Abstract
Hypoxia is encountered frequently by Candida albicans during systemic infection of the human host. We tested if hypoxia allows biofilm formation by C. albicans, which is a major cause of perseverance and antifungal resistance in C. albicans infections. Using an in vitro biofilm system, we unexpectedly discovered that several positive regulators of biofilm formation during normoxia, including Tec1, Ace2, Czf1, Och1, and Als3, had little or no influence on biofilm development during hypoxia, irrespective of the carbon dioxide level, indicating that C. albicans biofilm pathways differ depending on the oxygen level. In contrast, the Efg1 and Flo8 regulators were required for both normoxic and hypoxic biofilm formation. To explore the role of Efg1 during hypoxic and/or biofilm growth, we determined transcriptome kinetics following release of EFG1 expression by a system under transcriptional control of a doxycycline-inducible promoter. During hypoxia, Efg1 rapidly induced expression of all major classes of genes known to be associated with normoxic biofilm formation, including genes involved in glycolysis, sulfur metabolism, and antioxidative and peroxisome activities, as well as genes for iron uptake. The results suggest that hypoxic adaptation mediated by the Efg1 and Flo8 regulators is required even during normoxic biofilm development, while hypoxic biofilm formation in deep tissues or in organs may generate foci of C. albicans infections.
Candida albicans is an important human fungal pathogen that is able to cause superficial and also severe systemic infections. In recent years it has been recognized that the ability of C. albicans to form biofilms is a major factor that contributes to its virulence (for reviews, see references 12, 28, and 37). Biofilms consist of an inner layer of yeast form cells, mostly hyphal filaments in outer layers, and a surrounding extracellular matrix, and they develop in patients particularly on medical implants and devices (19), where they persist tenaciously because of their adherence and inherent antifungal resistance (for reviews, see references 8 and 9). Biofilm formation is studied mostly with in vitro systems using plastics, including polystyrene, silicone, polyvinyl chloride, or polymethylmethylacrylate, as adhesive and supporting surfaces, and the developing biofilm is incubated statically or using a flowthrough method (15) with various media supporting fungal growth and morphogenesis. In addition, in vivo animal models of biofilm formation using implants have been used (1).
About 25 proteins of C. albicans have been shown to significantly influence biofilm development, but there has been no genome-wide assessment of the genetic requirements during biofilm formation. The known components comprise two main classes of proteins, surface proteins and transcription factors. The surface proteins include covalently linked cell wall proteins specific for the hyphal growth form, such as the Als1, Als3, and Hwp1 adhesins (25, 30, 47), as well as hydrophobin-like CFEM proteins in the cytoplasmic membrane (34). The transcription factors Efg1, Tec1, and Ace2 are required for hypha formation (15, 18, 29, 36), while Bcr1 appears to specifically induce adhesin gene transcription within hyphae (30). Protein O mannosylation by the Pmt1 and Pmt4 isoforms is also important for biofilm formation, presumably because it alters the function of C. albicans surface proteins (33). Finally, the Mkc1 mitogen-activated protein (MAP) kinase pathway appears to signal and to mediate surface contact information for downstream transcriptional regulators involved in biofilm formation (20).
Several transcriptome analyses have established transcriptome patterns in developing and mature biofilms of C. albicans (15, 24, 46). Glycolytic genes were found to be induced during an early phase of biofilm formation (40, 46), which was confirmed by proteomic analyses (22, 45), and during biofilm formation by Candida parapsilosis (38). In agreement, some biofilm-defective mutants, including efg1, ace2, and pmt mutants, were reported to exhibit decreased glycolytic gene expression (6, 23, 42). The second prominent class of biofilm-induced genes encodes proteins for sulfate assimilation and the biosynthesis of cysteine and methionine (15, 24). Furthermore, proteomic analyses revealed upregulation of genes known to respond to oxidative stress (41) and genes involved in ergosterol or fatty acid biosynthesis (38, 40). The underlying functional purposes of the upregulation of these classes of genes during biofilm development have not been clarified yet.
While in vitro experimental systems have revealed many relevant aspects of C. albicans biofilm formation, they do not reflect other important parameters likely to influence biofilm development within the human host. The oxygen partial pressure of air-equilibrated media is high in vitro, but hypoxic environments may prevail in human tissues distant from arterial blood flow (for a review, see reference 13). Thus, in such niches in the body implants and other surfaces provide different conditions for biofilm formation. Furthermore, a decrease in the oxygen level in the human host is often associated with elevated carbon dioxide levels in body fluids, and the level of a transcript for a critical enzyme involved in CO2 utilization, carbonic anhydrase (Nce103), is increased in biofilms (24). Besides numerous effects of hypoxia on human host cells, C. albicans has been shown to alter its transcriptome and morphological phenotypes during hypoxia compared to its phenotypes during normoxia (42). While it is difficult to recreate the complex and variable molecular environment within the human host, in this study we focused on the ways in which oxygen and CO2 levels affect biofilm formation by C. albicans. The results demonstrate that hypoxia and CO2 levels allow significant in vitro biofilm formation, but surprisingly, under these conditions different C. albicans components were required compared to the components required under normoxic conditions. Furthermore, we found that the Efg1 regulator is a key element in induction of biofilm-specific classes of genes under hypoxic conditions, suggesting that Efg1-dependent biofilm formation is inherently associated with hypoxic adaptation of C. albicans.
MATERIALS AND METHODS
Strains and growth conditions.
The C. albicans strains used are listed in Table 1. Strains were grown as described previously (42) in liquid or on solid YPD (yeast extract peptone glucose medium) or in Roswell Park Memorial Institute (RPMI) 1640 medium containing 2% glucose buffered to pH 7 with 0.165 M MOPS (morpholinepropanesulfonic acid). Hypoxic growth with different concentrations of CO2 was carried out using an hypoxic workstation (INVIVO2 200; Ruskinn).
TABLE 1.
