Abstract
The objective of the study was to measure antiretroviral exposures in four physiological compartments during pregnancy, delivery, and postpartum. This prospective, open-label, longitudinal study collected paired blood plasma (BP) and genital tract (GT) aspirates antepartum, at delivery, and up to 12 weeks postpartum. Antiretroviral cord BP and amniotic fluid concentrations were also measured. Drug concentrations were analyzed by validated high-performance liquid chromatography/UV and liquid chromatography/tandem mass spectrometry methods, with secondary compartment concentrations presented as the percentage of BP. Fourteen women taking lamivudine plus zidovudine and either lopinavir-ritonavir (n = 7), nelfinavir (n = 6), or nevirapine (n = 1) were enrolled; four also received tenofovir. GT penetration relative to BP was highest for the nucleoside reverse transcriptase inhibitors compared to the protease inhibitors and nevirapine. Only antepartum nelfinavir GT penetration was significantly higher than in the second trimester (geometric mean ratio [GMR], 179.3) or third trimester (GMR, 41.9). Compared to nonpregnant historical controls, antepartum GT penetration was significantly lower (P < 0.05) for zidovudine (GMR, 0.25) and lopinavir (GMR, 0.03); postpartum lopinavir GT penetration continued to be significantly lower (GMR, 0.27). Cord BP exposures were highest for lamivudine and tenofovir (≥100%), with cord BP levels of the remaining drugs ranging from 49 to 86% of that of the respective BP level. Amniotic exposures for lamivudine, zidovudine, tenofovir, and nelfinavir were ≥100%, nevirapine exposure was 53%, and lopinavir and ritonavir exposures were ≤6% that of BP. We conclude that GT, cord BP, and amniotic fluid exposures vary within and between antiretroviral drug classes and biologic sites. Measurement of antiretroviral exposure in maternal genital secretions, cord BP, and amniotic fluid may be needed to identify signals of subtherapeutic or supratherapeutic drug exposure.
The widespread use of highly active antiretroviral (ARV) therapy in human immunodeficiency virus (HIV)-infected pregnant women and their infants has decreased mother-to-child transmission of HIV to less than 2% in developed countries (3, 16). Recent data for HIV-infected pregnant women suggest that plasma concentrations of protease inhibitors (PI) decrease during the third trimester (43, 50). Similar pregnancy-induced alterations in drug exposure have been observed for other therapeutic classes (e.g., antiepileptics, antidepressants, and antihypertensives) (19). These variations may be due to increased hepatic blood flow, increased volume of distribution, and/or alterations in drug-binding proteins (50, 55). Additionally, increased hormone production during pregnancy may cause induction of cytochrome P450 (CYP) enzyme activity (e.g., CYP3A4) (25, 40). These influences, taken together, may reduce concentrations of PIs and nonnucleoside reverse transcriptase inhibitors. Significant reductions in ARV concentrations increase the risk of incomplete suppression of viral replication and subsequent development of resistance (49, 54). Likewise, incomplete viral suppression may increase the risk of perinatal transmission (2).
Quantifying drug exposure in body compartments with which the infant has contact during pregnancy and delivery (genital tract, cord blood plasma, and amniotic fluid) may assist in selecting optimal drug regimens. This open-label, prospective, longitudinal, cohort study in HIV type 1 (HIV-1)-infected pregnant women initiating or continuing highly active ARV therapy was performed to measure the exposures of seven ARVs in four physiologic compartments during pregnancy, delivery, and postpartum.
(This research was previously presented in part at the 33rd Scientific Meeting of the Infectious Diseases Society for Obstetrics and Gynecology, Monterey, CA, 3 to 5 August 2006, and as a poster at the 46th Interscience Conference of Antimicrobial Agents and Chemotherapy, San Francisco, CA, 26 to 28 September 2006 [62].)
MATERIALS AND METHODS
HIV-1-infected pregnant women were recruited from the Infectious Diseases (ID) Clinic and the Obstetrics and Gynecology (OB/GYN) Clinic at the University of North Carolina at Chapel Hill (UNC) and enrolled in the study from February 2004 to December 2005. Subjects were included if they were ≥18 years of age, pregnant, had documented HIV-1 infection, and were planning to initiate or were currently receiving ARV therapy. All ARVs were selected by the subject's HIV health care provider. Subjects were excluded if they had active opportunistic or serious bacterial complications at the time of enrollment or had past or present obstetrical complications, including multiple gestation, placentia previa, eclampsia, confirmed birth defects, or chromosomal anomalies. The study protocol was approved by the UNC Biomedical Institutional Review Board, and all subjects provided written informed consent prior to any study procedure.
Subjects were eligible for enrollment after 10 weeks of gestation and completed at least monthly evaluations in conjunction with regularly scheduled OB/GYN or ID appointments until 12 weeks postpartum. Study visits occurred every 4 weeks from gestation weeks 10 through 34. After this time, samples were collected weekly until delivery. Postpartum visits occurred at 2, 6, and 12 weeks. At each study visit, subject weight, vital signs, and general health were assessed and paired blood plasma, cervicovaginal fluid, and urine specimens were collected. At delivery, blood plasma, cord blood plasma, and amniotic fluid (when possible) were obtained. Morning doses were withheld until after blood and genital tract sampling were completed. Adherence was assessed by a drug diary card at each visit which documented ARV dose timing for the previous 3 days. Direct questioning was utilized to assess adherence for the week prior to each study visit. Outpatient study visits were conducted at the UNC Verne S. Caviness General Clinical Research Center, ID clinic, or the OB/GYN clinic. Inpatient delivery stays were at the UNC Women's Hospital.
A separate daily drug dosing card was provided for documentation of ARV adherence from week 37 of gestation to the time of delivery. All women delivering at UNC Hospitals received intravenous zidovudine (ZDV) as a 2-mg/kg of body weight bolus over 1 h, administered at least 6 h prior to a scheduled elective cesarean section or at the onset of labor for vaginal delivery. A continuous ZDV 1-mg/kg/h infusion was maintained until the umbilical cord was ligated (15). Samples for ARV concentrations were obtained as follows: maternal blood on presentation to labor and delivery; mixed umbilical venous/arterial cord blood at delivery; maternal blood within 1 hour of cord blood collection. Amniotic fluid was obtained from women who underwent cesarean delivery.
Cervicovaginal fluid samples for ARV concentration were self-collected by direct aspiration of pooled fluid from the posterior fornix of the vagina using a cervicovaginal fluid collection device (Rovumeter; Recipe Pharmaceuticals, Munich, Germany) except for those collected during speculum pelvic examination at the baseline visit and week 35 or 36. Previous studies have demonstrated reproducible pharmacokinetic results with this collection method (18, 34). The cervicovaginal fluid was transferred into a 1-ml preweighed cryovial containing 1 ml 0.01 mM ammonium acetate buffer and stored at −80°C until analysis. Cervicovaginal fluid volumes typically ranged from 0.1 to 0.4 ml per sample.
