Abstract
Members of the phylum “Synergistetes” have frequently been detected in the human oral cavity at sites of dental disease, but they have rarely been detected in studies of oral health. Only two oral “Synergistetes” taxa are cultivable. The aims of this study were to investigate the diversity of “Synergistetes” in the oral cavity, to establish whether “Synergistetes” taxa are more strongly associated with periodontitis than with oral health, and to visualize unculturable “Synergistetes” in situ. Sixty samples (saliva, dental plaque, and mucosal swabs) were collected from five subjects with periodontitis and five periodontally healthy controls. Using phylum-specific 16S rRNA gene primers, “Synergistetes” were identified by PCR, cloning, and sequencing of 48 clones per PCR-positive sample. Subgingival plaque samples were labeled with probes targeting rRNA of unculturable oral “Synergistetes” using fluorescent in situ hybridization (FISH). Analysis of 1,664 clones revealed 12 “Synergistetes” operational taxonomic units (OTUs) at the 99% sequence identity level, 5 of which were novel. “Synergistetes” OTU 4.2 was found in significantly more subjects with periodontitis than controls (P = 0.048) and was more abundant in subgingival plaque at diseased sites than at healthy sites in subjects with periodontitis (P = 0.019) or controls (P = 0.019). FISH analysis revealed that unculturable oral “Synergistetes” cells were large curved bacilli. The human oral cavity harbors a diverse population of “Synergistetes.” “Synergistetes” OTU 4.2 is associated with periodontitis and may have a pathogenic role.
Periodontitis leads to the destruction of the tissues supporting the teeth and results from an inappropriate inflammatory response in susceptible individuals provoked by members of the normal oral microbiota (17). This disease results in loss of the attachment between the periodontium and the teeth, along with the formation of pockets which become heavily colonized with anaerobic bacteria, many of which possess virulence factors that can cause further damage to the host (21). The bacterial community found below the gum line, subgingival plaque, is extremely diverse, particularly in individuals with periodontitis, and over 400 species are estimated to inhabit the disease-associated periodontal ecosystem (29).
The candidate phylum “Synergistetes” (16) includes a wide variety of genera, including Aminobacterium, Aminomonas, Aminiphilus, Anaerobaculum, Cloacibacillus, Dethiosulfovibrio, Jonquetella, Pyramidobacter, Synergistes, Thermanaerovibrio, and Thermovirga (8, 43). “Synergistetes” are widely distributed in the environment, form part of the normal microbiota of animals (10), and have also been isolated from a variety of sites in humans, including the oral cavity. “Synergistetes” have been detected in subgingival plaque associated with diseased sites in periodontitis patients (7, 13, 15, 18-20, 29), in root canals and pus from abscesses in patients with endodontic infections (28, 31, 32, 36, 37, 44), and in dental caries (27, 30). Because the description of this candidate phylum is relatively recent, “Synergistetes” taxa have frequently been misidentified. For example, phylotypes D084, BH017, W028, W090, and BA121 were originally described as Deferribacteres (Paster), and phylotype E3_33 was originally described as Flexistipes (Munson). Despite the frequent detection of “Synergistetes” taxa at oral disease sites, they are almost never encountered in healthy individuals (1, 19, 29), suggesting that they have a pathogenic role. Furthermore, “Synergistetes” taxa among clones obtained from periodontitis patients and healthy subjects were found to be significantly associated with periodontitis (19). Specific “Synergistetes” phylotypes, including phylotypes W090, BH007, DO84, W028, and BH017, have also been associated with disease (18, 19, 29). However, the results of the different studies are not consistent, possibly because of heterogeneity in the clinical categorization of periodontal disease and health. Therefore, to date, it has not been possible to implicate specific taxa in periodontitis.
Human oral “Synergistetes” can be divided into two main groups: cluster B, which comprises the only two species that have been cultured to date, Jonquetella anthropi (16) and Pyramidobacter piscolens (8), and cluster A, which includes more than 20 other taxa for which no cultivable representatives are available (43). Unculturable “Synergistetes” in oral samples have been detected only by 16S rRNA gene sequence analysis, and the morphology of the cells remains unknown.
The aims of this study were (i) to investigate the diversity and distribution of “Synergistetes” in the human oral cavity using group-specific molecular primers, (ii) to compare the “Synergistetes” populations found in periodontally healthy and diseased subjects, and (iii) to use fluorescent in situ hybridization (FISH) to determine the cellular morphology of as-yet-uncultured “Synergistetes” taxa.
MATERIALS AND METHODS
Bacterial culture.
The bacterial strains listed in Table 1, with the exception of those indicated below, were cultured on fastidious anaerobe agar (Lab M) supplemented with 5% sterile defibrinated horse blood and incubated at 37°C in an anaerobic workstation (Don Whitley Scientific Ltd.) with an atmosphere consisting of 80% nitrogen, 10% hydrogen, and 10% carbon dioxide. The Neisseria mucosa strain was cultured aerobically at 37°C on blood agar base no. 2 (Lab M) with 5% sterile defibrinated horse blood. Denitrovibrio acetiphilus was grown anaerobically in peptone yeast extract glucose broth (11), and Dethiosulfovibrio peptidovorans and Aminobacterium mobile were cultured anaerobically on fastidious anaerobe agar supplemented with 0.2% yeast extract and 0.105% l-serine and supplemented with 0.1% yeast extract and 3% sodium chloride, respectively.
TABLE 1.
Bacterial strains and clones used for primer and probe validation
| Bacterial clone or strain | Used for validation of:
|
|
|---|---|---|
| PCR primers | FISH probes | |
| “Synergistetes” | ||
| “Synergistetes” OTU 1 A2G_1 | + | |
| “Synergistetes” OTU 3.2 A3A_4 | + | |
| “Synergistetes” OTU 3.3 A3G_46 | + | |
| “Synergistetes” OTU 4.4 A3G_41 | + | |
| “Synergistetes” OTU 6.1 A2G_67 | + | |
| Jonquetella anthropi E3_33 | + | + |
| Pyramidobacter piscolens DSM 21147T | + | + |
| Pyramidobacter piscolens AHN 1662 | + | |
| Dethiosulfovibrio peptidovorans DSM 11002T | + | |
| Aminobacterium mobile DSM 12262T | + | |
| Firmicutes | ||
| Parvimonas micra ATCC 33270T | + | + |
| Streptococcus mutans NCTC 10449T | + | |
| Lactobacillus casei ATCC 393T | + | |
| Eubacterium minutum ATCC 700079T | + | |
| Shuttleworthia satelles DSM 14600T | + | |
| Bulleidia extructa DSM 13220T | + | |
| Selenomonas noxia ATCC 43541T | + | |
| Bacteroidetes | ||
| Tannerella forsythensis FDC 338T | + | |
| Porphyromonas gingivalis ATCC 33277T | + | |
| Prevotella denticola ATCC 35308T | + | |
| Prevotella oris ATCC 33573T | + | |
| Prevotella baroniae DSM 16972T | + | |
| Prevotella oralis NCTC 11459T | + | |
| Prevotella intermedia DSM 20706T | + | |
| Actinobacteria | ||
| Slackia exigua ATCC 700122T | + | + |
| Actinomyces naeslundii NCTC 10301T | + | |
| Atopobium rimae ATCC 49626T | + | |
| Proteobacteria | ||
| Aggregatibacter actinomycetemcomitans ATCC 33384T | + | + |
| Campylobacter rectus ATCC 33238T | + | + |
| Neisseria mucosa NCTC 10777 | + | |
| Fusobacteria | ||
| Fusobacterium nucleatum subsp. nucleatum ATCC 25586 | + | + |
| Deferribacteres | ||
| Denitrovibrio acetiphilus DSM 12809T | + | |
“Synergistetes”-specific PCR primer design and validation.
