin and reach the epidermis and deep dermis. In the dermis, the infected macrophages phagocytose the PM-containing liposomes, and then PM is released by acidic lysosomal enzymes in the phagolysosome of the macrophage, where Leishmania parasites live and multiply (4, 16, 19, 22, 35, 41).
The effect of LPMFs on the course of L. major infection was significant, as the treated mice were completely cured and no relapse was seen at the end of the study. The parasite loads in the spleens of the treated mice were significantly lower than those in the spleens of the control mice at week 8 postinfection, and at week 12 postinfection, no parasite was observed in the spleens of treated animals (Fig. 2 and 3). In the in vivo experiment, the treatment of the lesions was started at week 4 after infection. As shown in Fig. 2, the LPMFs very quickly resolved the lesions and there was a significant reduction in lesion sizes within 2 weeks of the start of treatment (approximately 75%). After 4 weeks of treatment, six mice that had received Lip-PM-15 and two mice that had received Lip-PM-10 were completely cured and the lesion sizes in the rest of the treated mice were significantly smaller than those in the control groups. The treatment may have had an effect on the overall course of infection and might be the reason for the lower parasite burdens in the spleens of LPMF-treated mice. Furthermore, since some of these PM liposomes are very small, they can act as transdermal vehicles and pass from the epidermis intact and reach the bloodstream in the dermis. When liposomes are in the blood, spleen macrophages and Kupffer cells in the liver are the main cells that phagocytose the liposomal particles (14). According to data published by Foldvari (20), at least some of the liposomal PM reaches the blood and targets the spleen macrophages, and then PM might have some direct effect on the infected macrophages and L. major amastigotes. Future work in order to determine the amount of PM in the spleens of infected mice after the topical application of LPMFs might clear this up, although the immunomodulatory effect of PM might play a significant role.
The overall results obtained in this study show that liposomes are interesting carriers of PM and that the liposomal PM is an appropriate candidate for use for the treatment of CL. This is the first report on the utilization of topical liposomes containing PM for the treatment of CL. Since there were no statistically significant differences between the effects of Lip-PM-10 and Lip-PM-15, the Lip-PM-10 and formulations with lower concentrations of PM (7.5, 5.0, and 2.5%) will be used for further biodistribution and immunomodulatory studies and human efficacy trials.
Acknowledgments
This project was supported by a grant from the vice chancellor for research, Mashhad University of Medical Sciences, Mashhad, Iran.
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Anonymous. 2008. Data sheet 512731, revised 16 May. RFH paromomycin sulfate, catalog no. 512731. Merck, Darmstadt, Germany. http://www.emdbiosciences.com/product/512731.
- 2.Arana, B. A., C. E. Mendoza, N. R. Rizzo, and A. Kroeger. 2001. Randomized, controlled, double-blind trial of topical treatment of cutaneous leishmaniasis with paromomycin plus methylbenzethonium chloride ointment in Guatemala. Am. J. Trop. Med. Hyg. 65:466-470. [DOI] [PubMed] [Google Scholar]
- 3.Armijos, R. X., M. M. Weigel, M. Calvopina, M. Mancheno, and R. Rodriguez. 2004. Comparison of the effectiveness of two topical paromomycin treatments versus meglumine antimoniate for New World cutaneous leishmaniasis. Acta Trop. 91:153-160. [DOI] [PubMed] [Google Scholar]
- 4.Ashan, F., I. P. Rivas, M. A. Khan, and A. I. T. Suarez. 2002. Targeting to macrophages: role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J. Control. Release 79:29-40. [DOI] [PubMed] [Google Scholar]
- 5.Asilian, A., T. Jalayer, M. Nilforooshzadeh, R. L. Ghassemi, R. Peto, S. Wayling, et al. 2003. Treatment of cutaneous leishmaniasis with aminosidine (paromomycin) ointment: double-blind, randomized trial in the Islamic Republic of Iran. Bull. W. H. O. 81:353-359. [PMC free article] [PubMed] [Google Scholar]
- 6.Barry, B. W. 2001. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 14:101-114. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia, A., R. Kumar, and O. P. Katare. 2004. Tamoxifen in topical liposomes: development, characterization and in-vitro evaluation. J. Pharm. Pharm. Sci. 7:252-259. [PubMed] [Google Scholar]
- 8.Brain, K. F., K. A. Walters, and A. C. Watkinson. 2002. Methods for studying percutaneous absorption, p. 197-269. In K. A. Walters (ed.), Dermatological and transdermal formulation. Marcel Dekker Inc., New York, NY.