At study entry, during the second trimester (optional), and between 35 and 36 weeks of gestation, cervical fluid samples for HIV-1 RNA were collected by pelvic exam using five Sno-strips (Akron, Abita Spring, LA) placed through the cervical os into the distal endocervical canal. The strips were removed after absorbed secretions reached past the shoulder (approximately 3 min). At all other time points, five Sno-strips were touched to the cervicovaginal fluid collection device and held in place until fluid was fully wicked. The strips were cut at the shoulder (8 μl of specimen/strip) over a cryovial containing 1 ml NASBA buffer (bioMerieux, Inc., St. Louis, MO) and stored at −80°C until analysis.
All blood samples were collected in vacutainers (BD Diagnostics, Franklin Lakes, NJ) containing 8.55 mg K3EDTA, kept on ice, and centrifuged at 2,600 rpm at 4°C for 15 min. The resulting plasma was aliquoted and stored at −80°C until analysis.
To evaluate whether changes in ARV drug exposures were related to changes in CYP3A activity, morning spot urine collections were obtained. Subjects collected a first morning urine sample (∼30 ml) in a sterile urine container. This sample was refrigerated until transport to the clinic in an insulated carrier. CYP3A activity was estimated by measuring the urinary 6β-hydroxycortisol/cortisol ratio. Urine was mixed, aliquoted, and stored at −80°C until analysis.
Analytical methods.
All analytical work for drug concentrations was performed by the UNC Center for AIDS Research (CFAR) Clinical Pharmacology and Analytical Chemistry Core, which participates in quarterly national and international external ARV proficiency testing and for which >95% accuracy and precision have been reported (17, 20). Drug concentrations in blood plasma, cord blood plasma, and amniotic fluid were determined using validated high-performance liquid chromatography (HPLC)/UV methods (45-47), and HPLC/UV assay sensitivity was 25 ng/ml for nelfinavir (NFV), lopinavir (LPV), and ritonavir (RTV) and 10 ng/ml for the remaining drugs. Drug concentrations in cervicovaginal fluid were quantified using a validated HPLC/tandem mass spectrometry (MS/MS) method (22). The HPLC/MS/MS assay sensitivity was 1 ng/ml for NFV and nevirapine (NVP), 5 ng/ml for tenofovir (TNF), 10 ng/ml for ZDV and LPV, 40 ng/ml for RTV, and 50 ng/ml for lamivudine (3TC). ARV extraction efficiency ranged from 80 to 99%, with excellent intra- and interday precision (2.0 to 14.3%) and accuracy (88 to 113%).
HIV-1 RNA concentrations were measured by the UNC CFAR Virology Core. Blood plasma samples were analyzed with the Roche Amplicor Monitor test, version 1.5 (lower limit of quantitation [LLQ], 50 copies/ml). Genital tract HIV-1 RNA was quantified using the Organon Teknika NucliSens test (LLQ, 400 copies/ml).
An LC/MS/MS system (Sciex-4000; Applied Biosystems) was used to quantify urinary cortisol and 6β-hydroxycortisol concentrations. Briefly, samples were prepared by adding 25 μl of 10 nM 6-methylprednisolone solution (internal standard) and 100 μl acetonitrile to 50 μl urine. Solutions were vortexed and centrifuged, and the supernatants were evaporated to dryness under nitrogen. The residues were reconstituted with HPLC water and injected into a column for chromatographic separation of cortisol, 6β-hydroxycortisol, and 6-methylprednisolone. A gradient elution through a Zorbax SB C18 Aq column (150 by 3.0 mm; 3.5-μm particle size) over 14 min separated the analytes. Detection was performed using electrospray ionization in negative mode. The deprotonated molecules as parent and most suitable daughter ions for MS/MS transition for cortisol, 6β-hydroxycortisol, and the internal standard were m/z 361.17 to 331.04, m/z 377.2 to 347.0, and m/z 373.2 to 343.06, respectively. Standard curves were linear over a range of 1.5 nM to 1,500 nM for cortisol and 15 nM to 1,500 nM for 6β-hydroxycortisol. Intra- and interday precision levels were ≤20%, and accuracy was >80%.
Data analysis and statistical methods.
With the exception of delivery, samples were selected for analysis if they were obtained within 3 h of the end of each drug's dosing interval. For calculation purposes, HIV-1 RNA concentrations below the LLQ were imputed as equal to the LLQ; ARV concentrations below the LLQ were imputed as 1/2 the LLQ. The penetration of a drug into a secondary compartment (genital tract, cord blood plasma, and amniotic fluid) relative to blood plasma is represented as a ratio (genital tract/blood plasma, cord blood plasma/blood plasma, and amniotic fluid/blood plasma) (18, 44).
Linear mixed modeling was used to evaluate changes in individual drug concentrations in blood plasma, genital tract, and genital tract/blood plasma ratios during pregnancy (second and third trimesters) and postpartum, considering compound symmetry covariance structure to account for within-subject variation. Variability in drug concentrations is represented by the coefficient of variation. Drug concentration comparisons by pregnancy periods are presented as geometric mean ratios, with a 98.3% confidence interval with Bonferroni correction for multiple comparisons. Drug concentrations are presented as medians (and 25th and 75th percentiles) unless otherwise noted. Multivariable analyses were performed to evaluate predictors of HIV-1 RNA in blood for patients taking 3TC-ZDV-NFV and 3TC-ZDV-LPV-RTV.
Previously described cohorts were used for comparison (18, 34, 57) and are referred to as nonpregnant historical controls. These were 27 HIV-1-infected, nonpregnant women enrolled in an observational, open-label prospective, pharmacokinetic study that measured exposure to 11 ARVs (not including NFV) in cervicovaginal fluid and blood plasma.
RESULTS
Subject demographics.
The demographics of women (n = 14) enrolled into the study are shown in Table 1. Most were enrolled during their second trimester. One subject withdrew consent after delivery. Four subjects (29%) were taking ARVs before becoming pregnant (median, 91 weeks [range, 22 to 108] prior to study entry), and 10 started their ARV regimen during pregnancy (median, 18 weeks gestation [range, 12 to 36]). Most were on their first ARV regimen, with 13/14 (93%) on PI-based regimens and 1/14 on NVP. Women on LPV-RTV were taking the 133.3/33.3-mg soft-gel capsule formulation and those on NFV were taking the 250-mg tablets. All were prescribed nucleoside reverse transcriptase inhibitors (NRTIs), including 3TC, ZDV, stavudine (d4T), enteric coated didanosine (ddI EC), and tenofovir disoproxil fumarate (TDF). Average 7-day self-reported adherence was 96% antepartum and 86% postpartum.
TABLE 1.