PCR primers specific for the phylum “Synergistetes” were designed by visual inspection of an alignment of the 16S rRNA genes of representatives of all known “Synergistetes” taxa and their closest phylogenetic neighbor, the Deferribacteres. Candidate primers were selected on the basis of having less than three mismatches with members of the “Synergistetes” group and at least six mismatches with members of the Deferribacteres. The primers had G+C contents in the range from 45 to 55% and were checked for self-complementarity. They were validated in silico by interrogation of the Ribosomal Database Project-II 16S rRNA database (5) and a BLAST search of the GenBank nucleotide database and were synthesized by MWG Biotech AG. The primers were then checked for sensitivity and specificity with the panel of strains listed in Table 1. DNA was extracted from the isolates using a standard method, as described previously (28). PCR was performed using Reddy Mix PCR master mixture (ABgene United Kingdom) with 5 pmol of each primer and bacterial DNA as the template. The initial denaturation was performed at 95°C for 5 min, and this was followed by 30 cycles of denaturation at 95°C for 45 s, annealing at 56°C for 45 s, and extension at 72°C for 90 s. Reverse primer 806R (5′-CACACCCAGCATACATCGTTTACTGCCA 3′) used in conjunction with universal forward primer 27F (22) was found to amplify all of the representatives of the phylum “Synergistetes” tested but did not give products with any of the other strains tested and was therefore selected for use in this study.
Subjects.
Five subjects with chronic periodontitis and five periodontally healthy subjects matched for age and gender participated in the study with informed consent. Ethical approval was granted by Lewisham Research Ethics Committee (reference number 06/Q0701/35). All subjects were currently nonsmokers or former smokers (who had ceased smoking at least 6 months prior to participation in the study). Subjects excluded from the study included subjects who (i) had received periodontal or antimicrobial therapy within the previous 6 months, (ii) had a medical condition requiring antibiotic cover or affecting periodontitis severity or progression, or (iii) were pregnant. Subjects with chronic periodontitis had pocket probing depths of ≥6 mm, attachment loss of ≥4 mm, and alveolar bone loss of at least 40% of the root length at four or more posterior teeth. The periodontally healthy subjects had no probing depths that were >3 mm, negligible loss of attachment or bone loss, and minimal gingivitis. Partial mouth plaque and bleeding indices were recorded to obtain an overall measure of oral hygiene and marginal gingival inflammation for each subject (35). As specified by the plaque index (34), plaque was recorded as absent (0), detectable with a probe (1), just visible to the naked eye (2), or abundant (3). Bleeding was recorded as absent (0), visible within 30 s (1), or visible immediately with probing (2).
Saliva was collected by expectoration, and the cheek mucosa and dorsum of the tongue were sampled by scraping them five times with a sterile plastic spoon. Supra- and subgingival plaque samples were harvested with a sterile curette from mesial or distal surfaces of posterior teeth. In periodontally healthy “control” subjects, the supra- and subgingival plaque samples were collected from shallow sites with ≤3-mm probing depth which did not bleed. In subjects with periodontitis (referred to as “cases” below), supra- and subgingival plaque samples were collected not only from matched healthy sites but also from “diseased” sites (pockets at least 6 mm deep which bled upon probing). For each of the four plaque categories (supra- and subgingival for healthy and diseased sites), samples were obtained from four sites (one site in each quadrant of the mouth) and later pooled. Probing depth and bleeding status were ascertained only after microbiological sampling was complete to avoid contamination of the samples. All samples were suspended in Tris-EDTA buffer (pH 8.0), and DNA was extracted as previously described (28).
“Synergistetes” PCR cloning analysis.
“Synergistetes”-specific PCR was performed for each sample using primers 27F and 806R as described above. For each sample for which a product was obtained, PCR amplification was repeated a further five times, and the amplicons were pooled, to reduce stochastic variation, prior to cloning. The amplified genes were cloned using a TOPO TA cloning kit (Invitrogen) with the pCR4-TOPO plasmid vector and One Shot TOP10 chemically competent Escherichia coli cells according to the manufacturer's instructions. A library of 100 clones was prepared for each sample. Cloned inserts were reamplified by PCR using standard M13 primers and conditions. Forty-eight amplified inserts from each sample were sequenced by using a BigDye Terminator 3.1 cycle sequencing kit (Applied Biosystems) with primer 519R (22) and a 3730xl DNA analyzer (Applied Biosystems) according to the manufacturer's instructions. Sequences were checked for chimeras using Chimera_Check of Ribosomal Database Project-II (24) and excluded from further analysis if the presence of a chimera was suspected. The remaining sequences were aligned with Clustal X (42). The phylotypes were provisionally identified based on >99% sequence identity to 16S rRNA gene sequences in the Ribosomal Database Project-II 16S rRNA database (24). Using the Molecular Evolutionary Genetics Analysis software (version 3.1), a distance matrix was prepared (with Jukes-Cantor correction), and phylogenetic trees were constructed with the neighbor-joining method incorporating bootstrap analysis. To confirm the phylogenetic placement, a subset of clones (multiple representatives of each distinct taxon, selected from a range of subjects) were further sequenced with sequencing primers 357F (22), 27F, 806R, M13(-20)F, and M13R to obtain triple coverage (including both strands) for the complete length of the 16S rRNA gene insert. Assignment of sequences to operational taxonomic units (OTUs) was performed at both the 99 and 98% sequence identity levels.
Statistical analysis.
