Abstract
Previous studies have documented the capacity of European earthworms belonging to the family Lumbricidae to emit the greenhouse gas nitrous oxide (N2O), an activity attributed primarily to the activation of ingested soil denitrifiers. To extend the information base to earthworms in the Southern Hemisphere, four species of earthworms in New Zealand were examined for gut-associated denitrification. Lumbricus rubellus and Aporrectodea rosea (introduced species of Lumbricidae) emitted N2O, whereas emission of N2O by Octolasion cyaneum (an introduced species of Lumbricidae) and emission of N2O by Octochaetus multiporus (a native species of Megascolecidae) were variable and negligible, respectively. Exposing earthworms to nitrite or nitrate and acetylene significantly increased the amount of N2O emitted, implicating denitrification as the primary source of N2O and indicating that earthworms emitted dinitrogen (N2) in addition to N2O. The alimentary canal displayed a high capacity to produce N2O when it was supplemented with nitrite, and alimentary canal contents contained large amounts of carbohydrates and organic acids indicative of fermentation (e.g., succinate, acetate, and formate) that could serve as sources of reductant for denitrification. nosZ encodes a portion of the terminal oxidoreductase used in denitrification. The nosZ sequences detected in the alimentary canals of L. rubellus and O. multiporus were similar to those retrieved from soil and were distantly related to sequences of uncultured soil bacteria and genera common in soils (i.e., Bradyrhizobium, Azospirillum, Rhodopseudomonas, Rhodospirillum, Pseudomonas, Oligotropha, and Sinorhizobium). These findings (i) suggest that the capacity to emit N2O and N2 is a general trait of earthworms and not geographically restricted, (ii) indicate that species belonging to different earthworm families (i.e., Megascolecidae and Lumbricidae) may not have equal capacities to emit N2O, and (iii) also corroborate previous findings that link this capacity to denitrification in the alimentary canal.
Earthworms are dominant members of the soil fauna and affect the structure and fertility of soils (5, 20, 22, 23). Various species of European earthworms belonging to the family Lumbricidae (e.g., Aporrectodea caliginosa, Lumbricus rubellus, and Octolasion lacteum) emit dinitrogen (N2) and the greenhouse gas nitrous oxide (N2O), and their burrowing activities and feeding habits in combination with in situ conditions can influence the emission of nitrogenous gases from soils that they inhabit (1, 2, 13, 17, 25, 27, 39).
The microbiology of the earthworm alimentary canal has been addressed in numerous studies (3, 4, 6, 9, 14, 16, 32). The alimentary canal of the earthworm is anoxic, in marked contrast to the aerated material that earthworms ingest (14, 39). Anoxia and other in situ conditions of the alimentary canal appear to stimulate soil microbes capable of surviving under anaerobic conditions during passage through the gut (3, 4). Soils are rich in denitrifying bacteria (37), and the capacity of European earthworms to emit nitrogenous gases has been attributed primarily to the in situ activity of ingested denitrifying bacteria that appear to be highly active under the anoxic conditions of the earthworm alimentary canal (12, 15, 17, 25, 39). However, it is not known if the capacity to emit nitrogenous gases is a general trait of earthworms independent of their taxonomic family or geographic location. The main objectives of this study were to examine the capacity of Southern Hemisphere earthworms in New Zealand to emit N2O and to determine if this capacity was linked to denitrifying bacteria in the alimentary canal.
MATERIALS AND METHODS
Field sites and collection of earthworms.
Earthworms were collected in late August and early September 2008 in Palmerston North, New Zealand. Octochaetus multiporus (Megascolecidae; native to New Zealand [21, 34]) and Octolasion cyaneum (Lumbricidae; an introduced species [21, 33]) were collected from the upper 30 cm of a native forest soil (40°22′57″S, 175°37′07″E), and Aporrectodea rosea and Lumbricus rubellus (both Lumbricidae; introduced species [21, 33]) were collected from the upper 5 cm of a pasture soil (40°23′27″S, 175°37′20″E). Properties of the soils are summarized in Table 1. The forest soil was dominated by O. multiporus; i.e., the ratio of detected individuals of O. multiporus to detected individuals of O. cyaneum was approximately 20. In contrast, the pasture soil contained similar numbers of A. rosea and L. rubellus worms. Earthworms were stored in the dark at 4°C in containers filled with soil from the site from which they were collected. Earthworms were identified by using standard protocols and stored for a maximum of 1 week before they were used (21, 30). Nucleic acids were extracted from alimentary canal contents of freshly collected worms.