Demographics and antiretroviral regimen components for the 14 study women
| Characteristic | Value |
|---|---|
| At enrollment | |
| Median (IQR)a age (yrs) | 27.5 (22.0, 30.0) |
| Median (IQR) gestational age (wks) | 19.5 (13.5, 27.5) |
| Median (IQR) weight (kg) | 83.8 (68.9, 107.3) |
| Median (IQR) body mass index (kg/m2) | 31.4 (25.4, 41.2) |
| Median (range) HIV-1 RNA (copies/ml) in: | |
| Blood plasma | 1,360 (85, 195,000) |
| Genital tract | <400 (<400, 400,000) |
| Median (range) CD4+ T-cell count | |
| (cells/mm3) | 456 (230, 1,006) |
| Race or ethnicity | |
| No. (%) African-American | 9 (64) |
| No. (%) Caucasian | 3 (21) |
| No. (%) Hispanic | 2 (14) |
| No. (%) with indicated ARV experience | |
| First regimen | 10 (71) |
| Second regimen | 2 (14) |
| Multiple regimens | 2 (14) |
| No. (%) receiving indicated NRTI | |
| 3TC, 150 mg twice daily | 14 (100) |
| ZDV, 300 mg twice daily | 13 (93) |
| TDF, 300 mg once daily | 3 (21) |
| d4T, 40 mg twice daily | 1 (7) |
| ddI EC, 400 mg once daily | 1 (7) |
| No. (%) receiving indicated NNRTI and PI | |
| NVP, 200 mg twice daily | 1 (7) |
| LPV-RTV, 400 and 100 mg twice daily | 7 (50) |
| NFV, 1,250 mg twice daily | 6 (43) |
| Median (range) HIV-1 RNA (copies/ml)b in blood plasma during: | |
| Second trimester (n = 31) | 205 (<50, 11,300) |
| Third trimester (n = 52) | 67 (<50, 7,755) |
| Postpartum (n = 20) | <50 (<50, 13,181) |
| Median (range) HIV-1 RNA (copies/ml)b in genital tract | |
| Second trimester (n = 24) | <400 (<400, 33,000) |
| Third trimester (n = 33) | <400 (<400, 52,000) |
| Postpartum (n = 8) | <400 (<400, 26,000) |
IQR, interquartile range.
Data exclude enrollment values.
HIV RNA concentrations in blood plasma and the genital tract.
HIV-1 RNA concentrations were determined in 103 blood plasma and 65 genital tract samples from 14 women. In the four women starting ARVs prior to pregnancy, 23%, 40%, and 75% of blood plasma samples had HIV-1 RNA levels of ≤50 copies/ml in the second trimester (n = 13), third trimester (n = 15), and postpartum (n = 8), respectively. At these same sampling times, 43%, 20%, and 12% of blood plasma samples had HIV-1 RNA levels of >400 copies/ml, respectively. However, >80% of the samples with detectable HIV-1 RNA were from two women who required a change in their ARV regimen during pregnancy. At 33 weeks, one subject was switched from 3TC-ZDV-NFV to 3TC-ZDV-TDF-LPV-RTV (at standard doses) and had HIV-1 RNA levels of ≤50 copies/ml at 36 weeks. The second subject (who had a history of anemia) was switched from d4T-3TC-LPV-RTV to TDF-3TC-LPV-RTV at 21 weeks. Of 21 genital tract samples available for HIV-1 RNA quantitation, only 1 sample (4.8%) had a detectable HIV-1 RNA level of >400 copies/ml.
In the 10 women starting ARV therapy during pregnancy, 39%, 49%, and 33% of blood plasma samples had HIV-1 RNA levels of ≤50 copies/ml in the second trimester (n = 18), third trimester (n = 37), and postpartum (n = 12), respectively. Seven of the 10 women achieved plasma HIV-1 RNA levels of ≤50 copies/ml by a median of 7 weeks (range, 4 to 13) after initiation of therapy. At these same sampling times, 94%, 92%, and 50% of genital tract samples had HIV-1 RNA levels of ≤400 copies/ml, respectively. Three of the 10 women had detectable genital tract HIV-1 RNA at study entry, and 2 achieved undetectable genital tract HIV-1 RNA (<400 copies/ml) by 4 weeks (the third subject was lost to follow-up). No significant differences in HIV-1 RNA were observed in either blood plasma or genital tract by pregnancy period, and no significant correlations with ARV concentrations were noted.
Antiretroviral concentrations in the genital tract.
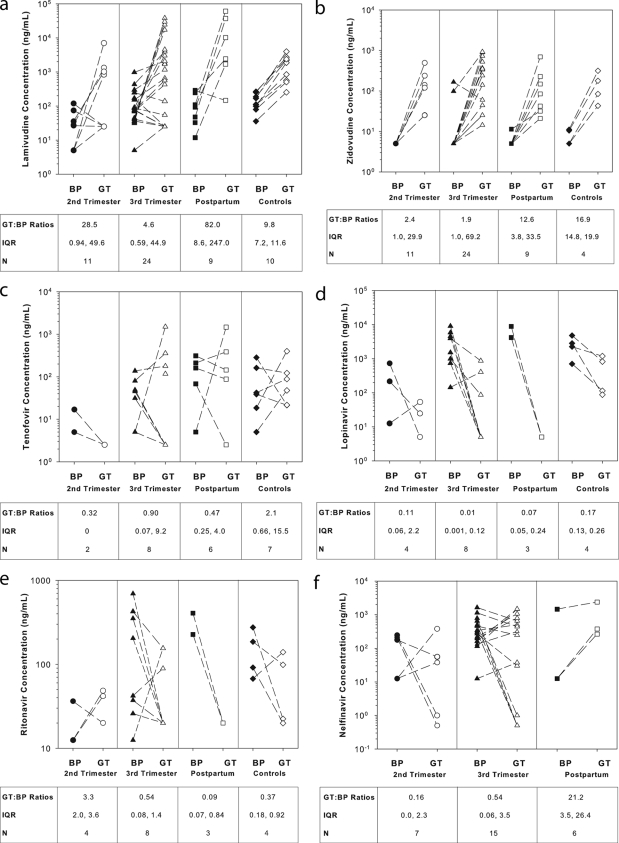
Drug concentrations were measured in 80 blood plasma and 46 genital tract samples. Individual paired blood plasma and genital tract concentrations for each drug are shown in Fig. 1a to f. Similar to nonpregnant historical controls (18, 34, 57), ARV concentrations were more variable in the genital tract than blood plasma: intra- and intersubject variabilities in the genital tract ranged from 47 to 223% and 92 to 317%, respectively, while intra- and intersubject variabilities in blood plasma ranged from 3 to 176% and 14 to 447%, respectively. No differences in variability were seen between antepartum and postpartum samples.
FIG. 1.