C. albicans strains
| Strain | Genotype | Reference |
|---|---|---|
| CAF2-1 | URA3/ura3::imm434 | 14 |
| CAI4 | ura3::imm434/ura3::imm434 | 14 |
| HLC67 | Like CAI4 but efg1::hisG/efg1::hisG | 31 |
| DSC10 | Like CAI4 but efg1::hisG/efg1::hisG ura3::imm434/ura3::imm434::URA3 | 31 |
| CM1613 | Like CAI4 but mkc1Δ::hisG-URA3-hisG/mkc1Δ::hisG | 27 |
| CSSK21 | Like CAI4 but ssk1::hisG/ssk1::hisG-URA3-hisG | 5 |
| MK 106 | Like SC5314 but ace2::FRT/ace2::FRT | 18 |
| C4/d63 | Like CAI4 but efh1::hisG/efh1::hisG-URA3-hisG | 11 |
| CCF3 | Like CAI4 but ura3::imm434/ura3::imm434 flo8::hisG/flo8::hisG-URA3-hisG | 7 |
| CaAS18 | Like CAI4 but tec1/tec1 (pVEC) | 39 |
| CKY230 | Like CAI4 but Δczf1::hisG/Δczf1::hisG ade2::pDB152 | 4 |
| CAYF178U | als3::ARG4/als3::HIS1 arg4::hisG/arg4::hisG his1::hisG/his1::hisG ura3 ura3::imm434/ura3::imm434::URA3 | 30 |
| NGY357 | Like CAI4 but och1Δ::hisG/och1Δ::hisG RPS1/rps1Δ::CIp10 | 2 |
| SPCa2 | Like CAI4 but pmt1Δ::hisG/pmt1Δ::hisG Δura3::imm434/URA3 | 33 |
| SPCa4 | Like CAI4 but pmt2Δ::hisG/PMT2 ura3::imm434/URA3 | 33 |
| SPCa6 | Like CAI4 but pmt4Δ::hisG/pmt4Δ::hisG ura3::imm434/URA3 | 33 |
Biofilm formation in polystyrene wells.
Strains were pregrown for 24 h at 37°C in YPD containing 2% glucose. Five milliliters of cells was pelleted by centrifugation and resuspended in 5 ml of phosphate-buffered saline (PBS). To separate cellular aggregates, the suspension was placed in a bath sonifier for 10 min; cells were harvested again by centrifugation and resuspended in 1 ml of PBS. Numbers of cells were estimated by measuring the optical density at 600 nm (OD600) using a standard curve, and cells were resuspended in RPMI 1640 medium at a concentration of 106 cells per ml. Each strain was inoculated into a separate 24-well culture dish. Culture dishes were weighed, and then 700 μl of a cell suspension was transferred into each well of each culture dish. The dishes were incubated for 60 h at 37°C. Following incubation the medium in the culture dishes was discarded, and each well was washed with 700 μl of PBS. The residual liquid was removed with a pipette, and the dishes were dried for 24 h at 37°C before determination of dry weights. Experiments were repeated three times with each strain. The statistical significance of differences between groups of data was analyzed using an unpaired t test and calculating two-tailed P values (GraphPad Prism 4 program).
Biofilms were visualized by scanning electron microscopy using a LEO 1430VP scanning electron microscope.
Adhesion of C. albicans strains to polystyrene.
A total of 0.5 × 106 cells in a 100-μl suspension were inoculated into wells of a 96-well polystyrene plate as described above for biofilm formation. Following incubation at 37°C for 2 h, nonadherent cells were removed, and adherent cells were washed with 100 μl PBS. One hundred microliters of a 1:5 mixture of 1.5 mM 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) and 0.4 mM menadione was added, and the plate was incubated in the dark for 2 h. The supernatant was removed, and its OD490 was determined (35). Pregrowth and adhesion were allowed to proceed either under normoxia or under an atmosphere containing 0.2% oxygen and 6% CO2 in an INVIVO2 200 hypoxic incubator.
Construction of doxycycline-inducible EFG1 gene and transcriptome kinetics.
To place EFG1 under transcriptional control of a doxycycline-inducible promoter (“tet-on”), we first amplified the HA-EFG1 gene from plasmid pBI-HAHYD (43) by PCR, using primers SalI-HA-EFG1 for (5′-TATATAGTCGACATGAGTCGATACCCATACGACG) and SalI-HA-EFG1 rev (5′-ATATATGTCGACTTACTTTTCTTCTTTGGCAACAGTGC) that add SalI sites to each end (underlined). The resulting SalI fragment was inserted into the single SalI site of pNIM1 (32) downstream of the promoter containing seven copies of the tet operator sequence, tetO. The 8.3-kb SacII-ApaI fragment of the plasmid (pCS1) containing the three expression units for SAT1, the reverse tetracycline-dependent transactivator, and HA-EFG was used to transform efg1 mutant HLC67, selecting for noursethricin resistance. Integration of the fragment into the ADH1 locus in transformants was verified by colony PCR using primer pTET (binding within the tetO-OP4 promoter) and primer ADH1-CA (binding outside the ADH1 region), and one transformant, designated CCS1, was selected. Similarly, the fragment of parental plasmid pNIM1 was transformed into HLC67, and correct integration was verified (control strain CCS2).
Strains CCS1 and CCS2 were grown overnight in YPD, and the cells were washed in YP (the same as YPD but without added glucose) and used to inoculate several shake flasks for each strain, which contained 100 ml of YP containing 10% horse serum, 0.01% glucose, and 0.002% uridine, to obtain an OD600 of 0.1. The flasks were prewarmed to 37°C for 10 min (either in air or under a stream of a mixture of 93.8% nitrogen, 6% CO2, and 0.2% oxygen), and the contents of one set of flasks were harvested by pouring the medium into ice-chilled centrifuge tubes and then centrifuging and washing the cells (at 4°C) (zero time). To the other flasks, 40 μg/ml doxycycline was added after the prewarming period, and the cells were incubated further aerobically or hypoxically and harvested as described above.
RNA was isolated from all cultures and transcriptome analyses were performed essentially as described previously (11). For each induction time and at zero time, labeled cDNAs generated from RNAs of strain CCS1 and strain CCS2 (using fluorescent dyes Cy3 and Cy5, respectively) were cohybridized to C. albicans genomic arrays (Eurogentec, Belgium). The arrays were read and evaluated by using the GeneSpring software as described previously. Genes were considered to be significantly regulated (i) if the expression ratios for strains CCS1 and CCS2 were ≥2, (ii) if the expression ratio for zero time and any induction time was ≥2, and (iii) if a consistent increase or decrease in expression during EFG1 induction was observed. A complete list of genes on the microarrays and their regulation at the different time points is available under Array Express accession numbers E-MEXP-2117 and E-MEXP-2119.
Selected transcript ratios determined in microarray experiments were verified by quantitative real-time PCR using methods described previously (6). Primer pair ECE-L1/ECE-R1 (5′-TTCAAAGACTCCCACAACTCA/5′-ACCGACAGTTTCAATGCTCTT) was used to amplify the ECE1 transcript, primer pair HWP1-L1/HWP1-R1 (5′-TCAGCCTGATGACAATCCTC/5′-GTAGCTGGAGTTGTTGGCTTT) was used to amplify the HWP1 transcript, and primer pair ACT1(RT)-f/ACT1(RT)-r (5′-TTGGATTCTGGTGATGGTGT/5′-TGGACAAATGGTTGGTCAAG) was used to amplify the ACT1 reference transcript. The relative transcript levels were determined for strains CCS1 and CCS2 at each time point during doxycycline induction, which was followed by calculation of the CCS1/CCS2 ratios.