TABLE 1.
Properties of soilsa
| Soil | pH | Moisture content (%) | NH4+ concn (mg liter [water content]−1) | NO3− concn (mg liter [water content]−1) | NO2− concn (mg liter [water content]−1) | Total carbon concn (mg g [dry wt]−1) | Total organic carbon concn (mg g [dry wt]−1) | Total nitrogen concn (mg g [dry wt]−1) |
|---|---|---|---|---|---|---|---|---|
| Pasture | 5.8 ± 0.1 | 26 ± 1 | 0.2 ± 0 | 1.1 ± 0.7 | NDb | 24 ± 1 | 23 ± 1 | 2 ± 0 |
| Forest | 6.7 ± 0.1 | 33 ± 2 | 0.2 ± 0 | 4.6 ± 0.4 | ND | 38 ± 7 | 37 ± 7 | 4 ± 0 |
The values are means ± standard deviations for triplicate analyses.
ND, not detected.
Emission of N2O by earthworms and alimentary canals.
Earthworms were washed with sterile water, dried with tissue paper, weighed, and placed in sterile gas-tight serum vials. In vivo emissions were assessed under an air atmosphere. Alimentary canals (i.e., crop to hindgut) were dissected out of washed earthworms that had been sacrificed by brief immersion in 70°C water. One or two alimentary canals were placed in gas-tight serum vials that were subsequently flushed with 100% argon. Earthworms and alimentary canals were incubated at room temperature (ca. 20°C) in the dark. Living worms and alimentary canals were wetted with small amounts (ca. 0.4 ml) of 2 mM sodium nitrite or 2 mM sodium nitrate, as described previously (25). Acetylene at a concentration of 15% (vol/vol) was provided in the gas phase of vials (40).
Extraction of soil and alimentary canal contents.
Earthworms were washed, sacrificed by brief immersion in 70°C water, and dissected under oxic conditions (16, 17). The alimentary canal contents of four to nine specimens were pooled to obtain approximately 500 mg (fresh weight) per sample. Soil and alimentary canal contents were extracted as previously described (39). Valeric acid was added as an internal standard (19). Solid matter was not removed for determination of the total nitrogen, total carbon, and organic carbon contents. Supernatant fluids were used for determination of the soluble organic compound, ammonium, nitrate, and nitrite contents.
Analytical techniques.
The moisture content was determined by weighing soil and alimentary canal contents before and after drying at 60°C for 72 h. Oven-dried solid matter was ground with a ball mill (MM2; Retsch, Haan, Germany) and analyzed to determine the total nitrogen content and the total carbon content with an NC analyzer (Flash EA 1112; CE Instruments, Wigan, United Kingdom). The inorganic carbon content was estimated using the amount of carbon lost by treatment with 8% HCl overnight followed by drying at 80°C for 1 to 2 h; the organic carbon content was defined as the difference between the total and inorganic carbon contents. Ammonium, nitrate, and nitrite contents were measured with a Lachat QuikChem 8000 flow injection analyzer (Lachat Instruments, Loveland, CO). The N2O content was determined with a 2010 gas chromatograph (Shimadzu, Columbia, MD) equipped with an electron capture detector and a 222XL autosampler (Gilson, Middleton, WI) (11). Organic compounds in alimentary canal contents and soil were analyzed with an LC10Ai high-performance liquid chromatograph equipped with an RID10A detector (Shimadzu, Kyoto, Japan) and a Resex 8 μ 8% H organic acid column (00H-0138-KO; Phenomenex, Torrance, CA) maintained at 45°C; the mobile phase was 5 mM H2SO4 at a flow rate of 0.8 ml per min (7). The detection limits for saccharides and organic acids were approximately 0.006 and 0.04 mM, respectively.
nosZ amplification and analysis.