Individual blood plasma (BP) and genital tract (GT) drug concentrations by pregnancy period (17, 33, 56). N, number of paired BP and GT samples by period (second trimester, third trimester, postpartum, and nonpregnant historical controls, respectively). For panel f, NFV concentrations in nonpregnant historical controls were not available. GT/BP ratios show the median genital tract drug penetration as a fraction of blood plasma drug concentration. IQR, interquartile range.
Overall, genital tract penetration in pregnant women, relative to drug levels in blood plasma, was highest for the NRTIs compared to the PIs and NVP. No statistically significant differences in genital tract penetration were observed for any of the ARVs between the second and third trimesters. During pregnancy, only NFV genital tract penetration was significantly higher postpartum compared to the second trimester (geometric mean ratio [GMR], 179.3; 98.3% confidence interval [CI], 38.6 to 903.6) and third trimester (GMR, 41.9; 98.3% CI, 3.7 to 470.6), respectively. Compared to nonpregnant historical controls, antepartum genital tract penetration was significantly lower (P < 0.05) for ZDV (GMR, 0.25; 98% CI, 0.08 to 0.75) and LPV (GMR, 0.03; 98% CI, 0.01 to 0.17). Postpartum LPV genital tract penetration continued to be significantly lower than in nonpregnant historical controls (GMR, 0.27; 98.3% CI, 0.08 to 0.91).
Antiretroviral concentrations in cord blood plasma.
Four (29%) subjects delivered at an outside hospital and delivery samples were not obtained. Of the remaining 10 women, 8 delivered by cesarean section and 2 by vaginal delivery (10 cord blood plasma samples were collected). Paired maternal and cord blood plasma samples were obtained a median of 15.5 hours (range, 11.7 to 54.7) after the last dose. Table 2 summarizes drug concentrations in maternal blood plasma, cord blood plasma, and amniotic fluid and corresponding ratios during delivery. NRTI concentrations in cord blood plasma were similar to those in maternal blood plasma except for TNF, for which cord blood plasma drug concentrations were six times higher than maternal blood plasma. For the PIs evaluated, median cord blood plasma drug concentrations were 43 to 51% lower than drug concentrations in maternal blood plasma. In the single subject receiving NVP, the cord blood plasma drug concentration was 41% lower than in maternal blood plasma.
TABLE 2.
Maternal blood plasma, cord blood plasma, and amniotic fluid drug concentrations during delivery
| ARV | No. of samplesa | Median drug concn (ng/ml) (IQR) in:
|
Median ratio (IQR) of drug concentrations
|
|||
|---|---|---|---|---|---|---|
| Maternal blood plasma | Cord blood plasma | Amniotic fluid | Cord blood/blood plasma | Amniotic fluid/blood plasma | ||
| Lamivudine | 10 (2), 10 (2), 6 (0) | 271 (32, 383) | 216 (42, 366) | 931 (422, 1,009) | 1.00 (0.91, 1.18) | 14.2 (10.4, 23.0) |
| Zidovudine | 10 (0), 10 (0), 6 (0) | 557 (326, 915) | 605 (344, 779) | 2,249 (1,413, 2,107) | 0.86 (0.72, 1.05) | 4.2 (3.2, 20.5) |
| Tenofovir | 3 (3), 3 (0), 1 (0) | 5 (5, 5) | 30 (18, 36) | 791 | 6.0 (3.5, 7.2) | 158.2b |
| Lopinavir | 6 (2), 6 (2), 3 (1) | 3,451 (786, 4,480) | 80 (24, 2,381) | 60 (43, 197) | 0.57 (0.05, 1.00) | 0.06 (0.04, 1.0) |
| Ritonavir | 6 (2), 6 (2), 3 (3) | 256 (73, 353) | 20 (13, 38) | 12 (6, 12) | 0.55 (0.09, 1.00) | 0.02 (0.01, 0.04) |
| Nelfinavir | 3 (1), 3 (1), 1 (1) | 65 (39, 543) | 25 (19, 262) | 12 | 0.49 (0.44, 0.74) | 1.00b |
| Nevirapine | 1 (0), 1 (0), 1 (0) | 2,595 | 1,538 | 1,385 | 0.59b | 0.53b |
Values are the number of maternal blood plasma, cord blood plasma, and amniotic fluid samples (and number of samples below limit of quantification), respectively.
Presented as the individual ratio.
Antiretroviral concentrations in amniotic fluid.
Although eight women underwent cesarean section, two subjects had amniotic membrane rupture prior to the procedure. Therefore, six amniotic fluid samples were collected a median of 15.5 hours (range, 13.2 to 53.8) after the last ARV dose. In amniotic fluid, NRTI concentrations were higher than in maternal blood plasma. 3TC and ZDV amniotic fluid concentrations were ∼14-fold and 4-fold higher than in maternal blood plasma, respectively. TNF amniotic fluid concentration (n = 1) was 158 times higher than in maternal blood plasma. NVP amniotic fluid concentration (n = 1) was 47% lower than in maternal blood plasma. Median amniotic fluid concentrations for RTV and LPV were 96% lower than in maternal blood plasma. The NFV concentration in amniotic fluid (n = 1) was similar to that in maternal blood plasma.
6β-Hydroxycortisol and cortisol concentrations in urine.
Urine samples were analyzed for 6β-hydroxycortisol and cortisol concentrations (n = 29, 43, and 16 in the second and third trimesters and postpartum, respectively). Median (range) urinary 6β-hydroxycortisol/cortisol ratios were 3.7 (0.7 to 14.9) in the second trimester, 5.4 (0.6 to 14.1) in the third trimester, and 9.0 (0 to 16.0) postpartum (P < 0.0001). Given that urinary cortisol ratios are usually <5.0 (13, 61), this suggests continued CYP3A induction throughout pregnancy and postpartum, despite the majority of subjects taking antiretrovirals that inhibit CYP3A activity (LPV-RTV and NFV). Across the phases of pregnancy, intrasubject variability in the ratios was 20 to 48% and intersubject variability was 40 to 72%. No correlations were noted between this ratio and antiretroviral drug exposure in any matrix.
DISCUSSION
Previous studies using intensive pharmacokinetic sampling of ARVs in pregnancy have noted significantly decreased third trimester systemic exposure to NRTIs (ZDV and TNF) (8, 59) and PIs (LPV and NFV) (7, 43, 50, 55, 56) compared to postpartum levels. Significant decreases in the area under the concentration-time curve from 0 h to time τ (time between dosing intervals) have been demonstrated for ZDV (59), TNF (8), LPV (50), and NFV (7, 43, 56), while decreased trough concentrations have been noted for TNF (8), LPV (50), and NFV (55, 56). ARV blood plasma concentrations observed in the present study were similar to those described in previous reports (8, 23, 36, 38, 50, 56, 59).