Statistical analysis was performed with nonparametric tests using Statistical Package for the Social Sciences (SPSS) 15.0 for Windows. Age and gender matching of the two cohorts (periodontally healthy and diseased subjects) was demonstrated with the Mann-Whitney U test. The same test was used to compare the significance of any differences between the cohorts in the plaque and bleeding indices and in the probing depths of the healthy sites sampled. The prevalence of “Synergistetes” phylotypes (defined at the 99% sequence identity level) in the two cohorts was compared by using the Mann-Whitney U test. In order to directly compare the diseased and healthy subjects, data were analyzed for the five sites common to both cohorts; the two samples taken from “diseased” sites in subjects with periodontitis were handled separately. “Synergistetes” taxon richness was assessed by comparing the mean numbers of OTUs per subject and per site for each cohort and examined with the Mann-Whitney U test. A similar comparison for the subgingival plaque samples from healthy and diseased sites in subjects with periodontitis was performed using the Wilcoxon matched-pair signed-rank test. Ordinal logistic regression (backward model) was used to identify any variables with a significant explanatory effect on differences in the number of “Synergistetes” OTUs. The variables considered were subject age, gender, plaque index, bleeding index, cohort (subject with periodontitis or periodontally healthy control), and site sampled (saliva, cheek mucosa, dorsum of tongue, and supra- or subgingival plaque at healthy sites). Data for plaque samples from diseased sites (only for subjects with periodontitis) were excluded from this analysis. Furthermore, “Synergistetes” species richness estimates were obtained and community structure comparisons were made by calculating Chao1 and abundance-based coverage estimators and Jaccard classic and Bray-Curtis similarity indices by using the program EstimateS (6). When the presence of specific “Synergistetes” OTUs was examined, the significance of differences between the cohorts was assessed using the Fisher exact test. The proportion of clones belonging to each “Synergistetes” OTU was compared for the different cohorts, and the results were assessed with the Mann-Whitney U test. A three-way comparison of the proportion of each “Synergistetes” OTU in subgingival plaque samples from subjects with periodontitis and healthy controls using the Kruskall-Wallis test was followed by explanatory Mann-Whitney tests comparing data for the diseased sites in subjects with periodontitis with data for the healthy sites in either subjects with periodontitis or healthy control subjects; as two comparisons were made for the same data in this case, the P value threshold was adjusted to 0.03 rather than the normal 0.05. Comparisons between the two cohorts for the presence and proportion of clones representing each “Synergistetes” OTU (see above) were repeated for OTUs defined at the 98% sequence identity level.
Visualization of “Synergistetes” cells by FISH.
Oligonucleotide probes targeting 16S rRNA were designed for oral “Synergistetes” clusters A (“unculturable”) and B, “Synergistetes” OTU 3.3, J. anthropi, and P. piscolens (Table 2). The probe selection criteria included an exact match with the target group, at least two base mismatches with other phylotypes, and brightness class I to III indicative of E. coli ribosome probe accessibility of >40% (9). Probe specificity was confirmed in silico, and probes were synthesized with one of three fluorophores at the 5′ end, Alexa Fluor 488, Cy3, or Cy5. The excitation and emission spectra of these probes were sufficiently distinct that multiple probes (one with each of the three chromes) could be used together in multi-FISH experiments without the risk of crossover. Probes were validated in vitro with narrow- and broad-range panels of bacteria (Table 1). Optimal conditions for probe hybridization stringency were determined by varying the formamide concentration in the hybridization buffer at 50°C. In brief, the buffer contained 18% (vol/vol) 5 M NaCl, 2% (vol/vol) 1 M Tris-HCl (pH 8.0), 0.1% (vol/vol) 10% sodium dodecyl sulfate, and formamide at a concentration of 0, 10, 20, 30, or 40%. After validation and optimization of individual probes, probes B_155, J.anth_63, and P1_70 were used in combination with a sample consisting of a mixture of J. anthropi and P. piscolens to confirm the expected patterns of hybridization and the overlap of fluorescent signals.
TABLE 2.
“Synergistetes” FISH probes
| FISH probea | Sequence (5′-3′) | 5′ Fluorophore | Length (bases) | G+C content (%) | Brightness classb | “Synergistetes” target | Optimal formamide concn (%)c |
|---|---|---|---|---|---|---|---|
| A_487 | CGGGGCTTATTCATGTGGT | Cy3 | 19 | 53 | III | Cluster A | 20-40 |
| A_845 | TAACTGCGGCACACCAACAT | Cy5 | 20 | 50 | III | Cluster A | NEd |
| B_155 | ATTAGCTCCGGTTTCCCGC | Alexa Fluor 488 | 19 | 58 | III | Cluster B | 0-30 |
| J.anth_63 | TCCTCGAGTATTCCCGTCCA | Cy5 | 20 | 55 | II | Jonquetella anthropi | 0-40 |
| P1_70 | CATAAGTCTCTCGAACCATCC | Cy3 | 21 | 48 | III | Pyramidobacter piscolens | 0-20 |
| 3.3_65 | CTCCACTTCATGGCACCGTCGTA | Alexa Fluor 488 or Cy3 | 23 | 57 | II | Synergistetes 3.3/BH007 | 10-40 |
As no isolates of uncultivated “Synergistetes” cluster A are available, clone FISH (33) was used to evaluate the validity of probes by hybridization to rRNA transcribed from the target “Synergistetes” 16S rRNA gene insert in E. coli clones treated with chloramphenicol. Clones (Table 1) were grown from a 1:20 dilution of an overnight culture in Luria-Bertani broth supplemented with 50 μl/ml kanamycin sulfate (Invitrogen) to an optical density at 600 nm of 0.4. After addition of 170 mg/liter chloramphenicol, the broth was incubated at 37°C overnight. Cells were harvested by centrifugation (4,200 × g, 10 min), resuspended in phosphate-buffered saline (PBS), and applied to microscope slides for FISH analysis. Although the clone FISH method provided distinguishable positive and negative results, the fluorescent signals were weak and did not allow assessment of optimal conditions for probe stringency. Therefore, optimization and further validation of the three probes targeting unculturable “Synergistetes” (A_487, A_845, and 3.3_65) were performed with clinical samples; extinction validation was used to infer optimal hybridization conditions by increasing the formamide concentration until there was a significant decrease in the frequency of positive reactions (14), and the three different FISH probes targeting members of unculturable “Synergistetes” cluster A were used simultaneously with single samples to confirm colocalization of positive reactions and therefore probe validity. For hybridization, 10 μl of a bacterial or plaque suspension in PBS was applied to each 6-mm-diameter well on 0.075% gelatin-coated, Shandon multispot microscope slides (Thermo Electron Corporation) and allowed to air dry. Cells were fixed with 30 μl of a 1:1 PBS-100% ethanol solution for 2 h at 4°C, after which the slides were washed twice with PBS. The slides were then dehydrated for 3 min each with 50%, 80%, and 96% ethanol. Hybridization was performed at 50°C for 2 h in the dark with 0.5 μl of each probe (16 μM) in 8 μl hybridization buffer at the optimal formamide concentration determined previously. This was followed by incubation in the dark at 52°C for 15 min in preheated posthybridization wash buffer containing 4.2% (vol/vol) 5 M NaCl, 2% (vol/vol) 1 M Tris-HCl (pH 8.0), 1% (vol/vol) 0.5 M EDTA, and 0.1% (vol/vol) 10% sodium dodecyl sulfate. Twenty-five microliters of Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc.) was applied to each sample after air drying, and the slides were subsequently sealed with a coverslip and varnish.
Microscopic detection of fluorescently labeled cells was performed using (i) an Olympus BH2-RFL fluorescence microscope (Olympus Optical Co.) with blue-green and UV-violet excitation filters and a ×40 objective and (ii) a Leica SP2 confocal laser scanning system (Leica Microsystems) fitted with an argon/argon-krypton laser (operating at 488 nm), a krypton laser (operating at 568 nm), and a helion-neon laser (operating at 633 nm), using a Leica DMIRE2 inverted microscope with a ×100 objective. For each multi-FISH experiment, checks were performed to ensure that the fluorescent emission was not due to “bleed through” as a result of excitation by lower-wavelength emissions of other fluorochromes.