RESULTS
C. albicans biofilm formation under hypoxia.
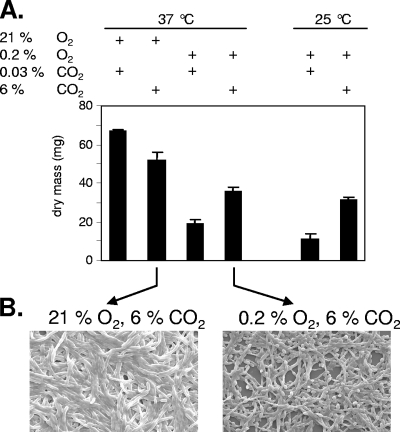
Current in vitro systems for C. albicans biofilm formation employ various air-equilibrated liquid media and plastic surfaces, and conflicting results have been obtained for biofilm formation under anoxic conditions (3, 44). Since C. albicans encounters numerous niches in which oxygen is depleted but not completely absent in the human host, we tested biofilm formation under hypoxic conditions. We used a polystyrene in vitro model and showed that under a low-oxygen atmosphere (0.2% O2) C. albicans wild-type strain CAF2-1 is able to form a significant biofilm, although the dry mass is about threefold less than that under normoxia (Fig. 1A). High CO2 levels (6%) doubled the mass but for the most part not the appearance of biofilms during hypoxia. The microscopic appearances of normoxic and hypoxic biofilms were similar, and the biofilms consisted of a mixture of yeast and hyphal cells, although less matrix material was visible under hypoxic conditions (Fig. 1B). We conclude that low concentrations of oxygen and elevated CO2 levels, conditions mimicking the conditions at many sites of systemic infection in the body, permit significant biofilm formation by C. albicans.
FIG. 1.
C. albicans biofilm formation in polystyrene cell culture wells. (A) Biofilm mass (dry weight) after growth at the temperatures indicated under an atmosphere containing the percentages of oxygen and carbon dioxide indicated. Wells were inoculated with 106 cells/ml of C. albicans strain CAF2-1 in RPMI 1640 medium and incubated in an hypoxic chamber for 60 h. Means and standard deviations were determined from the results of three independent experiments. (B) Appearance of biofilms after normoxic or hypoxic growth as determined by scanning electron microscopy.
Different requirements for hypoxic and normoxic biofilm formation.
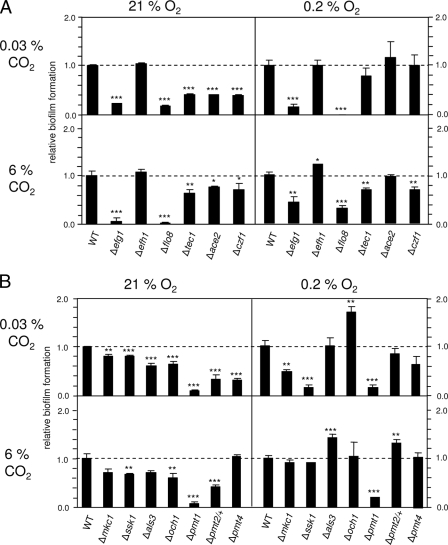
A selection of C. albicans mutants with known or suspected defects in normoxic biofilm formation were tested subsequently to determine their biofilm formation responses under low-oxygen and/or high-CO2 conditions. Mutations affect the C. albicans surface either by causing defects in structural components (Als3), by causing O mannosylation (Pmt isoforms), by causing a lack of signaling kinases (Mkc1, Ssk1), or by causing defects in the synthesis of transcription factors (Efg1, Flo8, and Ace2). According to the results obtained for biofilm formation under different conditions, the mutants can be classified in three groups: (i) mutants not affected by oxygen or CO2 levels (class 1), (ii) mutants showing normal biofilm formation under hypoxia but not under normoxia (class 2), and (iii) mutants affected by hypoxia and CO2 levels (class 3).
(i) Class 1 mutants: mutants that are biofilm defective irrespective of the oxygen and CO2 levels.
Efg1 is a transcriptional regulator known to regulate C. albicans morphology and metabolism, while its paralogue Efh1 has no major function (11). Importantly, Efg1 has been shown to be required for biofilm formation under standard (normoxic) conditions (36). Efg1 is functionally associated with the transcription factor Flo8 for coregulation of a common subset of genes (7). Using corresponding mutants, we showed that both Efg1 and Flo8 are factors that are essential for biofilm formation under all gas conditions, while an efh1 mutant behaved like the wild-type strain (Fig. 2A). Furthermore, a pmt1 mutant that lacks isoform 1 of Pmt proteins required for protein O mannosylation exhibited an equally complete block of biofilm formation (Fig. 2 B). Pmt1 has been shown previously to be required for normoxic biofilm formation (33), but here we report that this requirement is unconditional, in contrast to that of other Pmt isoforms (see below).
FIG. 2.
Biofilm formation by C. albicans mutant strains under different gas conditions. Biofilms of strains were allowed to form at 37°C for 60 h in the presence of the combinations of oxygen and CO2 levels indicated in an hypoxic chamber. Mutants with defective transcription factors (A) or defective cell surface structures (B) were compared. Relative biofilm formation was determined by determining the ratio of mutant biofilm mass (dry weight) to wild-type biofilm mass (dry weight). The strains tested include CAF2-1 (WT) and homozygous mutants DSC10 (Δefg1), C4/d63 (Δefh1), CCF3 (Δflo8), CaAS18 (Δtec1), MK106 (Δace2), CKY230 (Δczf1), CM1613 (Δmkc1), CSSK21 (Δssk1), CAYF178U (Δals3), NGY357 (Δoch1), SPCa2 (Δpmt1), and SPCa6 (Δpmt4) and heterozygous mutant SPCa4 (Δpmt2/+). For comparisons of mutant and control strains: *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
(ii) Class 2 mutants: mutants that are biofilm defective under normoxic conditions but not under hypoxic conditions.
The transcription factors Tec1, Ace2, and Czf1 contribute to C. albicans hyphal development; Ace2 and Tec1 also have been described to be partially required for (normoxic) biofilm formation (18, 29). Tec1 functions downstream of the Cek1 MAP kinase and allows hypha formation on certain solid media (39), while Czf1 permits hypha formation under embedded conditions, apparently counteracting functions of Efg1 (16). Ace2 allows derepressed pseudohyphal growth under normoxia, but filamentation under hypoxia is reduced; furthermore, similar to Efg1, it upregulates glycolytic gene expression but downregulates expression of respiratory genes (18). Here we show that these three transcription factors were required for normoxic biofilm formation but that the strong requirement for them was reduced or not apparent under hypoxia (Fig. 2 A).