Compared to nonpregnant historical controls, decreased genital tract drug concentrations and genital tract/blood plasma ratios were observed for ZDV and LPV in the antepartum period. These decreases extended to the postpartum period for LPV (18). The disproportionate decrease in genital tract exposure compared to systemic exposure may suggest that pregnancy-related alterations in ARV concentrations occur in compartments other than blood plasma. The mechanism driving these alterations has not been fully elucidated. Drug penetration into peripheral sites is strongly related to protein-binding capacity, among other physicochemical characteristics. However, hormonal fluctuations and other maternal physiological changes associated with pregnancy also influence drug transport protein or metabolizing enzyme activities (14). Whatever the mechanism, significantly decreased ARV concentrations in the pregnant female genital tract require some consideration.
It has been suggested that approximately 80% of HIV-1 vertical transmission occurs between 36 weeks of gestation and delivery in women not receiving antiretroviral therapy (26). HIV genital tract shedding has been seen in patients on ARV. Therefore, even lower exposure in the genital tract during pregnancy may result in increased risk of the mother shedding and transmitting HIV to the baby (1, 21). Fortunately, no infants in this study became infected with HIV despite these decreased ARV concentrations in the genital tract. However, our sample size was small; much larger studies will be needed to detect the clinical impact of these alterations.
Cord blood plasma ARV concentrations in this study were similar to previous reports, except for TNF (5, 6, 8, 10, 11, 30-32, 35, 36, 38, 48, 50, 55). Data suggest that TNF may cross the placenta by passive diffusion (31), although both TDF (the tenofovir prodrug) and TNF are substrates for various efflux transporters expressed in the human placenta which regulate drug transfer (24, 42, 52, 60). In the three subjects evaluated, all had cord blood plasma drug concentrations three- to sevenfold higher than blood plasma drug concentrations. Previous reports have not shown this level of exposure for either single dosing (range, 0.35 to 1.01) or multiple dosing (range, 0.35 to 1.7) (8, 31, 48). Variations in sample timing may play a role in the differential results seen. For the single-dose study, sampling was performed closer to the time of the maximum drug concentration in serum (median sampling time, 5.6 h postdose [range, 2 to 19.5]). Sample timing was not reported for multiple-dose studies. Sampling for the three maternal cord blood plasma pairs in this study occurred later in the dosing interval (closer to the time of the minimum serum drug concentration, 12 to 25 h after dosing).
Limited amniotic fluid samples were available. However, for drugs for which at least three paired samples were collected, amniotic fluid concentrations of 3TC and ZDV were up to 14-fold higher than blood plasma drug concentrations and similar to previously published data (10, 11, 30). Although previous investigations failed to do so (11), we were able to detect LPV concentrations in amniotic fluid by using a more sensitive assay. Median LPV concentrations in amniotic fluid were 4% those in maternal blood plasma. As HIV-1 RNA was not measured in amniotic fluid or cord blood, we cannot comment on the antiviral activities in these compartments. However, the measured concentrations for LPV were above the 50% inhibitory concentration for wild-type HIV-1 (41).
Previous reports have proposed the use of the endogenous urinary 6β-hydroxycortisol/cortisol ratio to demonstrate CYP3A induction during pregnancy (25, 51, 53). In our study, 13 women were taking antiretrovirals which inhibit CYP3A activity, and 1 was taking an antiretroviral which induces CYP3A activity. Despite this, 6β-hydroxycortisol/cortisol ratios from morning spot urine were similar to previous reports in healthy subjects (13, 61) and, unexpectedly, were highest postpartum. The inter- and intraindividual variabilities in our patients were similar to previous reports (12, 25). Both the high intrasubject variability (up to 50%) and the lack of correlation with drug exposure and postpartum changes in CYP3A suggest limited clinical utility for urinary 6β-hydroxycortisol/cortisol ratios as a marker of CYP3A induction in HIV-infected pregnant women.
Finally, reported adherence in our study decreased from approximately 96% antepartum to 86% postpartum. This is consistent with recent data showing that perfect adherence (no self-reported missed doses) rates decreased significantly from 75% antepartum to 65% postpartum (P < 0.01) (4). Since self-reported adherence tends to be higher than more objective measures (e.g., MEMS TrackCaps and pill counts), our postpartum adherence rate of 86% is likely an overestimate (9, 27, 28, 33). Despite adherence levels that may have been adequate for viral suppression (27, 29, 37), overall rates of women who had HIV RNA-1 levels of <50 copies/ml in blood plasma at each time point were relatively low (mean, 40%). This was likely due to the heterogeneity of treatment initiation and baseline HIV RNA concentrations: most women began therapy during the study period. In the women who initiated therapy during pregnancy, 70% achieved plasma HIV-1 RNA levels of ≤50 copies/ml by a median of 7 weeks (range, 4 to 13) after therapy initiation, consistent with findings in clinical trials (37, 58).
The exploratory nature of the study allowed pregnant women to be enrolled regardless of ARV regimen. Due to logistical difficulties in collecting timed samples in this population, only samples collected within 3 h of the end of each drug's dosing interval were included in the analysis. Particularly for drugs with short half-lives, this could have contributed to increased intra- and interindividual variabilities in drug exposures. However, even in nonpregnant, highly adherent, virologically suppressed patients, intraindividual variability in blood plasma ARV concentrations was high (ranging from 24 to 92%) and may have been due to factors beyond timing of sample collection (39).
In summary, measuring ARV penetration into the genital tract, cord blood plasma, and amniotic fluid may be needed to identify signals of subtherapeutic or supratherapeutic drug exposure. Our data are important in assisting the effort to understand the rate and extent of ARV exposure in secondary body compartments so that ARV regimens in pregnancy can be optimized for maximum efficacy and minimal toxicity.
Acknowledgments
This work was supported by the Society of Infectious Diseases Pharmacists Pfizer Fellowship Award (R.F.Y. and A.D.M.K.) and grants AI54980 (A.D.M.K.), AIO77355 (K.B.P.), Building Interdisciplinary Research Careers in Women's Health (BIRCWH) K12 HD 01441-01 (K.B.P.), the UNC Center for AIDS Research (AI50410), and the UNC General Clinical Research Center (RR00046).
We gratefully acknowledge Arlene Bridges for her work on the cortisol assay, the women volunteers and the nurses and staff of the UNC Infectious Diseases Clinic, UNC Obstetrics and Gynecology Clinic, the Verne S. Caviness General Clinical Research Center, and the UNC CFAR Clinical Pharmacology and Analytical Chemistry Core and Biostatistics Core.
R.F.Y. receives ongoing research support from Abbott Laboratories. A.D.M.K. and K.B.P. have received research support from Tibotec, Abbott Laboratories, Pfizer, Gilead Sciences, Boehringer Ingelheim, Merck, and Bristol-Myers Squibb. H.L.T., Y.C., and M.V. received research fellowships supported by GlaxoSmithKline. M.V. is currently employed by Pfizer. The remaining authors do not have conflicts of interest related to this research or manuscript.