FISH was performed with PBS suspensions of pooled subgingival plaque samples collected from two deep (6 to 10 mm) periodontal pockets from each of nine subjects (ages, 29 to 73 years; five males and four females) with localized or generalized severe periodontitis. In addition to the “Synergistetes”-specific probes (Table 2), the universal bacterial probe EUB338 (5′-GCTGCCTCCCGTAGGAGT-3′) (2) with Cy5 or rhodamine red modifications at the 5′ end and the nonsense probe NON338 (reverse sequence of EUB338) with 5′-fluorescein isocyanate were used. Hybridizations were performed with all nine samples using the following combinations of probes: 3.3_65, A_487, and EUB338; 3.3_65, A_487, and A_845; and J.anth_63, P1_70, and B_155.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the 16S rRNA genes of novel OTUs sequenced in this study are as follows: “Synergistetes” OTU 2 A6A_39, FJ490412; “Synergistetes” OTU 3.1 A2G_10, FJ490413; “Synergistetes” OTU 3.2 A3G_2, FJ490415; “Synergistetes” OTU 4.2 A2F_22, FJ490414; and “Synergistetes” OTU 5 A3G_7, FJ490416.
RESULTS
The five periodontitis cases and five periodontally healthy controls were closely matched for age and gender, and there was no statistically significant difference between the cohorts (Table 3). The cases had chronic periodontitis ranging from localized severe disease to more generalized forms. The median bleeding index for the periodontitis cases was 0.5 and significantly different (P = 0.015) from that for the healthy controls (0.04). The median plaque indices were not significantly different for the two cohorts (0.79 and 0.71, respectively). The mean probing depths for healthy sites from which samples of supra- and subgingival plaque were obtained were also not significantly different (Table 3).
TABLE 3.
Demographic and clinical data for cases and controls
| Characteristic | Periodontitis cases (n = 5) | Healthy controls (n = 5) |
|---|---|---|
| Age (yr)a | ||
| Mean (SD) | 48.20 (10.04) | 47.20 (8.29) |
| Median | 43 | 45 |
| Range | 39-63 | 40-61 |
| No. of males/no. of females | 2/3 | 2/3 |
| Plaque indexb | ||
| Median | 0.79 | 0.71 |
| Range | 0.71-1.00 | 0.54-0.96 |
| Bleeding indexc | ||
| Median | 0.50 | 0.04 |
| Range | 0.17-1.17 | 0.00-0.17 |
| Probing depth for healthy sample sites (mm)d | ||
| Mean (SD) | 2.52 (0.46) | 2.70 (0.41) |
| Range | 2.00-3.00 | 2.00-3.00 |
| Probing depth for “diseased” sample sites (mm) | ||
| Mean (SD) | 6.98 (0.61) | NAe |
| Range | 6.00-9.00 | NA |
The P value for age is 1.000.
The P value for the plaque index is 0.402.
The P value for the bleeding index is 0.015.
The P value for the probing depth for healthy sample sites is 0.663.
NA, not applicable.
The “Synergistetes”-specific PCR primers successfully amplified sequences of the cultivable oral “Synergistetes” species J. anthropi and P. piscolens. J. anthropi E3_33 could be detected at a level of 3.0 × 103 cells, and P. piscolens DSM 21147T and AHN 1662 could be detected at levels of 1.2 × 103 and 8.6 × 102 cells, respectively. PCR products were obtained with these primers for 36 of the 60 samples. Good-quality sequences were obtained from at least 45 of the 48 clones sequenced from each library, resulting in a total of 1,664 16S rRNA gene sequences. The inserts obtained were the expected size, 796 to 797 bp. Sequence analysis confirmed that the 27F/806R primer combination successfully amplified 16S rRNA genes from members of the phylum “Synergistetes” in the mixed-template patient samples. However, sequences of some members of the family Acidaminococcaceae in the phylum Firmicutes, particularly members of the genus Selenomonas, were also amplified. A total of 742 of the 1,209 sequences obtained from cases and 128 of the 455 sequences obtained from controls were found to be “Synergistetes” sequences.
“Synergistetes” were detected in 9 of the 10 subjects; the one exception was a healthy control subject. All habitats except cheek mucosa were positive for “Synergistetes” in at least one subject. Subgingival plaque was the site found to have detectable “Synergistetes” in the highest proportion of subjects (8/10), closely followed by saliva (7/10). A comparison of periodontitis cases and periodontally healthy subjects revealed a greater proportion of the sites per subject positive for “Synergistetes” in the cases (median, six of seven sites) than in the controls (two of five sites). Although the difference was statistically significant (P = 0.045), for the analysis limited to the five sample sites common to both cases and controls the difference was not statistically significant (P = 0.193), indicating that the diseased sites (deep, bleeding pockets) may have been the main source of the microbiological difference between cases and controls. Saliva and the dorsum of tongue were found to harbor “Synergistetes” more frequently in the cases (five and four subjects, respectively) than in the controls (two and one subjects, respectively); however, the differences were not statistically significant.
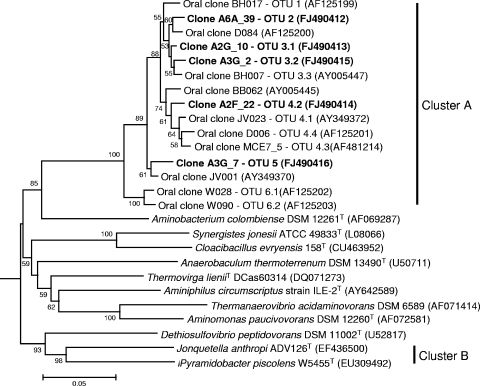
The “Synergistetes” sequences identified fell into 12 OTUs at the 99% sequence identity level in cluster A (Fig. 1). On the basis of the clustering in the phylogenetic tree, the OTUs were placed into six higher-level groups, and groups 3, 4, and 6 were subdivided into three, four, and two subgroups, respectively. OTU 1 included previously described phylotypes BH017 and JV006; OTU 3.3 included phylotype BH007; OTU 4.1 included JV023; OTU 4.3 included “Synergistetes” phylotype MCE7_5; OTU 4.4 included phylotype D006; OTU 6.1 included phylotype W028 and “Synergistetes” phylotype MCE3_120; and OTU 6.2 included phylotype W090. The phylogenetic positions of the five novel OTUs identified in this study are shown in Fig. 1. Each of these OTUs was detected in more than one sample, providing strong evidence that they were genuinely novel and not chimeras.
FIG. 1.
Phylogenetic tree based on 16S rRNA gene sequence comparisons, showing the relationships between novel “Synergistetes” taxa identified in this study and related species. The tree was constructed using the neighbor-joining method following distance analysis of aligned sequences using the Jukes-Cantor correction and was rooted with Treponema socranskii (accession no. AF033307). The novel sequences were around 750 bp long and were compared in a pairwise manner with virtually full-length sequences of reference strains with unaligned bases for each pair deleted. The numbers at the nodes are bootstrap values for the branches based on data for 500 trees. The accession number for the 16S rRNA sequence is given for each strain. The scale bar indicates 0.05 nucleotide substitutions per site.
Within OTUs, there was significant sequence microvariation, even within samples. For example, although the 214 clones belonging to “Synergistetes” OTU 3.3 had less than 1% overall sequence variation, there were 59 different genotypes in the eight subjects positive for this OTU. Subject A7 had 20 “Synergistetes” OTU 3.3 gene sequence types, and in the saliva sample alone there were a number of subclusters with microvariation of up to two base substitutions over 435 bases compared with phylotype BH007 (Fig. 2).