Four additional class 2 mutants have defects in C. albicans surface structures. Lack of the Als3 cell wall protein reduces adhesive properties and biofilm formation (29, 47); an och1 mutant has shortened N-glycosyl chains (2), while a pmt2/PMT2 heterozygous mutant and a pmt4 homozygous mutant lack Pmt isoforms that O mannosylate subsets of glycoproteins and show reduced biofilm formation under normoxia (33). These mutant strains, although defective for biofilm formation under normoxia (irrespective of the CO2 level), showed wild-type levels of biofilm formation under hypoxia (Fig. 2 B). Strikingly, biofilm formation by the als3 and och1 mutants was even significantly enhanced during hypoxia compared to the results for the wild-type strain (for the als3 mutant, this was true only under hypoxia in the presence of 6% CO2).
(iii) Class 3 mutants: mutants rescued by CO2 under hypoxia.
The two mkc1 and ssk1 kinase mutants showed yet a different pattern of biofilm formation. The Mkc1 MAP kinase functions downstream of protein kinase C, which is needed for surface sensing, filamentation, and biofilm formation (20), while Ssk1 is a kinase in the cascade activating the Hog1 MAP kinase that regulates hypha formation and resistance to oxidative stress (5). Under normoxia and to an even greater extent under hypoxia, both mutant strains were biofilm defective, but the biofilm levels were completely restored to wild-type levels if 6% CO2 was present during hypoxia (but not during normoxia) (Fig. 2B). Thus, the combination of hypoxia and a high CO2 content permits biofilm formation by these mutants. It should be noted that the observed positive CO2 effect was not caused by a significant decrease in the medium pH during the course of the biofilm experiment.
In conclusion, the biofilm formation patterns for the class 2 and 3 mutants indicate the importance of environmental conditions for biofilm formation by C. albicans. A decrease in the oxygen level and an increase in the CO2 level (i.e., conditions that occur in the human host during systemic infection) recruit undescribed default pathways for biofilm construction that are not detectable during normoxia.
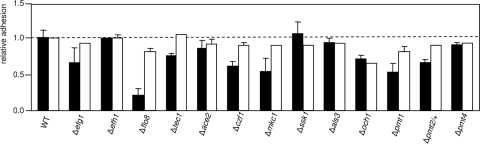
Adhesive properties of C. albicans mutants following normoxic or hypoxic growth.
For several mutants the experiments described above revealed significant differences with respect to biofilm formation under normoxic and hypoxic conditions. Biofilm formation consists of an early phase of adhesion to the supporting surface, followed by later developmental phases, in which the three-dimensional layered biofilm structure is formed (12). Although identically grown (normoxic) cells were used to initiate the biofilms, it appeared that it was possible that the greater biofilm mass of some mutants under hypoxia was related to improved adhesiveness compared to that of the wild-type strain during hypoxic biofilm growth. To explore this possibility, we pregrew the mutants either in normoxia as in the experiments described above or under 0.2% oxygen-6% CO2 and subsequently tested adhesion to the polystyrene support matrix under the same conditions for 2 h. Adhering cells were washed, and the numbers of these cells were estimated by measuring the XTT absorbance at 490 nm (35). The results for wild-type strain CAF2-1 showed that the adhesion to polystyrene was about threefold greater following normoxic pregrowth than following hypoxic pregrowth (OD490 for normoxic growth, 0.99; OD490 for hypoxic growth, 0.37), suggesting that the two conditions result in different surface structures and properties.
Several mutants have a greater tendency to adhere to the polystyrene support following hypoxic growth than following normoxic growth (Fig. 3). The biofilm performance of class 1 mutants was not improved since these mutants were biofilm-defective under all conditions. For some class 2 mutants improved adhesiveness may have contributed to greater biofilm formation under hypoxia, since mutants lacking transcription factors Tec1 and Czf1 adhered better following hypoxic growth than following normoxic growth. However, hypoxic growth did not affect adhesion of other mutants (ace2, als3, pmt4, ssk1, och1, and pmt2 mutants).
FIG. 3.
Adhesion of C. albicans strains to polystyrene. Strains were inoculated into polystyrene wells as they were for biofilm experiments. Following 2 h of incubation nonadherent cells were removed by washing, and the numbers of cells were assessed by using an XTT reduction assay. The relative adhesion was expressed as the ratio of the OD490 of a mutant to the OD490 of the wild-type strain (WT). Pregrowth and adhesion were allowed to proceed either under normoxia (filled bars) or under an atmosphere containing 0.2% oxygen and 6% CO2 in an hypoxic incubator (open bars).
We conclude that the improved biofilm performance of some, but not all, mutant strains during hypoxic growth may be due in part to increased adhesion. The adhesion of such mutants may be different than the adhesion of the wild-type strain, because of altered surface composition.
Efg1 rapidly upregulates biofilm-specific classes of genes during hypoxia.
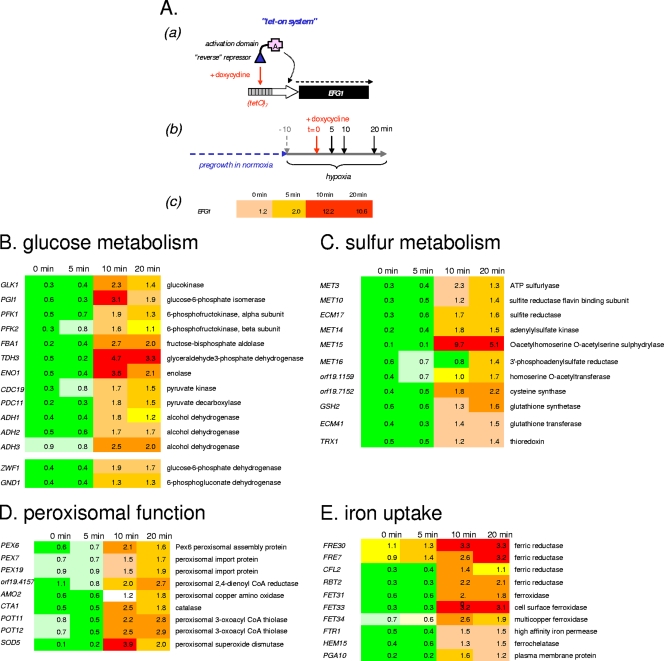
Previous transcriptome comparisons of efg1 mutants and wild-type strains have revealed numerous genes that are differentially expressed during normoxia and hypoxia (11, 21, 26, 42). However, comparisons of mutant and wild-type strains suffer from the drawback that transient or early regulatory functions cannot be distinguished from secondary regulatory events occurring during long-term adaptation. Therefore, to clarify the essential role of Efg1 in biofilm formation, we assessed transcriptome kinetics following release of Efg1 biosynthesis during hypoxia or normoxia. It appeared that it was possible that Efg1 rapidly and/or transiently regulates genes which have been shown to be regulated during biofilm formation (15, 24, 38, 40, 46). Such genes were found to include glycolytic genes, genes involved in sulfur metabolism, and genes for antioxidative functions.