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Anderson, B. L., and S. Cu-Uvin. 2008. Determinants of HIV shedding in the lower genital tract of women. Curr. Infect. Dis. Rep. 10:505-511. [DOI] [PubMed] [Google Scholar]
- 2.Angel, J. B., Y. Khaliq, M. L. Monpetit, D. W. Cameron, and K. Gallicano. 2001. An argument for routine therapeutic drug monitoring of HIV-1 protease inhibitors during pregnancy. AIDS 15:417-419. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. 2008. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. U.S. Department of Health and Human Services, Washington, DC. http://aidsinfo.nih.gov/contentfiles/PerinatalGL.pdf.
- 4.Bardeguez, A. D., J. C. Lindsey, M. Shannon, R. E. Tuomala, S. E. Cohn, E. Smith, A. Stek, S. Buschur, A. Cotter, L. Bettica, and J. S. Read. 2008. Adherence to antiretrovirals among US women during and after pregnancy. J. Acquired Immune Defic. Syndr. 48:408-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhadrakom, C., R. J. Simonds, J. V. Mei, S. Asavapiriyanont, V. Sangtaweesin, N. Vanprapar, K. H. Moore, N. L. Young, W. H. Hannon, T. D. Mastro, N. Shaffer, et al. 2000. Oral zidovudine during labor to prevent perinatal HIV transmission, Bangkok: tolerance and zidovudine concentration in cord blood. AIDS. 14:509-516. [DOI] [PubMed] [Google Scholar]
- 6.Bonora, S., D. Gonzalez de Requena, E. Chiesa, A. Maccabruni, M. Forleo, F. Vichi, S. Fiore, T. Bini, E. Ferrazzi, A. d'Arminio Monforte, and TARGET Study Group. 2007. Transplacental passage of tenofovir and other antiretrovirals at delivery, abstr. 738a. 14th Conf. Retrovir. Opportunistic Infect., Los Angeles, CA, 25 to 28 February 2007.
- 7.Bryson, Y., A. Stek, M. Mirochnick, L. Mofenson, J. Connor, H. Watts, S. Huang, M. Hughes, B. Cunningham, L. Purdue, Y. Asfaw, E. Smith, and for the PACTG 353 Team. 2000. Pharmacokinetics, antiviral activity, and safety of nelfinavir with ZDV/3TC in pregnant HIV-infected women and their infants: PACTG 353 cohort 2, abstr. 715. 7th Conf. Retrovir. Opportunistic Infect., San Francisco, CA, 30 January to 4 February 2000.
- 8.Burchett, S. K., B. Best, M. Mirochnick, C. Hu, E. Capparelli, D. Holland, E. Smith, B. Sheeran, J. S. Read, A. Stek, and PACTG 1026s Study Team. 2007. Tenofovir pharmacokinetics during pregnancy, at delivery, and postpartum, abstr. 738b. 14th Conf. Retrovir. Opportunistic Infect., Los Angeles, CA, 25 to 28 February 2007.
- 9.Burney, K. D., K. Krishnan, M. T. Ruffin, D. Zhang, and D. E. Brenner. 1996. Adherence to single daily dose of aspirin in a chemoprevention trial. An evaluation of self-report and microelectronic monitoring. Arch. Fam. Med. 5:297-300. [DOI] [PubMed] [Google Scholar]
- 10.Chappuy, H., J. M. Treluyer, V. Jullien, J. Dimet, E. Rey, M. Fouche, G. Firtion, G. Pons, and L. Mandelbrot. 2004. Maternal-fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob. Agents Chemother. 48:4332-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappuy, H., J. M. Treluyer, E. Rey, J. Dimet, M. Fouche, G. Firtion, G. Pons, and L. Mandelbrot. 2004. Maternal-fetal transfer and amniotic fluid accumulation of protease inhibitors in pregnant women who are infected with human immunodeficiency virus. Am. J. Obstet. Gynecol. 191:558-562. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Y. C., S. K. Gotzkowsky, A. N. Nafziger, R. W. Kulawy, M. L. Rocci, Jr., J. S. Bertino, Jr., and A. D. Kashuba. 2006. Poor correlation between 6β-hydroxycortisol:cortisol molar ratios and midazolam clearance as measure of hepatic CYP3A activity. Br. J. Clin. Pharmacol. 62:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, Z., L. A. Dunning, K. E. Anderson, J. L. Holtzman, and W. Zheng. 2004. Within-person variability of urinary 6β-hydroxycortisol to urinaryl ratios in Caucasian women. Steroids 69:67-70. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, M. S., C. Gay, A. D. Kashuba, S. Blower, and L. Paxton. 2007. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann. Intern. Med. 146:591-601. [DOI] [PubMed] [Google Scholar]
- 15.Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O'Sullivan, R. VanDyke, M. Bey, W. Shearer, R. L. Jacobson, et al. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N. Engl. J. Med. 331:1173-1180. [DOI] [PubMed] [Google Scholar]
- 16.Cooper, E. R., M. Charurat, L. Mofenson, I. C. Hanson, J. Pitt, C. Diaz, K. Hayani, E. Handelsman, V. Smeriglio, R. Hoff, and W. Blattner. 2002. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J. Acquired Immune Defic. Syndr. 29:484-494. [DOI] [PubMed] [Google Scholar]
- 17.Droste, J. A., R. E. Aarnoutse, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2003. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J. Acquired Immune Defic. Syndr. 32:287-291. [DOI] [PubMed] [Google Scholar]
- 18.Dumond, J. B., R. F. Yeh, K. B. Patterson, A. H. Corbett, B. H. Jung, N. L. Rezk, A. S. Bridges, P. W. Stewart, M. S. Cohen, and A. D. Kashuba. 2007. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 21:1899-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodge, L. S., and T. S. Tracy. 2007. Alterations in drug disposition during pregnancy: implications for drug therapy. Expert Opin. Drug Metab. Toxicol. 3:557-571. [DOI] [PubMed] [Google Scholar]
- 20.Holland, D. T., R. DiFrancesco, J. D. Connor, and G. D. Morse. 2006. Quality assurance program for pharmacokinetic assay of antiretrovirals. ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004: a requirement for therapeutic drug monitoring. Ther. Drug Monit. 28:367-374. [DOI] [PubMed] [Google Scholar]
- 21.Iversen, A. K., J. Attermann, J. Gerstoft, L. Fugger, J. I. Mullins, and P. Skinhoj. 2004. Longitudinal and cross-sectional studies of HIV-1 RNA and DNA loads in blood and the female genital tract. Eur. J. Obstet. Gynecol. Reprod. Biol. 117:227-235. [DOI] [PubMed] [Google Scholar]
- 22.Jung, B. H., N. L. Rezk, A. S. Bridges, A. H. Corbett, and A. D. Kashuba. 2007. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 21:1095-1104. [DOI] [PubMed] [Google Scholar]
- 23.Khuong-Josses, M., A. Boussairi, M. Herida, S. Abbas, and D. Mechali. 2006. Nelfinavir plasma concentrations in 40 pregnant women, abstr. 707. 13th Confer. Retrovir. Opportunistic Infect., Denver, CO, 5 to 8 February 2006.