FIG. 2.
Phylogenetic tree based on 16S rRNA gene sequence comparisons, showing the variation among representatives of “Synergistetes” OTU 3.3 in a single sample (saliva from subject A7A). The tree was constructed using the neighbor-joining method following distance analysis of aligned sequences using the Jukes-Cantor correction, was rooted with “Synergistetes” phylotype D084, and was based on 435 unambiguously aligned bases.
Chao 1 and abundance-based coverage estimators provided estimates of OTU richness remarkably similar to the actual number of “Synergistetes” OTUs detected in this investigation (12 OTUs in the periodontitis cohort and 7 OTUs in the periodontally healthy cohort), suggesting that there was adequate coverage of the communities sampled (Table 4). The Chao 1 95% confidence intervals for the two cohorts (Table 4) did not overlap, from which one can surmise that the “Synergistetes” communities in subjects with periodontitis and in the periodontally healthy subjects are significantly different from each other. Statistical comparison of the “Synergistetes” community structures for the two cohorts provided a similarity index value, 0.583 (Jaccard classic coefficient) based on the presence of specific “Synergistetes” OTUs, that was slightly higher than that for the abundance of OTUs (0.294, Bray-Curtis method).
TABLE 4.
Estimates of species richness for subjects with periodontal disease and healthy controls
| Cohort | No. of clones | No. of OTUs | Abundance-based coverage estimator mean | Chao 1 mean (SD) | Chao 1 95% confidence interval |
|---|---|---|---|---|---|
| Subjects with periodontitis | 742 | 12 | 12.75 | 12.00 (0.47) | 11.99-12.00 |
| Healthy controls | 128 | 7 | 7.69 | 7.00 (0.25) | 7.00-7.00 |
“Synergistetes” communities were more OTU rich in periodontitis cases (mean, 7.8 OTUs; range, 7 to 10 OTUs) than in the controls (mean, 3.4 OTUs; range, 0 to 6 OTUs). Whereas the difference was highly statistically significant (P = 0.008), in a comparison limited to the five sites common to both cases and controls (for which the means were 6.4 and 3.4 OTUs, respectively) the difference was found to only “approach” statistical significance (P = 0.055). It appears, therefore, that, like the difference in the prevalence of “Synergistetes,” the difference in “Synergistetes” OTU richness between cases and controls may to a large extent be explained by the data for diseased sites. In addition, however, there was a significant difference between the cohorts in the mean number of “Synergistetes” OTUs in saliva (5.2 OTUs in cases and 1.4 OTUs in controls) and samples from the dorsum of tongue (2.6 OTUs in cases and 0.2 OTU in controls) (P = 0.029 and P = 0.034, respectively). In all subjects, the subgingival plaque sample had the highest number of “Synergistetes” OTUs, and in one case there were 10 OTUs. There was little difference between the cohorts in “Synergistetes” OTU richness in subgingival plaque from healthy sites (the means were 3.4 OTUs in cases and 3.0 OTUs in controls). However, in the subjects with periodontitis, there was a significantly greater number of “Synergistetes” OTUs in subgingival plaque from diseased sites (mean, 6.8 OTUs) than in subgingival plaque from healthy sites (mean, 3.4 OTUs) (P = 0.039). Finally, regression analysis applied to all samples except plaque samples from diseased sites revealed that the bleeding index was a significant explanatory variable (P = 0.008) for the number of “Synergistetes” OTUs present.
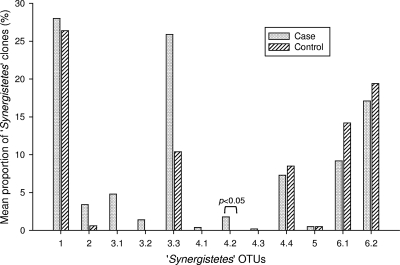
Investigation of specific “Synergistetes” OTUs revealed that “Synergistetes” OTUs 1, 3.3, 6.1, and 6.2 were detected in at least 8 of the 10 subjects, while certain other OTUs (“Synergistetes” OTUs 3.1, 3.2, 4.1, and 4.3) were found in only one subject, indicating that there was some subject specificity (Fig. 3). Furthermore, the relative proportions of “Synergistetes” OTUs in subjects were found to differ considerably. For example, the “Synergistetes” clones of case A5 were dominated by “Synergistetes” OTU 3.3 (64.1%), whereas case A3 harbored an apparently more diverse “Synergistetes” population, including the greatest number of “Synergistetes” OTUs (10 OTUs) with a fairly even distribution. When the prevalence of specific “Synergistetes” OTUs at different sites was examined, it was found that whereas certain OTUs (“Synergistetes” OTUs 1, 3.3, 6.1, and 6.2) colonized most sites tested in each of the synergistetes-positive subjects, “Synergistetes” OTU 4.2 in particular was found almost exclusively in the samples from “diseased” pockets (supra- and subgingival plaque). A comparison of the periodontitis cases and healthy controls revealed that 7 of the 12 “Synergistetes” OTUs were present in both cohorts, while the remaining 5 OTUs were present exclusively in the periodontitis patients (Fig. 3). Furthermore, novel “Synergistetes” OTU 4.2, which was found in four of the five cases and none of the controls, was significantly associated with disease (P = 0.048). When relative abundance was examined, a comparison of the mean values for the percentage of clones belonging to each “Synergistetes” OTU expressed as a proportion of the total number of “Synergistetes” clones (Fig. 4) revealed a statistically significant difference between cases and controls only for “Synergistetes” OTU 4.2 (P = 0.018). Since it is a reasonable assumption that the primary habitat of a putative periodontal “pathogen” is plaque at sites of disease and it was noted that “Synergistetes” OTU 4.2 was found almost exclusively in such habitats, a comparison of the proportions of clones belonging to each OTU was made between cases and controls, particularly for subgingival plaque samples (Fig. 5). This three-way comparison (Kruskall-Wallis test) gave statistically significant results (P = 0.007) for “Synergistetes” OTU 4.2, for which the proportion of clones in subgingival plaque obtained from diseased sites in periodontitis cases was significantly greater than the proportion of clones in subgingival samples obtained either from healthy sites in the same patients (P = 0.019) or from healthy sites in the control subjects (P = 0.019). OTUs were also defined at a less stringent level, 98% sequence identity, which resulted in a reduction in the number of groups to six, involving merging of OTUs 2, 3.1, 3.2, and 3.3; OTUs 4.1 and 4.2; OTUs 4.3 and 4.4; and OTUs 6.1 and 6.2. For the six groups, statistically significant differences between the two cohorts were found only for OTUs 4.1 and 4.2 combined, a significantly greater number of which was present in periodontitis cases than in healthy controls (P = 0.048) and which comprised a significantly larger proportion of the clones in cases (P = 0.018) and a significantly greater percentage of the clones from subgingival plaque from deep periodontal pockets in cases than from subgingival plaque from shallow sites in cases (P = 0.019) or controls (P = 0.019).
FIG. 3.
Detection of 12 “Synergistetes” OTUs in cases and controls.