- 9.Bryceson, A. D., A. Murphy, and A. H. Moody. 1994. Treatment of ‘Old World’ cutaneous leishmaniasis with aminosidine ointment: results of an open study in London. Trans. R. Soc. Trop. Med. Hyg. 88:226-228. [DOI] [PubMed] [Google Scholar]
- 10.Callahan, H. L., A. C. Portal, R. Devereaux, and M. Grogl. 1997. An axenic amastigote system for drug screening. Antimicrob. Agents Chemother. 41:818-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauchetier, E., M. Paul, D. Rivollet, H. Fessi, A. Astier, and M. Deniau. 2000. Therapeutic evaluation of free and liposome-encapsulated atovaquone in the treatment of murine leishmaniasis. Int. J. Parasitol. 30:777-783. [DOI] [PubMed] [Google Scholar]
- 12.Croft, S. L. 2001. Monitoring drug resistance in leishmaniasis. Trop. Med. Int. Health 6:899-905. [DOI] [PubMed] [Google Scholar]
- 13.Croft, S. L., and V. Yardley. 2002. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 8:319-342. [DOI] [PubMed] [Google Scholar]
- 14.Drummond, D. C., C. O. Noble, M. E. Hayes, J. W. Park, and D. B. Kirpotin. 2008. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J. Pharm. Sci. 97:4696-4740. [DOI] [PubMed] [Google Scholar]
- 15.Dutta, A., S. Bandyopadhyay, C. Mandal, and M. Chatterjee. 2005. Development of a modified MTT assay for screening antimonial resistant field isolates of Indian visceral leishmaniasis. Parasitol. Int. 54:119-122. [DOI] [PubMed] [Google Scholar]
- 16.El Maghraby, G. M., B. W. Barry, and A. C. Williams. 2008. Liposomes and skin: from drug delivery to model membranes. Eur. J. Pharm. Sci. 34:203-222. [DOI] [PubMed] [Google Scholar]
- 17.El-On, J., G. P. Jacobs, E. Witzum, and C. L. Greenblatt. 1984. Development of topical treatment for cutaneous leishmaniasis caused by Leishmania major in experimental animals. Antimicrob. Agents Chemother. 26:745-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faghihi, G., and R. Tavakoli-kia. 2003. Treatment of cutaneous leishmaniasis with either topical paromomycin or intralesional meglumine antimoniate. Clin. Exp. Dermatol. 28:13-16. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira, L. S., G. A. Ramaldes, E. A. Nunan, and L. A. M. Ferreira. 2004. In vitro skin permeation and retention of paromomycin from liposomes for topical treatment of the cutaneous leishmaniasis. Drug Dev. Ind. Pharm. 30:289-296. [DOI] [PubMed] [Google Scholar]
- 20.Foldvari, M. December 1998. Biphasic multilamellar lipid vesicles. U.S. patent 5,853,755.
- 21.Foldvari, M., A. Gesztes, and M. Mezei. 1990. Dermal drug delivery by liposome encapsulation: clinical and electron microscopic studies. J. Microencapsul. 4:479-489. [DOI] [PubMed] [Google Scholar]
- 22.Ghyczy, M., and J. Gareiss. 1994. Liposomes from vegetable phosphatidylcholine: their production and effects on the skin. Cosmet. Toiletr. 109:75-81. [Google Scholar]
- 23.Goncalves, G. S., A. P. Fernandes, R. C. C. Souza, J. E. Cardoso, F. de Oliveira-Silva, F. C. Maciel, et al. 2005. Activity of a paromomycin hydrophilic formulation for topical treatment of infections by Leishmania (Leishmania) amazonensis and Leishmania (Viannia) braziliensis. Acta Trop. 93:161-167. [DOI] [PubMed] [Google Scholar]
- 24.Gregoriadis, G., C. K. P. Large, A. Meehan, and J. Senior. 1981. Targeting of liposomes: study of influence factors, p. 155-184. In G. Gregoriadis and J. Senior (ed.), Targeting of drugs. Plenum Press, New York, NY.
- 25.Grogl, M., B. G. Schuster, W. Y. Ellis, and J. D. Berman. 1999. Successful topical treatment of murine cutaneous leishmaniasis with a combination of paromomycin (aminosidine) and gentamicin. J. Parasitol. 85:354-359. [PubMed] [Google Scholar]
- 26.Honeywell-Nguyen, P. L., A. M. de Graaff, H. W. Groenink, and J. A. Bouwstra. 2002. The in vivo and in vitro interactions of elastic and rigid vesicles with human skin. Biochim. Biophys. Acta 1573:130-140. [DOI] [PubMed] [Google Scholar]
- 27.Institute for OneWorld Health. Visceral leishmaniasis. http://www.oneworldhealth.org/diseases/leishmaniasis.php. Institute for OneWorld Health, San Francisco, CA.