To test the functions of Efg1, we first constructed a strain in which expression of the EFG1 coding region is inducible by doxycycline (strain CCS1). After pregrowth without doxycycline, the cells were transferred to induction medium and equilibrated for 10 min, and then doxycycline was added. In the case of hypoxic induction, the culture was flushed for 10 min with a gas mixture containing 93.8% nitrogen, 6% CO2, and 0.2% oxygen before doxycycline was added, which was followed by incubation under a stream of the same gas mixture. At different times after EFG1 induction, total RNA of strain CCS1 and, in parallel, total RNA of control strain CCS2 lacking the EFG1 coding region were isolated. Transcriptome analyses were performed by cohybridizing labeled cDNA derived from RNA of strains CCS1 and CCS2 at each time point to genome-wide C. albicans microarrays. We used stringent criteria to assign genes regulated by Efg1 in these experiments, including at least twofold up- or downregulation of genes in strains CCS1 and CCS2; importantly, we also considered genes significantly regulated only if they showed a consistent pattern of up- or downregulation during the course of the experiment.
(i) Genes rapidly induced by Efg1 under hypoxia.
During 10 min of doxycycline induction, in strain CCS1 the EFG1 transcript levels increased about 10-fold compared to the background levels in strain CCS2 (Fig. 4A). Concomitantly, the transcripts of 45 genes were found to be upregulated, while only 13 transcripts were downregulated. The 45 upregulated genes fell into four distinct classes, three of which are known hallmarks of biofilm formation. First, most glycolytic genes were induced by Efg1 (Fig. 4B). The TDH3 and ENO1 genes encoding glyceraldehyde-3-phosphate dehydrogenase and enolase, respectively, were upregulated 5- to 10-fold following doxycycline addition. Besides glycolytic genes, genes encoding enzymes of the pentose phosphate cycle (glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase) were highly induced as well. Second, genes involved in sulfur metabolism, including genes required for sulfate reduction and cysteine or methionine biosynthesis, were highly induced (Fig. 4C). These genes were found to be upregulated in transcriptome analyses of biofilms, although their functional significance in biofilms is unknown. We speculate that reactive oxygen species (ROS) arising during hypoxic growth phases in biofilms (10) are detoxified by reducing compounds, including methionine and cysteine, or by proteins, such as thioredoxin. The induction of the third class of genes, which are involved in the assembly of peroxisomes and peroxisome functions, including catalase and superoxide dismutase, supports this view (Fig. 4D). Recent proteomic studies also support the view that antioxidative functions are increased, at least in mature biofilms (41). The fourth class of genes induced by Efg1 under hypoxia includes genes involved in iron uptake (Fig. 4 E). Expression of these genes has been detected during planktonic growth of C. albicans in the presence of low levels of oxygen (42) but not in transcriptomes of biofilms under normoxia. We presume that upregulation of iron uptake genes occurs in biofilms under special conditions (for example, if the biofilms develop under hypoxia in a medium containing low iron levels).
FIG. 4.
Transcriptome kinetics following EFG1 induction under hypoxia. (A) (Panel a) Diagram of an expression unit. In plasmid pCS1 EFG1 is under transcriptional control of a minimal OP4 promoter that is regulated by repeats of the tet operator; pCS1 also encodes the reverse Tet repressor fused to an activation domain that binds to the tet operators upon addition of doxycycline. pCS1 and pNIM1, which does not contain the EFG1 insert, were both integrated into the ADH1 locus of strain HLC67 (efg1). (Panel b) Diagram showing the experimental protocol. Transformants containing either pCS1 (strain CCS1) or pNIM as a control (strain CCS2) were preincubated for 10 min in hypha-inducing medium (10% serum, 37°C) under a stream containing 93.8% nitrogen, 6% CO2, and 0.2% oxygen, and then doxycycline was added (40 μg/ml) and cells were incubated for the times indicated. At zero time (before doxycycline addition) and after the times indicated the cells were harvested, and RNA was prepared and used for preparation of Cy3- and Cy5-labeled cDNA that was used for transcriptome analyses. (Panel c) Expression of EFG1 following doxycycline addition. (B to E) Genes regulated by EFG1 induction. For each time point, cDNAs of the pCS1 transformant (CCS1) and the pNIM1 transformant (CCS2) were cohybridized to microarrays, and the ratio of expression was calculated for each gene. Genes that were consistently up- or downregulated by a factor of at least 2 (for comparisons of pCS1 and pNIM1 transformants and for comparisons of zero time and a later time point) were considered (the ratios are indicated by numbers and the colors of boxes). EFG1 ratios were used as a control, which verified that induction of EFG1 occurred.
An unexpected feature of transcriptome kinetics under hypoxia (unlike the kinetics under normoxia [see below]) was a low initial transcript ratio for many Efg1-regulated genes (ratio of the transcript level in strain CCS1 to the transcript level in strain CCS2 at zero time). This characteristic may be related to background Efg1 levels in strain CCS1 even before doxycycline induction and/or to the short exposure to air during harvesting of cells (although cells were chilled immediately). Whatever the reason, upregulation of transcript levels at all subsequent time points clearly defines these genes as genes that are induced by Efg1 during hypoxia.
(ii) Genes rapidly induced by Efg1 under normoxia.
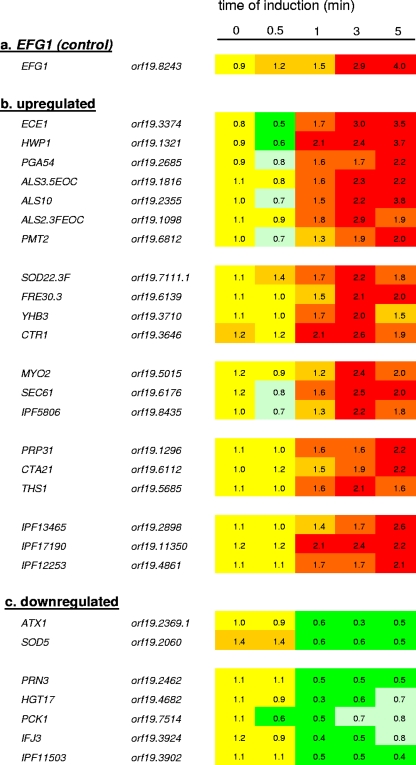
A similar transcriptome analysis was carried out during normoxia. Under these conditions the EFG1 transcript was upregulated fourfold compared with background levels shortly after doxycycline addition, along with the transcripts of 20 other genes (Fig. 5A). The most prominent group of coinduced genes includes genes encoding cell wall proteins (ECE1, HWP1, PGA54, ALS2, and ALS3) and a gene encoding a protein O-mannosyltransferase that is involved in O mannosylation of cell wall proteins (PMT2). ALS3 and its allele previously designated ALS10 were both significantly upregulated. Upregulation of ECE1 and HWP1 transcripts during doxycycline induction was verified by a quantitative real-time PCR analysis, in which the relative ECE1 (HWP1) transcript levels at zero time and during induction at 0.5, 1, 3, and 5 min were determined to be 1.2 (0.8), 0.8 (0.9), 2.4 (2.2), 3.4 (2.2), and 2.8 (2.0), respectively. These genes are a subset of genes that have been identified previously in comparisons of the transcriptomes of efg1 mutant and wild-type strains (11, 21, 26, 42); their presence among rapidly Efg1-upregulated genes strongly suggests that they are direct or very early indirect targets of Efg1. Some upregulated genes also appear to contribute to a reductive state of molecules; these genes include genes encoding a cytosolic superoxide dismutase (SOD22/SOD3), Fe3+ reductases (FRE30), a flavohemoglobin activity (YHB3/YHB5), and a copper transport protein (CTR1) that possibly is related to uptake of Fe2+. The FRE30 gene was also upregulated by Efg1 under hypoxia, like SOD5. In addition, the gene encoding transcription factor Cta21, which belongs to a conserved class of genes of unknown function situated close to telomeres (TLO genes), was upregulated. While most Efg1-regulated genes were induced (20 genes), a few genes (7 genes) appeared to be downregulated during hypoxic kinetics as described above. However, the short induction times used in our transcriptome experiments may not be reflected in the reduced levels of long-lived mRNA species.