FIG. 4.
Percentage of clones representing each “Synergistetes” OTU as a proportion of the total “Synergistetes” clones from all sites for cases and controls.
FIG. 5.
Percentage of clones representing each “Synergistetes” OTU in subgingival plaque samples from cases and controls.
All six of the FISH probes designed in this study were sensitive to their designated targets. Low-level nonspecific hybridization was detected between the A_487 probe and the species Aggregatibacter actinomycetemcomitans, Prevotella oralis, and Prevotella baroniae when hybridization was performed using buffer with less than 20% formamide. There was also weak hybridization between the 3.3_65 probe and the clone belonging to “Synergistetes” OTU 6.1 in hybridization buffer not containing any formamide. The optimal formamide concentrations shown in Table 2 represent the conditions under which each probe was both specific and sensitive. A formamide concentration of 20% was the lowest formamide concentration at which all probes collectively were reliable; therefore, multi-FISH reactions using a combination of probes simultaneously were performed with this concentration.
The validity of probes B_155, J.anth_63, and P1_70 was confirmed by hybridization to a mixed sample containing J. anthropi and P. piscolens; the cluster B probe bound all bacteria present, whereas the species-specific probes each labeled only their target cells. There was colocalization of fluorescent signals when plaque samples were probed with A_487 and A_845 together, showing that these probes correctly targeted the same organisms. Probe 3.3_65 labeled a subset of the bacteria labeled with both of the cluster A probes. Figure 6 shows the colocalization of probes 3.3_65 and A_487 in the same target cells in subgingival plaque. Furthermore, additional cells were labeled with probe A_487 (Fig. 6b and 6c), indicating the presence of other cluster A “Synergistetes,” as well as “Synergistetes” OTU 3.3.
FIG. 6.

Confocal FISH micrographs of subgingival plaque showing (a) “Synergistetes” OTU 3.3 (green) (probe 3.3_65 and Alexa Fluor 488), (b) cluster A “Synergistetes” (red) (probe A_487 and Cy3), and (c) an overlay for “Synergistetes” OTU 3.3 (yellow), cluster A “Synergistetes” (yellow and pink), and total bacteria (blue) (probe EUB338 and Cy5).
FISH analysis of the subgingival plaque samples did not detect any cluster B “Synergistetes.” However, cluster A “Synergistetes” were present in the deep-pocket plaque samples collected from all nine subjects. Counts of cells staining positive with A_487 and EUB338 were determined for five of the subjects' samples, and the results showed that the cluster A “Synergistetes” counts were between 3.2 and 11.3% (mean, 7.6%) of the total counts. Figure 7 shows the result of probing plaque from sample F4 with probe 3.3_65 (Fig. 7a) and an overlay image of the same sample also probed with the universal probe EUB338 (Fig. 7b), demonstrating the relative abundance of these as-yet-unculturable “Synergistetes” bacteria. The cell morphology of cluster A “Synergistetes” was found to be curved bacilli that are 3 to 7 μm long and 1 to 1.5 μm wide (Fig. 6).
FIG. 7.
FISH images of subgingival plaque showing (a) “Synergistetes” OTU 3.3 (green) (probe 3.3_65 and Cy3) and (b) an overlay of “Synergistetes” OTU 3.3 (yellow or green) with total bacteria (red) (probe EUB338 and Cy5).
DISCUSSION
The work described in this paper confirmed that members of the candidate phylum “Synergistetes” are part of the human oral microbiota. 16S rRNA gene sequences representing 12 “Synergistetes” OTUs (5 of which were novel) were detected in this study, revealing that there is an OTU-rich “Synergistetes” population in the human oral cavity. It was noteworthy that all phylotypes detected in the current study were members of “Synergistetes” cluster A comprising the as-yet-unculturable “Synergistetes,” and neither of the two cultivable oral “Synergistetes” taxa (cluster B) was detected. A possible explanation for this, that 16S rRNA genes of cluster B phylotypes were not amplified by the primer set used, can be excluded in light of the facts that these taxa (i) were used successfully in this study as the positive controls for PCR and (ii) have been identified by molecular culture-independent methods in plaque and root canal samples (20, 31). Therefore, a more likely explanation is absence or low relative abundance of cluster B “Synergistetes” in the clinical samples.
Indeed, the 806R primer designed for this study successfully targeted a range of “Synergistetes” OTUs from clinical samples. However, there was some cross-reaction with some members of the family Acidaminococcaceae in the phylum Firmicutes, which have a similar region in the 16S rRNA gene, but the region has four to six base mismatches with the 806R primer. Experimentation with primer annealing temperatures higher than 56°C (to reduce the tolerance of the primers) significantly reduced the amount of amplicon and was therefore not pursued further. There is some evidence to suggest that if the target of a specific primer is present only at low levels or is absent from a sample, then the primer more readily selects other taxa which are less stringently matched (37). Accordingly, the larger proportion of “Synergistetes” sequences (of the total number of sequences) in subjects with periodontitis (61.4%) than in healthy controls (28.1%) may perhaps imply that there was a greater abundance of “Synergistetes” in the cases.
Sequence analysis revealed marked microvariation among individual cloned sequences. It has been suggested that PCR microvariation artifacts are not uncommon for mixed templates of closely related 16S rRNA gene sequences (39); however, without comparison with a control group involving a mixed template with a broader range, whether the composition of a PCR template plays a significant role remains unknown. Although sequence microvariation may also be the result of PCR errors attributable to the use of a nonproofreading polymerase, the absence of chimeras and the generation of identical sequences representing novel taxa from multiple samples and subjects make this unlikely. The phylogenetic trees with highly branched topology and relatively low bootstrap values and the presence of significant sequence microvariation suggest that recombination events are common for this group of bacteria, which may be indicative of natural competence. Furthermore, in a genome survey of P. piscolens, it was found that some P. piscolens genes were highly similar to genes in Fusobacterium nucleatum and Treponema denticola, two species which are members of distinct phyla but which frequently inhabit subgingival plaque, and that genes encoding mobile genetic elements were present (8). Horizontal gene transfer may be common among bacterial inhabitants of dental plaque, perhaps due to their close proximity (25, 26).
In agreement with the sequencing results, FISH revealed that the cluster A (but not cluster B) “Synergistetes” were highly prevalent in subgingival plaque. This confirms that DNA sequences representing these “unculturable” bacteria originate from intact whole cells present in dental plaque. The cells of cluster A “Synergistetes” were visualized for the first time and were found to be large curved bacilli. Large curved bacilli have been observed frequently in subgingival plaque from subjects with periodontitis viewed by dark-field microscopy (23), but no cultivable examples have been described. It is possible that these unidentified bacteria belong to “unculturable” cluster A “Synergistetes.” The “Synergistetes” species that have been cultured to date are straight or curved gram-negative bacilli, and some of them are motile (43). One particular “Synergistetes” taxon has been shown to occur in a symbiotic relationship with the protist Caduceia versatilis in the gut of termites, where the flagella of the motile “Synergistetes” bacterium propel the protozoan (12, 41). In the absence of any culturable representatives of cluster A “Synergistetes,” it has not been confirmed that these bacteria also are motile, although this is probable based on the morphology of the cells.