Earthworms (25 and 3 specimens of L. rubellus and O. multiporus, respectively) were washed, sacrificed by brief immersion in 70% ethanol, and dissected under oxic conditions (16, 17). Crop-gizzard or hindgut sections (denitrification is very active in these sections of the alimentary canal [39]) from several worms were pooled to obtain approximately 0.5 g (fresh weight) per type of section. RNA and DNA were coextracted from soil or alimentary canal sections by bead beating lysis, organic solvent extraction, and precipitation (10). PCR amplification of nosZ (a structural gene for N2O reductase, the terminal oxidoreductase in denitrification) was performed with primers nosZ-F-1181 and nosZ-R-1880 (26) using a T-Gradient cycler (Biometra, Göttingen, Germany) and Mastermix (5 Prime, Hamburg, Germany) (12). The PCR conditions were 40 cycles of 94°C for 30 s, 58 to 52°C for 60 s, and 72°C for 60 s. Touchdown PCR was performed using an initial annealing temperature of 58°C, which was decreased 0.5°C per cycle for the first 10 cycles until the final annealing temperature, 52°C, was reached. The final elongation was at 72°C for 10 min. The PCR products for crop-gizzard and hindgut sections were pooled to obtain equal concentrations, ligated into pGEM-T vector plasmids (Promega, Mannheim, Germany), and transformed into competent cells of Escherichia coli JM109 (Promega, Mannheim, Germany) using the protocol described in the manufacturer's instructions. Clones with the correct insert were selected for sequencing at Macrogen (Seoul, South Korea). Analysis of nosZ sequences (700 bp) was performed with MEGA 4.0 (18) and BLAST (http://blast.ncbi.nlm.nih.gov/). DOTUR-1.53 was used to define operational taxonomic units (OTUs) (31). A threshold level of similarity of 14% (nosZ sequence) was used to define OTUs. The threshold dissimilarity value, 14%, was chosen because comparative sequence analyses of nosZ and 16S rRNA genes indicated that it corresponds with 90% probability to a level of 16S rRNA sequence similarity of ≥97% (K. Palmer, H. L. Drake, and M. A. Horn, unpublished data), which is a conservative estimate for species-level differentiation (35, 36). Coverage was calculated as described previously (12).
A phylogenetic tree was based on amino acid sequences and was constructed using the neighbor-joining method (28), and the analysis included a bootstrap test with 10,000 replicates (8). All positions with alignment gaps and missing data were eliminated only in pairwise sequence comparisons (pairwise deletion option).
Nucleotide sequence accession numbers.
The sequences obtained in this study have been deposited in the EMBL nucleotide sequence database under accession numbers FM993316 to FM993330, FM993332 to FM993353, FM993355 to FM993383, FM993385, FM993387 to FM993403, FM993405 to FM993411, FM993413 to FM993420, FN295833 to FN295863, FN295865 to FN295945, and FN295947 to FN295951.
RESULTS
In vivo emission of N2O by earthworms.
L. rubellus and A. rosea (introduced species [21, 33]) produced 0.29 ± 0.31 and 0.22 ± 0.16 nmol N2O per g (fresh weight) worms, respectively, after 6 h of incubation under air (the values are the means for four worms per species; each worm was positive for emission of N2O). Only one of four individuals of O. cyaneum (an introduced species [21, 33]) emitted N2O; this worm produced 3.74 nmol N2O per g (fresh weight) during the 6 h of incubation. The values obtained for L. rubellus, A. rosea, and O. cyaneum were similar to or slightly less than those obtained for earthworms collected in Europe (17, 25). The native species O. multiporus (21, 34) displayed essentially no capacity to emit N2O (n = 12). These results demonstrated that earthworms collected in New Zealand emitted N2O but that their capacity to do so was variable.
In vivo emission of N2O by earthworms exposed to nitrite.
Nitrite, a precursor of nitrogenous gases formed by denitrifiers (37, 41), greatly stimulates the emission of N2O by European earthworms (25). The amount of N2O emitted by earthworms increased with time when earthworms were exposed to (i.e., wetted with) 2 mM sodium nitrite (Fig. 1). At the end of 6 h of incubation, the amounts of N2O emitted by A. rosea, L. rubellus, and O. multiporus exposed to nitrite were approximately 110, 54, and 6 nmol N2O per g (fresh weight) worm, respectively, which were significantly greater than the amounts obtained without exposure to nitrite (see above). The values for A. rosea and L. rubellus were similar to the values obtained for nitrite-exposed L. rubellus samples in Europe (25). The fresh weights of specimens of O. multiporus were approximately 10-fold greater than the fresh weights (maximal weight, 21 g) of the specimens of the other species. Thus, worms exposed to nitrite emitted similar amounts of N2O on a per worm basis.
FIG. 1.