FIG. 5.
Transcriptome kinetics following EFG1 induction under normoxia. Transcriptome kinetics analyses were performed as described in the legend to Fig. 4, but preincubation and doxycycline treatment were carried out with aeration.
To summarize, we report that the induction of Efg1 biosynthesis under hypoxia (but not under normoxia) closely mimics the transcriptome response that occurs during the formation of biofilms under normoxia. We conclude that (i) biofilm formation is associated with hypoxic growth and (ii) Efg1 is a central regulator of hypoxic adaptation and biofilm formation.
DISCUSSION
C. albicans cells grown under hypoxia have different metabolic activities, alternative pathways of cellular morphogenesis, and, as shown here, different requirements for biofilm formation compared to cells grown under normoxia. Recent transcriptome analyses have revealed a fermentative mode of energy metabolism under hypoxia, leading to increased expression of glycolytic genes and reduced expression of respiratory genes (42). In addition, genes specifying enzymatic functions requiring molecular oxygen, including genes for the biosynthesis of unsaturated fatty acids and ergosterol, were upregulated. On the other hand, biofilm development under standard (normoxic) conditions was also reported to upregulate glycolytic genes and genes for ergosterol biosynthesis (22, 38, 40, 46). These results suggest that the transcriptome patterns during biofilm formation and hypoxic exposure are related, a notion that is supported further by transcriptome kinetics dependent on Efg1 (see below). Conceivably, three-dimensional growth and cellular crowding in biofilms limit oxygen concentrations and result in hypoxic responses, especially near the supporting surface. Besides the major influence on metabolism, some morphological phenotypes of C. albicans are known to be influenced by oxygen-limited growth. Chlamydospore formation and stabilization of the opaque cellular phenotype require hypoxia. Furthermore, hypoxic surface growth at temperatures less than 37°C triggers formation of true hyphae in the absence of known inducer molecules (42). This unusual type of filamentous growth requires transcription factor Czf1, but it is suppressed by the Efg1 and Flo8 regulators that stimulate hypha formation under normoxia (7, 42). With regard to biofilm formation, our results show that several factors that have been described as factors that are essential under normoxia are not required under hypoxia. Thus, the Tec1, Ace2, and Czf1 transcriptional regulators have no or only slight effects on hypoxic biofilm formation, while a defect is clearly apparent under normoxia (15, 18, 29, 36). The lack of a hypoxic biofilm phenotype is especially surprising for the ace2 mutant, which under hypoxia is defective in glycolytic gene expression and in filamentation (18). Likewise, a czf1 mutant is able to form a biofilm, although it is filamentation defective during embedding (oxygen limitation) (16). Other mutants with mutations related to surface properties and signaling also do not show biofilm deficiency under hypoxia. The Als3 cell wall protein (30, 47) and glycosyltransferases involved in N glycosylation (Och1) or O glycosylation (Pmt2 and Pmt4) (2, 33) are required under normoxia, but they are dispensable under hypoxia. Correspondingly, an als3 mutant, although blocked for in vitro biofilm formation, could form an intact biofilm in an in vivo model (30), suggesting that the in vivo gaseous environment is able to suppress the mutant phenotype. Interestingly, mutants lacking members of the protein kinase C/Mkc1 and Hog1 signaling pathways (Ssk1) respond to both hypoxia and CO2 levels, because under hypoxia they are biofilm defective, yet hypoxia in combination with an elevated CO2 level permits efficient biofilm formation. Collectively, the results indicate (i) that biofilm formation is highly sensitive to the gas environment and (ii) that several regulators of biofilm development are not absolutely required as assumed previously but affect this process merely in certain environments.
The efg1 mutant is defective in biofilm formation under normoxic conditions (15, 36), and we found here that this defect occurs at all oxygen or CO2 levels. It has also been shown that a flo8 mutant has an identical phenotype, suggesting that Efg1 and Flo8 cooperate in biofilm formation, as they do for other characteristics (7). Although transcriptome comparisons between efg1 mutants and wild-type strains have been described previously (11, 21, 26, 42), we determined transcriptome kinetics dependent on Efg1 with the hope of discovering genes rapidly and/or transiently regulated by Efg1 in the presence of high or low oxygen levels. The results were unexpected and relevant for biofilm formation since it was found that all major classes of genes known to be induced in biofilms were also upregulated rapidly by Efg1 under hypoxia (but not under normoxia). First, almost all glycolytic genes were upregulated under hypoxia, which is at variance with previous comparisons of efg1 mutant and wild-type transcriptomes (42). The different results obtained for kinetic and steady-state transcriptome comparisons very likely reflect different Efg1 functions during short- and long-term hypoxic adaptation, although the mechanisms by which Efg1 upregulates glycolytic genes transiently in hypoxia and permanently during normoxia are currently not known. Interestingly, a pmt1 mutant defective in O mannosylation, which also showed a lower level of glycolytic gene expression (6), is highly defective for biofilm formation as well (33; this study). In summary, these results strongly suggest that an elevated glycolytic flow is essential for biofilm formation by C. albicans, supporting previous transcriptome and proteome results (22, 38, 40, 46). On the other hand, ethanol produced as a consequence of glycolysis appears to restrict the extent of biofilm development (22).