- 28.Iraji, F., and A. Sadeghinia. 2005. Efficacy of paromomycin ointment in the treatment of cutaneous leishmaniasis: results of a double-blind, randomized trial in Isfahan, Iran. Ann. Trop. Med. Parasitol. 99:3-9. [DOI] [PubMed] [Google Scholar]
- 29.Jaafari, M. R., A. Ghafarian, A. Farrokh-Gisour, A. Samiei, M. T. Kheiri, F. Mahboudi, et al. 2006. Immune response and protection assay of recombinant major surface glycoprotein of Leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine 24:5708-5717. [DOI] [PubMed] [Google Scholar]
- 30.Khan, G. M., Y. Frum, O. Sarheed, G. M. Eccleston, and V. M. Meidan. 2005. Assessment of drug permeability distributions in two different model skins. Int. J. Pharm. 303:81-87. [DOI] [PubMed] [Google Scholar]
- 31.Klaus, S. N. and D. Kafka. 1992. Topical paromomycin: a safe and effective therapy for cutaneous leishmaniasis, abstr. p. 410-411. Dermatology: progress and perspective. Abstr. Proc. 18th World Congr. Dermatol.
- 32.Lunn, G. 2000. HPLC methods for pharmaceutical analysis, p.36-39. John Wiley & Sons, Inc., New York, NY.
- 33.Markle, W. H., and K. Makhoul. 2004. Cutaneous leishmaniasis: recognition and treatment. Am. Fam. Physician 69:1455-1460. [PubMed] [Google Scholar]
- 34.Mauel, J. 1990. Macrophage parasite interactions in Leishmania infections. J. Leukoc. Biol. 47:187-193. [DOI] [PubMed] [Google Scholar]
- 35.Mezei, M. 1993. Liposomes and the skin, p. 125-135. In G. Gregoriadis, A. T. Florence and H. M. Patel (ed.), Liposomes in drug delivery. Harwood Academic Publishers, Langhorne, PA.
- 36.Mezei, M., and U. Gulasekharam. 1980. Liposomes, a selective drug delivery system for the topical route of administration of lotion dosage forms. Life Sci. 26:1437-1477. [DOI] [PubMed] [Google Scholar]
- 37.Murray, H. W., J. D. Berman, C. R. Davies, and N. G. Saravia. 2005. Advances in leishmaniasis. Lancet 366:1561-1577. [DOI] [PubMed] [Google Scholar]
- 38.Neal, R. A., S. Allen, N. McCoy, P. Olliaro, and S. Croft. 1995. The sensitivity of Leishmania species to aminosidine. J. Antimicrob. Chemother. 35:577-584. [DOI] [PubMed] [Google Scholar]
- 39.Neal, R. A., A. G. Murphy, P. Olliaro, and S. Croft. 1994. Aminosidine ointments for the treatment of experimental leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 88:223-225. [DOI] [PubMed] [Google Scholar]
- 40.Pal, S., R. Ravindran, and N. Ali. 2004. Combination therapy using sodium antimony gluconate in stearylamine-bearing liposomes against established and chronic Leishmania donovani infection in BALB/c mice. Antimicrob. Agents Chemother. 48:3591-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel, H. M. and S. M. Moghimi. 1993. Liposomes and the skin permeability barrier, 137-148. In G. Gregoriadis, A. T. Florence, and H. M. Patel (ed.), Liposomes in drug delivery. Harwood Academic Publishers, Langhorne, PA.
- 42.Rahman, A. U., M. I. Choudhary, and W. J. Thomsen. 2001. Bioassay techniques for drug development, p.62-64. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 43.Shazad, B., B. Abbaszadeh, and A. Khamesipour. 2005. Comparison of topical paromomycin sulfate (twice/day) with intralesional meglumine antimoniate for the treatment of cutaneous leishmaniasis caused by L. major. Eur. J. Dermatol. 15:85-87. [PubMed] [Google Scholar]
- 44.Soto, J. M., J. T. Toledo, P. Gutierrez, M. Arboleda, R. S. Nicholls, J. R. Padilla, et al. 2002. Treatment of cutaneous leishmaniasis with a topical antileishmanial drug (WR279396): phase 2 pilot study. Am. J. Trop. Med. Hyg. 66:147-151. [DOI] [PubMed] [Google Scholar]
- 45.Tempone, A. G., D. Perez, S. Rath, A. L. Vilavinho, R. A. Mortara, and H. F. Andradr. 2004. Targeting Leishmania chagasi amastigotes through macrophage scavenger receptors: the use of drug entrapped in liposomes containing phosphatidylserine. J. Antimicrob. Chemother. 54:60-68. [DOI] [PubMed] [Google Scholar]
- 46.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limited dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 47.Touitou, E., H. E. Junginger, W. D. Weiner, T. Nagai, and M. Mezei. 1994. Liposomes as carriers for topical and transdermal delivery. J. Pharm. Sci. 83:1189-1203. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. 1990. Control of leishmaniasis, p.1-158. World Health Organization Technical Report Series 793. World Health Organization, Geneva, Switzerland. [PubMed]
- 49.World Health Organization. 2004. Scientific working group on leishmaniasis. Meeting report. World Health Organization, Geneva, Switzerland.
- 50.Yardley, V., and S. L. Croft. 2000. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int. J. Antimicrob. Agents 13:243-248. [DOI] [PubMed] [Google Scholar]