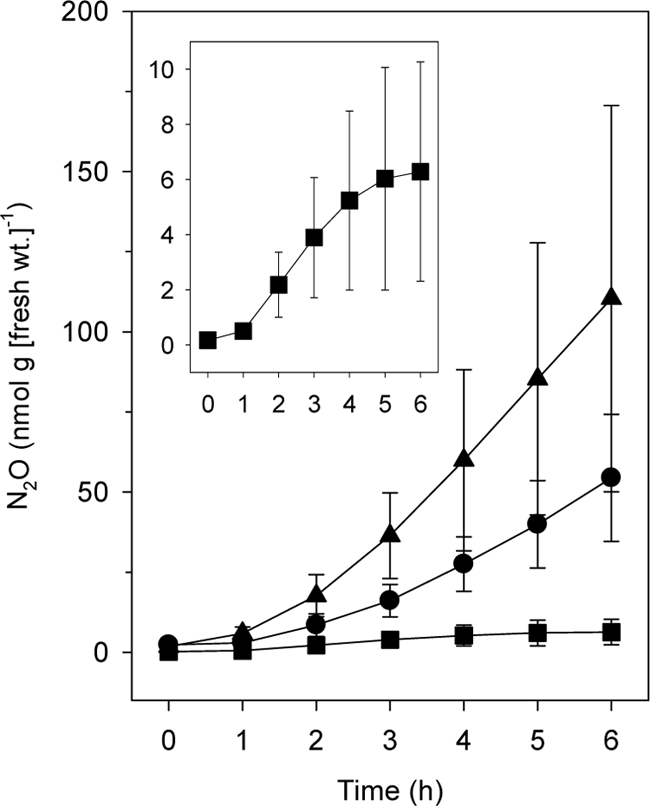
In vivo emission of N2O by New Zealand earthworms wetted with sodium nitrite (2 mM). The symbols indicate the means for triplicate determinations; the error bars indicate standard deviations. •, L. rubellus; ▴, A. rosea; ▪, O. multiporus. The inset shows the emission of N2O by O. multiporus with an expanded scale (the units for the axes are the same as those in the large graph).
These results demonstrated that O. multiporus had the capacity to emit N2O even though emission was not apparent in the absence of nitrite. However, the time-dependent increase in nitrite-stimulated emission of N2O by O. multiporus was not as prolonged as that for L. rubellus or A. rosea.
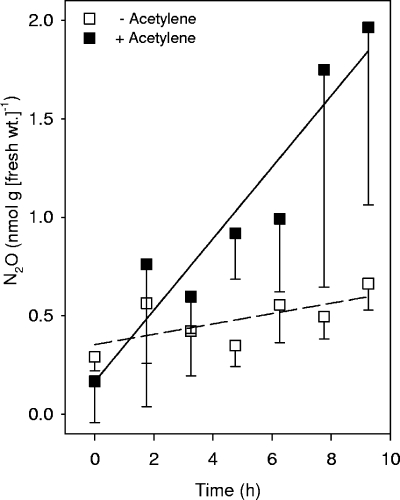
Effect of acetylene on the in vivo emission of N2O by earthworms.
Acetylene blocks the terminal step of denitrification (i.e., the reduction of N2O to N2 by N2O reductase [40]). Thus, acetylene-dependent stimulation of N2O production is evidence of denitrification and can also be used to estimate the amount of denitrification-derived N2. Acetylene significantly enhanced the emission of N2O by O. multiporus exposed to nitrate (Fig. 2). The acetylene-stimulated emission was approximately 0.18 nmol N2O per h per g (fresh weight) worm, whereas the emission in the absence of acetylene was approximately 0.03 nmol N2O per h per g (fresh weight) worm, suggesting that N2 was the main end product of denitrification in the alimentary canal of O. multiporus. In contrast, acetylene only slightly increased the amount of N2O emitted by L. rubellus (the production rates with and without acetylene were approximately 0.35 and 0.26 nmol N2O per h per g [fresh weight] worm, respectively, for individuals exposed to 2 mM sodium nitrate), indicating that N2O was the main end product of denitrification in the alimentary canal of L. rubellus. Nitrate was far less stimulatory than nitrite, a result consistent with previous findings (25), as well as with the fact that nitrite is a closer precursor of N2O in denitrification than nitrate is (41).
FIG. 2.
Effect of acetylene (15%, vol/vol) on the in vivo emission of N2O by O. multiporus wetted with sodium nitrate (2 mM). The symbols indicate the means for triplicate determinations; the error bars indicate negative standard deviations.