A second class of genes, genes involved in sulfur assimilation and biosynthesis of sulfur-containing amino acids, has repeatedly been shown to be upregulated in transcriptome analyses of biofilms (15, 24); this is also apparent from Efg1 transcriptome kinetics under hypoxia. As observed for glycolytic genes, this regulation appears to be a transient response, since it was not discovered previously for efg1 mutant transcriptomes (42). Upregulation of sulfur assimilatory genes under hypoxia may provide a clue to the functionality of these genes since it is known that a malfunctioning respiratory chain (due to the lack of molecular oxygen) generates ROS that require detoxification (10). Compounds containing SH groups (Met, Cys, thioredoxin, and coenzyme A) may be instrumental for this purpose. Thus, even under seemingly aerobic conditions, cells within biofilms appear to experience and respond to hypoxia by Efg1 signaling. The third class of genes upregulated by Efg1 under hypoxia also appears to represent a response to ROS since it comprises genes for peroxisome assembly and function, including genes encoding enzymes that directly detoxify ROS, including catalase and superoxide dismutase. This finding also is in agreement with previous proteomic results for biofilm formation (41). The fourth class of transiently Efg1-dependent genes upregulated during hypoxia includes several genes for reductive iron uptake, including genes encoding ferric reductases, ferroxidase, and ferrochelatase. Upregulation of these genes could reflect an attempt by cells to cope with hypoxia by optimizing the use of residual oxygen through improved functioning of iron proteins in the respiratory chain. An iron starvation-like response occurring during hypoxia could also upregulate EFG1 expression itself and thereby allow filamentation and biofilm formation (17). In summary, the congruence of genes induced in biofilms and genes induced by Efg1 under hypoxia is striking and indicates that cells in biofilms undergo Efg1-dependent hypoxic adaptation. It should be stressed that most biofilm-regulated genes were regulated by Efg1 during planktonic growth only under hypoxia and not during normoxia. Under normoxia, Efg1 induction led to rapid induction of subsets of genes identified in previous efg1 transcriptomes (11, 21, 26, 42), including genes encoding cell wall proteins and some antioxidative functions. Although our kinetic approach to transcriptome analysis revealed only a limited number of Efg1-regulated genes, we were unable to clearly define shared regulatory motifs in their promoter regions by computer analyses, possibly because these sequences are relatively variable or redundant. In ongoing experiments we are characterizing direct and/or indirect Efg1 regulation of target genes by performing promoter binding and deletion experiments.
Hypoxic adaptation and the potential functions of alternative signaling pathways during biofilm formation by C. albicans are important for developing approaches to combat C. albicans biofilm formation using specific inhibitors. Systemic C. albicans infections of internal organs, including the liver and kidney, typically produce foci of C. albicans colonial growth, presumably reflecting local biofilms. Inhibitor screening systems should attempt to mimic such in vivo conditions by limiting oxygen, increasing CO2 levels, and providing host compounds that the fungal invader cannot synthesize in the absence of oxygen, such as unsaturated fatty acids and sterols.
Acknowledgments
We thank G. Butler, R. Calderone, S. Filler, N. Gow, C. Kumamoto, H. Liu, J. Morschhäuser, J. Pla, and K. Schröppel for providing strains or plasmids. We thank G. Schuster for help with the scanning electron microscopy.
This project was funded by the Deutsche Forschungsgemeinschaft (grants SFB590 and SPP 1160) and by EU project “Galar Fungail II” (grant MRTN-CT-2003-504148).
Footnotes
Published ahead of print on 3 April 2009.
REFERENCES
- 1.Andes, D., J. Nett, P. Oschel, R. Albrecht, K. Marchillo, and A. Pitula. 2004. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 72:6023-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates, S., H. B. Hughes, C. A. Munro, W. P. Thomas, D. M. MacCallum, G. Bertram, A. Atrih, M. A. Ferguson, J. M. Bain, A. Brand, S. Hamilton, C. Westwater, L. M. Thomson, A. J. Brown, F. C. Odds, and N. A. Gow. 2006. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J. Biol. Chem. 281:90-98. [DOI] [PubMed] [Google Scholar]
- 3.Biswas, S. K., and W. L. Chaffin. 2005. Anaerobic growth of Candida albicans does not support biofilm formation under similar conditions used for aerobic biofilm. Curr. Microbiol. 51:100-104. [DOI] [PubMed] [Google Scholar]
- 4.Brown, D. H., Jr., A. D. Giusani, X. Chen, and C. A. Kumamoto. 1999. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol. Microbiol. 34:651-662. [DOI] [PubMed] [Google Scholar]
- 5.Calera, J. A., X. J. Zhao, and R. Calderone. 2000. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect. Immun. 68:518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantero, P. D., C. Lengsfeld, S. K.-H. Prill, M. Subanović, E. Román, J. Pla, and J. F. Ernst. 2007. Transcriptional and physiological adaptation to defective protein-O-mannosylation in Candida albicans. Mol. Microbiol. 64:1115-1128. [DOI] [PubMed] [Google Scholar]
- 7.Cao, F., S. Lane, P. P. Raniga, Y. Lu, Z. Zhou, K. J. Ramon, J. Chen, and H. Liu. 2006. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell 17:295-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra, J., D. M. Kuhn, P. K. Mukherjee, L. L. Hoyer, T. McCormick, and M. A. Ghannoum. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Enfert, C. 2006. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr. Drug Targets 7:465-470. [DOI] [PubMed] [Google Scholar]
- 10.Dirmeier, R., K. M. O'Brien, M. Engle, A. Dodd, E. Spears, and R. O. Poyton. 2002. Exposure of yeast cells to anoxia induces transient oxidative stress. J. Biol. Chem. 277:34773-34784. [DOI] [PubMed] [Google Scholar]
- 11.Doedt, T., S. Krishnamurthy, D. P. Bockmühl, B. Tebarth, C. Stempel, C. L. Russell, A. J. P. Brown, and J. F. Ernst. 2004. APSES proteins regulate morphogenesis and metabolism in Candida albicans. Mol. Biol. Cell 15:3167-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 13.Ernst, J. F., and D. Tielker. 2009. Responses to hypoxia in fungal pathogens. Cell. Microbiol. 11:183-190. [DOI] [PubMed] [Google Scholar]
- 14.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Sánchez, S., S. Aubert, I. Iraqui, G. Jambon, J.-M. Ghigo, and C. D'Enfert. 2004. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot. Cell 3:536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giusani, A. D., M. Vinces, and C. A. Kumamoto. 2002. Invasive filamentous growth of Candida albicans is promoted by Czf1p-dependent relief of Efg1p-mediated repression. Genetics 160:1749-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hameed, S., T. Prasad, D. Banerjee, A. Chandra, C. K. Mukhopadhyay, S. K. Goswami, A. A. Lattif, J. Chandra, P. K. Mukherjee, M. A. Ghannoum, and R. Prasad. 2008. Iron deprivation induces EFG1-mediated hyphal development in Candida albicans without affecting biofilm formation. FEMS Yeast Res. 8:744-755. [DOI] [PubMed] [Google Scholar]