The FISH abundance estimates for cluster A “Synergistetes” in subgingival plaque from deep periodontal pockets were between 3 and 11% of the total bacteria hybridized with the EUB338 probe; this figure is higher than the 1.4% of the total clones from deep-pocket plaque previously reported to be cluster A “Synergistetes” (19). Although both methods have inherent biases and the “Synergistetes” proportions in the present FISH study may be slight overestimates due to the difficulty of accurately determining numbers of total bacteria where they are closely apposed, it is clear that the cluster A “Synergistetes,” which were prevalent and abundant in the plaque from all nine subjects in this study, are prominent members of the subgingival microbiota.
“Synergistetes” were detected in all of the intraoral habitats investigated with the exception of cheek mucosa; thus, bacteria belonging to this group may colonize supra- and subgingival plaque, saliva, and the dorsum of the tongue. Although the time of onset and the duration of disease in the subjects with chronic periodontitis were unknown, the collection of plaque samples from different subjects at sites with comparable clinical probing depths and bleeding status reduced the potential for heterogeneity. Furthermore, plaque samples from four sites per subject were pooled in an effort to render the results representative of each plaque category. Subgingival plaque (particularly that taken from deep periodontal pockets) was the habitat most frequently colonized and contained the greatest range of OTUs, making it a possible source of the “Synergistetes” found at other sites in the oral cavity. Furthermore, the number of saliva and tongue dorsum samples positive for “Synergistetes” was greater for the cases than for the periodontally healthy controls, and the samples from the cases were statistically significantly more OTU rich. There is evidence of dissemination of periodontitis-associated bacteria into saliva and onto the tongue following mechanical periodontal therapy, implying that the periodontal pocket is the source of putative periodontal pathogens in the oral cavity (3).
Despite comparable plaque indices in subjects with disease and healthy controls, a greater proportion of sites were colonized with “Synergistetes” and a more OTU-rich “Synergistetes” population was present in periodontitis cases than in controls, and the difference between the cohorts was due mainly to the plaque taken from “diseased” sites in the cases. Thus, the “Synergistetes” phylum of bacteria appears to have a stronger association with periodontitis than with health. This is in agreement with previous reports of “Synergistetes” detected in humans, which have found that these bacteria are related to sites of disease, including dental sites (7, 13, 27, 29, 36) and sites involving the foot, scalp, and blood (13). Furthermore, the “Synergistetes” species characterized to date have been found to be proteolytic and strictly anaerobic, a profile matched by the typical periodontal “pathogen.” It is interesting that irrespective of periodontal disease or health categorization, the overall gingival bleeding status of the subjects in this study had a significant impact on the “diversity” of “Synergistetes” at other oral sites; perhaps “Synergistetes,” like Porphyromonas gingivalis belonging to the “red cluster” of potentially periodontopathic bacteria (38), are metabolically adapted to prefer an environment where there is inflammation and bleeding.
Naturally, it is possible that some bacteria in any particular phylum are harmless commensals, whereas others (whether endogenous or exogenous) may possess particular virulence mechanisms predisposing them to pathogenesis in a susceptible host. Therefore, it is more useful to investigate an association with disease or health for individual species or clusters of species in a phylum rather than for the phylum as a whole. The results revealed that certain “Synergistetes” OTUs, “Synergistetes” OTU 1 (including phylotypes BH017 and JV006), “Synergistetes” OTU 3.3 (including phylotype BH007), “Synergistetes” OTU 6.1 (including phylotypes W028 and MCE3_120), and “Synergistetes” OTU 6.2 (including phylotype W090), were present in the majority of both cases and controls, at most of the intraoral sites investigated and at high “relative abundances” in both cohorts (where the relative proportions of sequences representing each “Synergistetes” OTU were used to indicate the relative abundance of the OTU in the clinical sample). These OTUs are likely to be oral commensals. It has been reported that phylotype BH017 was not associated with periodontitis (19); however, other studies (18, 29) found that this phylotype and other phylotypes mentioned above have a strong association with disease. The possible reasons for the conflicting results are (i) designation of “disease association” based on prevalence alone (29), as opposed to a combination of prevalence and relative abundance, and (ii) utilization of a single primer pair (for nested PCR) targeting both “Synergistetes” phylotypes D084 and BH017 (18), although these two phylotypes fell into distinct OTUs in the current study. The prevalence and abundance of the novel taxon “Synergistetes” OTU 4.2, which was found almost exclusively in plaque from “diseased” sites, were significantly higher in periodontitis cases than in healthy controls, indicating an association with disease rather than with health. OTUs in this study were defined using a 99% sequence identity threshold, as recommended on the basis of correlation with DNA-DNA reassociation values (40). When the 98% sequence identity threshold was used to define taxa, the enlarged group which included OTU 4.2 was also significantly associated with periodontitis, providing further support that this OTU represents a disease marker for periodontitis and may potentially play a pathogenic role in the disease.
In conclusion, there is a diverse population of cluster A “Synergistetes” which appears to make up a significant part of the oral microbiota. There is still little information on these as-yet-uncultivated bacteria besides their cell morphology and the implication that they are subject to recombination events. One “Synergistetes” OTU has been identified as a disease marker for periodontitis. It would be of value to attempt to isolate this novel species and ultimately to begin the search for possible virulence mechanisms possessed by this putative periodontal pathogen.
Acknowledgments
This study was supported by King's College London Dental Institute. We acknowledge financial support from the Department of Health via a National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St. Thomas' NHS Foundation Trust in partnership with King's College London.
Ron Wilson is thanked for assistance with the statistical analysis.
Footnotes
Published ahead of print on 3 April 2009.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beikler, T., G. Abdeen, S. Schnitzer, S. Salzer, B. Ehmke, A. Heinecke, and T. F. Flemmig. 2004. Microbiological shifts in intra- and extraoral habitats following mechanical periodontal therapy. J. Clin. Periodontol. 31:777-783. [DOI] [PubMed] [Google Scholar]
- 4.Brosius, J., M. L. Palmer, P. J. Kennedy, and H. F. Noller. 1978. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc. Natl. Acad. Sci. USA 75:4801-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, J. R., B. Chai, R. J. Farris, Q. Wang, A. S. Kulam-Syed-Mohideen, D. M. McGarrell, A. M. Bandela, E. Cardenas, G. M. Garrity, and J. M. Tiedje. 2007. The ribosomal database project (RDP-II): introducing myRDP space and quality controlled public data. Nucleic Acids Res. 35:D169-D172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colwell, R. K. 2005. EstimateS: statistical estimation of species richness and shared species from samples. Version 7.5. http://purl.oclc.org/estimates.