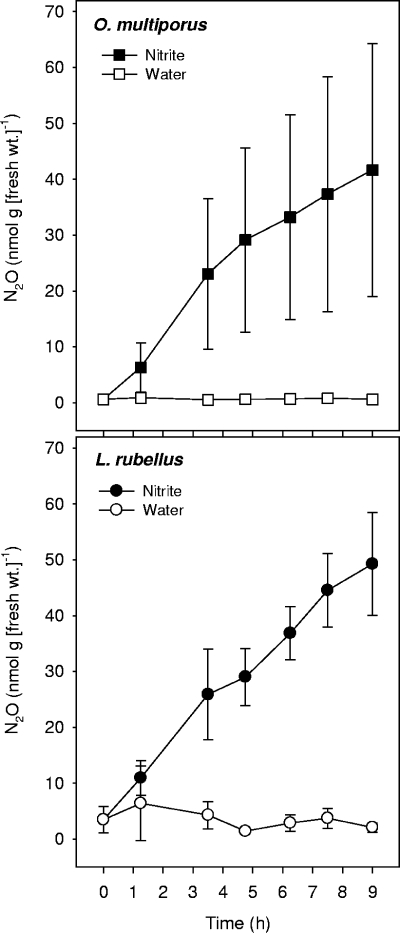
Production of N2O by alimentary canals.
Nitrite greatly increased the capacity of alimentary canals of O. multiporus and L. rubellus to produce N2O under anoxic conditions with acetylene (Fig. 3). (Acetylene was provided to maximize the detection of denitrification-derived nitrogenous gases [both N2O and N2].) Although the nitrite-stimulated N2O production capacities of alimentary canals of O. multiporus were more variable than those of alimentary canals of L. rubellus, the mean emission values for alimentary canals of these two species were similar. These findings demonstrated that the alimentary canals of these earthworms had equally high capacities to produce N2O under anoxic conditions.
FIG. 3.
Effect of sodium nitrite (2 mM) on the production of N2O by alimentary canals of L. rubellus and O. multiporus. The gas phase contained argon (85%, vol/vol) and acetylene (15%, vol/vol). The symbols indicate the means for triplicate determinations; the error bars indicate standard deviations.
Organic compounds in alimentary canal contents.
Alimentary canal contents were rich in readily utilizable organic compounds, including glucose and maltose, as well as several fatty acids (e.g., acetate, propionate, and succinate) indicative of fermentation (Table 2). In comparison, soils contained very small or undetectable amounts of these compounds, demonstrating that they were produced in situ in the alimentary canal. The amounts of formate and propionate in alimentary canal contents of O. multiporus were greater than the amounts detected in alimentary canal contents of L. rubellus; in contrast, the amounts of glucose, maltose, and succinate in alimentary canal contents of O. multiporus were less than the amounts detected in alimentary canal contents of L. rubellus. These results suggest that the in situ parameters that affect the activities of the microbial biomes in the alimentary canals of these contrasting species were dissimilar.
TABLE 2.
Concentrations of organic compounds detected in alimentary canal contents from L. rubellus and O. multiporus and soils from which they were collected (pasture soil and forest soil, respectively)a
| Compound | Concn (mmol liter [water content]−1)
|
|||
|---|---|---|---|---|
| Alimentary canal contents of L. rubellus | Pasture soil | Alimentary canal contents of O. multiporus | Forest soil | |
| Maltose | 1.3 ± 0.3 | NDb | 0.5 ± 0.0 | ND |
| Glucose | 17.7 ± 4.6 | ND | 6.0 ± 1.1 | ND |
| Succinate | 5.9 ± 1.1 | ND | 2.5 ± 0.5 | ND |
| Lactate | 0.4 ± 0.1 | ND | ND | ND |
| Formate | 1.9 ± 0.7 | ND | 5.7 ± 0.6 | ND |
| Acetate | 2.2 ± 0.5 | ND | 1.8 ± 0.4 | ND |
| Propionate | 8.6 ± 2.4 | 0.7 ± 1.3 | 15.2 ± 1.7 | 0.3 ± 0.5 |
The values are the means ± standard deviations for triplicate determinations; each replicate consisted of alimentary canal contents from nine L. rubellus worms or four O. multiporus worms.
ND, not detected.
nosZ diversitiy in the alimentary canal and soil.