- 18.Kelly, M. T., D. M. MacCallum, S. D. Clancy, F. C. Odds, A. J. Brown, and G. Butler. 2004. The Candida albicans CaAce2 gene affects morphogenesis, adherence and virulence. Mol. Microbiol. 53:969-983. [DOI] [PubMed] [Google Scholar]
- 19.Kojic, E. M., and R. O. Darouiche. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumamoto, C. 2005. A contact-activated kinase signals Candida albicans invasive growth and biofilm development. Proc. Natl. Acad. Sci. USA 102:5576-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane, S., C. Birse, S. Zhou, R. Matson, and H. Liu. 2001. DNA array studies demonstrate convergent regulation of virulence factors by Cph1, Cph2, and Efg1 in Candida albicans. J. Biol. Chem. 276:48988-48996. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee, P. K., S. Mohamed, J. Chandra, D. Kuhn, S. Liu, O. S. Antar, R. Munyon, A. P. Mitchell, D. Andes, M. R. Chance, M. Rouabhia, and M. A. Ghannoum. 2006. Alcohol dehydrogenase restricts the ability of the pathogen Candida albicans to form a biofilm on catheter surfaces through an ethanol-based mechanism. Infect. Immun. 74:3804-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulhern, S. M., M. E. Logue, and G. Butler. 2006. Candida albicans transcription factor Ace2 regulates metabolism and is required for filamentation in hypoxic conditions. Eukaryot. Cell 5:2001-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murillo, L. A., G. Newport, C.-Y. Lan, S. Habelitz, J. Dungan, and N. M. Agabian. 2005. Genome-wide transcription profiling of the early phase of biofilm formation by Candida albicans. Eukaryot. Cell 4:1562-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nailis, H., R. Vandenbroucke, K. Tilleman, D. Deforce, H. Nelis, and T. Coenye. 2009. Monitoring ALS1 and ALS3 gene expression during in vitro Candida albicans biofilm formation under continuous flow conditions. Mycopathologia 167:9-17. [DOI] [PubMed] [Google Scholar]
- 26.Nantel, A., D. Dignard, C. Bachewich, D. Harcus, A. Marcil, A. P. Bouin, C. W. Sensen, H. Hogues, M. van het Hoog, P. Gordon, T. Rigby, F. Benoit, D. C. Tessier, D. Y. Thomas, and M. Whiteway. 2002. Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol. Biol. Cell 13:3452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro-Garcia, F., M. Sanchez, J. Pla, and C. Nombela. 1995. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol. Cell. Biol. 15:2197-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobile, C. J., and A. P. Mitchell. 2006. Genetics and genomics of Candida albicans biofilm formation. Cell. Microbiol. 8:1382-1391. [DOI] [PubMed] [Google Scholar]
- 29.Nobile, C. J., and A. P. Mitchell. 2005. Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr. Biol. 15:1150-1155. [DOI] [PubMed] [Google Scholar]
- 30.Nobile, C. J., D. R. Andes, J. E. Nett, F. J. Smith, Jr., F. Yue, Q.-T. Phan, J. E. Edwards, S. G. Filler, and A. P. Mitchell. 2006. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, H., C. L. Myers, D. C. Sheppard, Q. T. Phan, A. A. Sanchez, J. Edwards, and S. G. Filler. 2005. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell. Microbiol. 7:499-510. [DOI] [PubMed] [Google Scholar]
- 32.Park, Y.-N., and J. Morschhäuser. 2005. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot. Cell 4:1328-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peltroche-Llacsahuanga, H., S. Goyard, C. D'Enfert, S. K. Prill, and J. F. Ernst. 2006. Protein O-mannosyltransferase isoforms regulate biofilm formation in Candida albicans. Antimicrob. Agents Chemother. 50:3488-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez, A., B. Pedrós, A. Murgui, M. Casanova, J. L. López-Ribot, and J. P. Martínez. 2006. Biofilm formation by Candida albicans mutants for genes coding fungal proteins exhibiting the eight-cysteine-containing CFEM domain. FEMS Yeast Res. 6:1074-1084. [DOI] [PubMed] [Google Scholar]
- 35.Ramage, G., K. VandeWalle, B. L. Wickes, and J. López-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramage, G., K. VandeWalle, J. L. López-Ribot, and B. L. Wickes. 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol. Lett. 214:95-100. [DOI] [PubMed] [Google Scholar]
- 37.Ramage, G., S. P. Saville, D. P. Thomas, and J. L. López-Ribot. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossignol, T., C. Ding, A. Guida, C. d'Enfert, D. G. Higgins, and G. Butler. 2009. Correlation between biofilm formation and the hypoxic response in Candida parapsilosis. Eukaryot. Cell 8:550-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweizer, A., S. Rupp, B. N. Taylor, M. Röllinghoff, and K. Schröppel. 2000. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol. Microbiol. 38:435-445. [DOI] [PubMed] [Google Scholar]
- 40.Sellam, A., T. Al-Niemi, K. McInnerney, S. Brumfield, A. Nantel, and P. A. Suci. 2009. A Candida albicans early stage biofilm detachment event in rich medium. BMC Microbiol. 9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seneviratne, C. J., Y. Wang, L. Jin, Y. Abiko, and L. P. Samaranayake. 2008. Candida albicans biofilm formation is associated with increased anti-oxidative capacities. Proteomics 8:2936-2947. [DOI] [PubMed] [Google Scholar]
- 42.Setiadi, E. R., T. Doedt, F. Cottier, C. Noffz, and J. F. Ernst. 2006. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen-sensing- and Efg1p-regulatory networks. J. Mol. Biol. 361:399-411. [DOI] [PubMed] [Google Scholar]
- 43.Stoldt, V. R., A. Sonneborn, C. E. Leuker, and J. F. Ernst. 1997. Efg1p, an essential regulator of morphogenesis of the human fungal pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thein, Z. M., Y. H. Samaranayake, and L. P. Samaranayake. 2007. In vitro biofilm formation of Candida albicans and non-albicans Candida species under dynamic and anaerobic conditions. Arch. Oral Biol. 52:761-767. [DOI] [PubMed] [Google Scholar]
- 45.Thomas, D. P., S. P. Bachmann, and J. L. Lopez-Ribot. 2006. Proteomics for the analysis of the Candida albicans biofilm lifestyle. Proteomics 6:5795-5804. [DOI] [PubMed] [Google Scholar]
- 46.Yeater, K. M., J. Chandra, G. Cheng, P. K. Mukherjee, X. Zhao, S. L. Rodriguez-Zas, K. E. Kwast, M. A. Ghannoum, and L. L. Hoyer. 2007. Temporal analysis of Candida albicans gene expression during biofilm development. Microbiology 153:2373-2385. [DOI] [PubMed] [Google Scholar]
- 47.Zhao, X., K. J. Daniels, S.-H. Oh, C. B. Green, K. Yeater, D. R. Soll, and L. L. Hoyer. 2006. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology 152:2287-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]