- 24.Kiser, J. J., M. L. Carten, C. L. Aquilante, P. L. Anderson, P. Wolfe, T. M. King, T. Delahunty, L. R. Bushman, and C. V. Fletcher. 2008. The effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin. Pharmacol. Ther. 83:265-272. [DOI] [PubMed] [Google Scholar]
- 25.Kosel, B. W., K. P. Beckerman, S. Hayashi, M. Homma, and F. T. Aweeka. 2003. Pharmacokinetics of nelfinavir and indinavir in HIV-1-infected pregnant women. AIDS 17:1195-1199. [DOI] [PubMed] [Google Scholar]
- 26.Kourtis, A. P., F. K. Lee, E. J. Abrams, D. J. Jamieson, and M. Bulterys. 2006. Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect. Dis. 6:726-732. [DOI] [PubMed] [Google Scholar]
- 27.Liu, H., C. E. Golin, L. G. Miller, R. D. Hays, C. K. Beck, S. Sanandaji, J. Christian, T. Maldonado, D. Duran, A. H. Kaplan, and N. S. Wenger. 2001. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann. Intern. Med. 134:968-977. [DOI] [PubMed] [Google Scholar]
- 28.Liu, H., L. G. Miller, R. D. Hays, G. Wagner, C. E. Golin, W. Hu, K. Kahn, R. Haubrich, A. H. Kaplan, and N. S. Wenger. 2006. A practical method to calibrate self-reported adherence to antiretroviral therapy. J. Acquired Immune Defic. Syndr. 43(Suppl. 1):S104-S112. [DOI] [PubMed] [Google Scholar]
- 29.Maggiolo, F., L. Ravasio, D. Ripamonti, G. Gregis, G. Quinzan, C. Arici, M. Airoldi, and F. Suter. 2005. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin. Infect. Dis. 40:158-163. [DOI] [PubMed] [Google Scholar]
- 30.Mandelbrot, L., G. Peytavin, G. Firtion, and R. Farinotti. 2001. Maternal-fetal transfer and amniotic fluid accumulation of lamivudine in human immunodeficiency virus-infected pregnant women. Am. J. Obstet. Gynecol. 184:153-158. [DOI] [PubMed] [Google Scholar]
- 31.Martelli, S., K. Long, S. Berdot, P. Faucher, F. Damond, A. Bourgeois-Moine, A. Devidas, J. L. Delassus, S. Matheron, and G. Peytavin. 2007. Placental transfer of tenofovir in HIV-infected women treated with, T. D. F., and protease inhibitors containing regimen, poster P14.7/01. 11th Eur. AIDS Conf., Madrid, Spain, 24 to 27 October 2007.
- 32.Marzolini, C., C. Rudin, L. A. Decosterd, A. Telenti, A. Schreyer, J. Biollaz, and T. Buclin. 2002. Transplacental passage of protease inhibitors at delivery. AIDS 16:889-893. [DOI] [PubMed] [Google Scholar]
- 33.Mason, B. J., J. R. Matsuyama, and S. G. Jue. 1995. Assessment of sulfonylurea adherence and metabolic control. Diabetes Educ. 21:52-57. [DOI] [PubMed] [Google Scholar]
- 34.Min, S. S., A. H. Corbett, N. Rezk, S. Cu-Uvin, S. A. Fiscus, L. Petch, M. S. Cohen, and A. D. Kashuba. 2004. Protease inhibitor and nonnucleoside reverse transcriptase inhibitor concentrations in the genital tract of HIV-1-infected women. J. Acquired Immune Defic. Syndr. 37:1577-1580. [DOI] [PubMed] [Google Scholar]
- 35.Mirochnick, M., A. Dorenbaum, D. Holland, B. Cunningham-Schrader, C. Cunningham, R. Gelber, L. Mofenson, M. Culnane, J. Connor, and J. L. Sullivan. 2002. Concentrations of protease inhibitors in cord blood after in utero exposure. Pediatr. Infect. Dis. J. 21:835-838. [DOI] [PubMed] [Google Scholar]
- 36.Mirochnick, M., A. Stek, E. Capparelli, B. M. Best, D. Holland, J. Connor, S. K. Burchett, C. Hu, E. Smith, J. S. Read, and PACTG 1026s Study Team. 2006. Adequate lopinavir (LPV) exposure achieved with a higher dose during the 3rd trimester of pregnancy, poster 710. 13th Conf. Retrovir. Opportunistic Infect., Denver, CO.
- 37.Molina, J. M., T. J. Podsadecki, M. A. Johnson, A. Wilkin, P. Domingo, R. Myers, J. M. Hairrell, R. A. Rode, M. S. King, and G. J. Hanna. 2007. A lopinavir/ritonavir-based once-daily regimen results in better compliance and is non-inferior to a twice-daily regimen through 96 weeks. AIDS Res. Hum. Retrovir. 23:1505-1514. [DOI] [PubMed] [Google Scholar]
- 38.Moodley, J., D. Moodley, K. Pillay, H. Coovadia, J. Saba, R. van Leeuwen, C. Goodwin, P. R. Harrigan, K. H. Moore, C. Stone, R. Plumb, and M. A. Johnson. 1998. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J. Infect. Dis. 178:1327-1333. [DOI] [PubMed] [Google Scholar]
- 39.Nettles, R. E., T. L. Kieffer, T. Parsons, J. Johnson, J. Cofrancesco, Jr., J. E. Gallant, K. A. Carson, R. F. Siliciano, and C. Flexner. 2006. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin. Infect. Dis. 42:1189-1196. [DOI] [PubMed] [Google Scholar]
- 40.Ohkita, C., and M. Goto. 1990. Increased 6-hydroxycortisol excretion in pregnant women: implication of drug-metabolizing enzyme induction. DICP 24:814-816. [DOI] [PubMed] [Google Scholar]
- 41.Parkin, N. T., N. S. Hellmann, J. M. Whitcomb, L. Kiss, C. Chappey, and C. J. Petropoulos. 2004. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray, A. S., T. Cihlar, K. L. Robinson, L. Tong, J. E. Vela, M. D. Fuller, L. M. Wieman, E. J. Eisenberg, and G. R. Rhodes. 2006. Mechanism of active renal tubular efflux of tenofovir. Antimicrob. Agents Chemother. 50:3297-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Read, J. S., B. Best, A. Stek, C. Hu, E. Capparelli, D. Holland, S. K. Burchett, E. Smith, B. Sheeran, and M. Mirochnick. 2007. Nelfinavir pharmacokinetics (625-mg tablets) during the third trimester of pregnancy and post-partum, abstr. 740. 14th Conf. Retrovir. Opportunistic Infect., Los Angeles, CA, 25 to 28 February 2007.