- 7.de Lillo, A., F. P. Ashley, R. M. Palmer, M. A. Munson, L. Kyriacou, A. J. Weightman, and W. G. Wade. 2006. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol. Immunol. 21:61-68. [DOI] [PubMed] [Google Scholar]
- 8.Downes, J., S. R. Vartoukian, F. E. Dewhirst, J. Izard, T. Chen, W.-H. Yu, I. C. Sutcliffe, and W. G. Wade. 2009. Pyramidobacter piscolens gen. nov., sp. nov., a member of the phylum “Synergistetes” isolated from the human oral cavity. Int. J. Syst. Evol. Microbiol. 59:972-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs, B. M., G. Wallner, W. Beisker, I. Schwippl, W. Ludwig, and R. Amann. 1998. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 64:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godon, J. J., J. Moriniere, M. Moletta, M. Gaillac, V. Bru, and J. P. Delgenes. 2005. Rarity associated with specific ecological niches in the bacterial world: the “Synergistes” example. Environ. Microbiol. 7:213-224. [DOI] [PubMed] [Google Scholar]
- 11.Holdeman, L. V. H., E. P. Cato, and W. E. C. Moore. 1977. Anaerobe laboratory manual, 4th ed. Virginia Polytechnic Institute and State University, Blacksburg.
- 12.Hongoh, Y., T. Sato, M. F. Dolan, S. Noda, S. Ui, T. Kudo, and M. Ohkuma. 2007. The motility symbiont of the termite gut flagellate Caduceia versatilis is a member of the “Synergistes” group. Appl. Environ. Microbiol. 73:6270-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horz, H. P., D. M. Citron, Y. A. Warren, E. J. Goldstein, and G. Conrads. 2006. Synergistes group organisms of human origin. J. Clin. Microbiol. 44:2914-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugenholtz, P., G. W. Tyson, R. I. Webb, A. M. Wagner, and L. L. Blackall. 2001. Investigation of candidate division TM7, a recently recognized major lineage of the domain Bacteria with no known pure-culture representatives. Appl. Environ. Microbiol. 67:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutter, G., U. Schlagenhauf, G. Valenza, M. Horn, S. Burgemeister, H. Claus, and U. Vogel. 2003. Molecular analysis of bacteria in periodontitis: evaluation of clone libraries, novel phylotypes and putative pathogens. Microbiology 149:67-75. [DOI] [PubMed] [Google Scholar]
- 16.Jumas-Bilak, E., J. P. Carlier, H. Jean-Pierre, D. Citron, K. Bernard, A. Damay, B. Gay, C. Teyssier, J. Campos, and H. Marchandin. 2007. Jonquetella anthropi gen. nov., sp. nov., the first member of the candidate phylum “Synergistetes” isolated from man. Int. J. Syst. Evol. Microbiol. 57:2743-2748. [DOI] [PubMed] [Google Scholar]
- 17.Kornman, K. S. 2008. Mapping the pathogenesis of periodontitis: a new look. J. Periodontol. 79:1560-1568. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, P. S., A. L. Griffen, J. A. Barton, B. J. Paster, M. L. Moeschberger, and E. J. Leys. 2003. New bacterial species associated with chronic periodontitis. J. Dent. Res. 82:338-344. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, P. S., A. L. Griffen, M. L. Moeschberger, and E. J. Leys. 2005. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J. Clin. Microbiol. 43:3944-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar, P. S., E. J. Leys, J. M. Bryk, F. J. Martinez, M. L. Moeschberger, and A. L. Griffen. 2006. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J. Clin. Microbiol. 44:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, United Kingdom.
- 23.Listgarten, M. A., and L. Hellden. 1978. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J. Clin. Periodontol. 5:115-132. [DOI] [PubMed] [Google Scholar]
- 24.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, R. J. Farris, G. M. Garrity, G. J. Olsen, T. M. Schmidt, and J. M. Tiedje. 2001. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcy, Y., C. Ouverney, E. M. Bik, T. Losekann, N. Ivanova, H. G. Martin, E. Szeto, D. Platt, P. Hugenholtz, D. A. Relman, and S. R. Quake. 2007. Dissecting biological “dark matter” with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc. Natl. Acad. Sci. USA 104:11889-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mira, A., R. Pushker, B. A. Legault, D. Moreira, and F. Rodriguez-Valera. 2004. Evolutionary relationships of Fusobacterium nucleatum based on phylogenetic analysis and comparative genomics. BMC Evol. Biol. 4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munson, M. A., A. Banerjee, T. F. Watson, and W. G. Wade. 2004. Molecular analysis of the microflora associated with dental caries. J. Clin. Microbiol. 42:3023-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munson, M. A., T. Pitt Ford, B. Chong, A. J. Weightman, and W. G. Wade. 2002. Molecular and cultural analysis of the microflora associated with endodontic infections. J. Dent. Res. 81:761-766. [DOI] [PubMed] [Google Scholar]
- 29.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preza, D., I. Olsen, J. A. Aas, T. Willumsen, B. Grinde, and B. J. Paster. 2008. Bacterial profiles of root caries in elderly patients. J. Clin. Microbiol. 46:2015-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rocas, I. N., and J. F. Siqueira, Jr. 2005. Detection of novel oral species and phylotypes in symptomatic endodontic infections including abscesses. FEMS Microbiol. Lett. 250:279-285. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto, M., J. F. Siqueira, Jr., I. N. Rocas, and Y. Benno. 2008. Molecular analysis of the root canal microbiota associated with endodontic treatment failures. Oral Microbiol. Immunol. 23:275-281. [DOI] [PubMed] [Google Scholar]
- 33.Schramm, A., B. M. Fuchs, J. L. Nielsen, M. Tonolla, and D. A. Stahl. 2002. Fluorescence in situ hybridization of 16S rRNA gene clones (clone-FISH) for probe validation and screening of clone libraries. Environ. Microbiol. 4:713-720. [DOI] [PubMed] [Google Scholar]
- 34.Silness, J., and H. Loe. 1964. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 22:121-135. [DOI] [PubMed] [Google Scholar]
- 35.Silness, J., and T. Roynstrand. 1988. Partial mouth recording of plaque, gingivitis and probing depth in adolescents. J. Clin. Periodontol. 15:189-192. [DOI] [PubMed] [Google Scholar]
- 36.Siqueira, J. F., Jr., and I. N. Rocas. 2005. Uncultivated phylotypes and newly named species associated with primary and persistent endodontic infections. J. Clin. Microbiol. 43:3314-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siqueira, J. F., Jr., I. N. Rocas, C. D. Cunha, and A. S. Rosado. 2005. Novel bacterial phylotypes in endodontic infections. J. Dent. Res. 84:565-569. [DOI] [PubMed] [Google Scholar]
- 38.Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent, Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]
- 39.Speksnijder, A. G., G. A. Kowalchuk, S. De Jong, E. Kline, J. R. Stephen, and H. J. Laanbroek. 2001. Microvariation artifacts introduced by PCR and cloning of closely related 16S rRNA gene sequences. Appl. Environ. Microbiol. 67:469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stackebrandt, E., and J. Ebers. 2006. Taxonomic parameters revisited: tarnished gold standards. Microbiol. Today 8:152-155. [Google Scholar]
- 41.Tamm, S. L. 1982. Flagellated ectosymbiotic bacteria propel a eucaryotic cell. J. Cell Biol. 94:697-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vartoukian, S. R., R. M. Palmer, and W. G. Wade. 2007. The division “Synergistes.” Anaerobe 13:99-106. [DOI] [PubMed] [Google Scholar]
- 44.Vianna, M. E., G. Conrads, B. P. Gomes, and H. P. Horz. 2007. Quantification and characterization of Synergistes in endodontic infections. Oral Microbiol. Immunol. 22:260-265. [DOI] [PubMed] [Google Scholar]