- 44.Reddy, Y. S., S. K. Gotzkowsky, J. J. Eron, J. Y. Kim, W. D. Fiske, S. A. Fiscus, L. Petch, M. S. Cohen, and A. D. Kashuba. 2002. Pharmacokinetic and pharmacodynamic investigation of efavirenz in the semen and blood of human immunodeficiency virus type 1-infected men. J. Infect. Dis. 186:1339-1343. [DOI] [PubMed] [Google Scholar]
- 45.Rezk, N. L., R. D. Crutchley, and A. D. Kashuba. 2005. Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. J. Chromatogr. B 822:201-208. [DOI] [PubMed] [Google Scholar]
- 46.Rezk, N. L., R. D. Crutchley, R. F. Yeh, and A. D. Kashuba. 2006. Full validation of an analytical method for the HIV-protease inhibitor atazanavir in combination with 8 other antiretroviral agents and its applicability to therapeutic drug monitoring. Ther. Drug Monit. 28:517-525. [DOI] [PubMed] [Google Scholar]
- 47.Rezk, N. L., R. R. Tidwell, and A. D. Kashuba. 2003. Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet absorbance detection. J. Chromatogr. B 791:137-147. [DOI] [PubMed] [Google Scholar]
- 48.Rodman, J., P. Flynn, D. Shapiro, A. Bardeguez, S. Huang, S. Fiscus, K. VanRompey, J. F. Rooney, L. Mofenson, H. Watts, P. Jean-Phillipe, and PACTG 394 Protocol Team. 2006. Pharmacokinetics (PK) and safety of tenofovir disoproxil fumarate (TDF) in HIV-1 infected pregnant women and their infants, abstr. 708. 13th Conf. Retrovir. Opportunisitic Infect., Denver, CO, 5 to 8 February 2006.
- 49.Si-Mohamed, A., M. D. Kazatchkine, I. Heard, C. Goujon, T. Prazuck, G. Aymard, G. Cessot, Y. H. Kuo, M. C. Bernard, B. Diquet, J. E. Malkin, L. Gutmann, and L. Belec. 2000. Selection of drug-resistant variants in the female genital tract of human immunodeficiency virus type 1-infected women receiving antiretroviral therapy. J. Infect. Dis. 182:112-122. [DOI] [PubMed] [Google Scholar]
- 50.Stek, A. M., M. Mirochnick, E. Capparelli, B. M. Best, C. Hu, S. K. Burchett, C. Elgie, D. T. Holland, E. Smith, R. Tuomala, A. Cotter, and J. S. Read. 2006. Reduced lopinavir exposure during pregnancy. AIDS 20:1931-1939. [DOI] [PubMed] [Google Scholar]
- 51.Streetman, D. S., J. S. Bertino, Jr., and A. N. Nafziger. 2000. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics 10:187-216. [DOI] [PubMed] [Google Scholar]
- 52.Syme, M. R., J. W. Paxton, and J. A. Keelan. 2004. Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 43:487-514. [DOI] [PubMed] [Google Scholar]
- 53.Tracy, T. S., R. Venkataramanan, D. D. Glover, and S. N. Caritis. 2005. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am. J. Obstet Gynecol. 192:633-639. [DOI] [PubMed] [Google Scholar]
- 54.Tuomala, R. E., P. T. O'Driscoll, J. W. Bremer, C. Jennings, C. Xu, J. S. Read, E. Matzen, A. Landay, C. Zorrilla, W. Blattner, M. Charurat, and D. J. Anderson. 2003. Cell-associated genital tract virus and vertical transmission of human immunodeficiency virus type 1 in antiretroviral-experienced women. J. Infect. Dis. 187:375-384. [DOI] [PubMed] [Google Scholar]
- 55.van Heeswijk, R. P., Y. Khaliq, K. D. Gallicano, M. Bourbeau, I. Seguin, E. J. Phillips, and D. W. Cameron. 2004. The pharmacokinetics of nelfinavir and M8 during pregnancy and post partum. Clin. Pharmacol. Ther. 76:588-597. [DOI] [PubMed] [Google Scholar]
- 56.Villani, P., M. Floridia, M. F. Pirillo, M. Cusato, E. Tamburrini, A. F. Cavaliere, G. Guaraldi, C. Vanzini, A. Molinari, A. Degli Antoni, and M. Regazzi. 2006. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br. J. Clin. Pharmacol. 62:309-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vourvahis, M., H. Tappouni, K. Patterson, Y. Chen, N. L. Rezk, S. Fiscus, B. P. Kearney, J. F. Rooney, M. Cohen, and A. D. M. Kashuba. 2006. A pharmacologic basis for the use of tenofovir In pre- and post-exposure prophylaxis: Intra and extracellular genital tract pharmacokinetics and pharmacodynamics from first dose to steady state in HIV-1 infected men and women, poster 569. 13th Conf. Retrovir. Opportunistic Infect., Denver, CO, 5 to 8 February 2006.
- 58.Walmsley, S., B. Bernstein, M. King, J. Arribas, G. Beall, P. Ruane, M. Johnson, D. Johnson, R. Lalonde, A. Japour, S. Brun, and E. Sun. 2002. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N. Engl. J. Med. 346:2039-2046. [DOI] [PubMed] [Google Scholar]
- 59.Watts, D. H., Z. A. Brown, T. Tartaglione, S. K. Burchett, K. Opheim, R. Coombs, and L. Corey. 1991. Pharmacokinetic disposition of zidovudine during pregnancy. J. Infect. Dis. 163:226-232. [DOI] [PubMed] [Google Scholar]
- 60.Weiss, J., D. Theile, N. Ketabi-Kiyanvash, H. Lindenmaier, and W. E. Haefeli. 2007. Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and nonnucleoside reverse transcriptase inhibitors. Drug Metab. Dispos. 35:340-344. [DOI] [PubMed] [Google Scholar]
- 61.Yasui-Furukori, N., T. Kondo, T. Kubota, H. Otake, T. Ohkubo, T. Nagasaki, K. Sugawara, K. Chiba, K. Otani, and S. Kaneko. 2001. No correlations between the urinary ratio of 6β-hydroxycortisol to free cortisol and pharmacokinetics of alprazolam. Eur. J. Clin. Pharmacol. 57:285-288. [DOI] [PubMed] [Google Scholar]
- 62.Yeh, R. F., K. B. Patterson, J. B. Dumond, H. L. Tappouni, Y. Chen, M. Vourvahis, K. A. Boggess, A. L. Horton, S. A. Fiscus, and A. D. M. Kashuba. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-378.