Forty-nine and 50 nosZ sequences (700 bp) were obtained from the alimentary canals (i.e., crop-gizzard and hindgut) of L. rubellus and O. multiporus, respectively (Fig. 4). The coverage was approximately 98% for both L. rubellus-derived sequences and O. multiporus-derived sequences. The average nucleotide sequence similarity of all L. rubellus-derived sequences was 93.7% ± 6.6%, while the average nucleotide sequence similarity of all O. multiporus-derived sequences was 92.4% ± 6.1%.
FIG. 4.
Phylogenetic tree of nosZ sequences retrieved from the alimentary canal of earthworms and from soil and related nosZ sequences. The phylogenetic tree was based on amino acid sequences. The nosZ sequence of Haloarcula marismortui (accession number NC_006396) was used as an outgroup. Only representative sequences are shown for OTUs 1, 2, 3, 6, 7, and 8 (which contained the majority of the sequences). The values at the nodes are the percentages of replicate trees in which the associated taxa clustered together in the bootstrap test; branches corresponding to partitions in less than 50% of the bootstrap replicates are drawn as multifurcations. Abbreviations: L, L. rubellus-derived sequences; O, O. multiporus-derived sequences; PS, pasture soil-derived sequences; FS, forest soil-derived sequences.
Eighty-eight and 29 nosZ sequences were obtained from the pasture and forest soils, respectively (Fig. 4). The coverage was approximately 99% and 97% for pasture soil- and forest soil-derived sequences, respectively. The average nucleotide sequence similarity of all pasture soil-derived sequences was 92.3% ± 0.1%, while the average nucleotide sequence similarity of all forest soil-derived sequences was 88.1% ± 0.1%.
Eleven nosZ OTUs were identified for the 216 nosZ sequences, and 6 of these OTUs (OTUs 1, 2, 3, 7, 8, and 10) were found in both soil and the alimentary canal. However, these six OTUs contained 92% of the total sequences. The average nucleotide sequence similarity between L. rubellus- and pasture soil-derived sequences was 92.7% ± 0.1%, and the average nucleotide sequence similarity between O. multiporus- and forest soil-derived sequences was 89.7% ± 0.1%, indicating that there was a high degree of similarity between the worm- and soil-derived nosZ sequences.
nosZ sequences obtained from alimentary canal sections and soils were distantly related to nosZ sequences of uncultured soil bacteria and nosZ sequences of genera common in soils. nosZ sequences of OTUs 1 to 7 were related to sequences of Bradyrhizobium japonicum and Rhodopseudomonas palustris and more distantly related to the sequence of Oligotropha carboxidovorans. In contrast to the results obtained with BLAST, OTU 6 was affiliated with Sinorhizobium meliloti in the phylogenetic tree. The sequences of OTUs 9 and 10 were closely related to Azospirillum lipoferum sequences. The closest cultured relatives of OTU 8 and OTU 11 were Rhodospirillum centenum and Pseudomonas brassicacearum, respectively. These results indicated that soil denitrifiers were present in the alimentary canal of New Zealand earthworms and underscored the likelihood that ingested soil denitrifiers were the primary source of the N2O that they emitted.
DISCUSSION
Emission of N2O and link to denitrification.
The in vivo emission of N2O and the production of N2O by alimentary canals were significantly stimulated by nitrate, nitrite, and acetylene, indicating that N2O was derived primarily from denitrification. However, the capacity of earthworms to emit N2O was variable. The emission of N2O by earthworms collected in Europe is likewise variable; approximately 5% of individual worms do not emit N2O at the time of collection (unpublished data). Earthworms have three feeding guilds, namely anecic (worms living in deeper soil zones, ingesting moderate amounts of mineral soil, and feeding on litter dragged into their burrows), endogeic (worms feeding in the rhizosphere, ingesting substantial amounts of mineral soil, and preferentially living in upper mineral soil), and epigeic (worms feeding on litter and preferentially living above the mineral soil) (5, 20, 22, 23). Although emission is variable at the time of collection, all three guilds in Europe emit N2O along with N2 (13, 17, 25), suggesting that the capacity to emit N2O is independent of feeding guilds. However, O. multiporus (endogeic) did not emit N2O unless it was provided with something that stimulated denitrification, and even then the capacity to emit N2O was much lower than that of other species (Fig. 1). It is therefore interesting that previous studies with European earthworms have been restricted to species belonging to the Lumbricidae, the family to which the three introduced species (L. rubellus [epigeic], A. rosea [endogeic], and O. cyaneum [endogeic]) evaluated in the present study belong.
One might speculate that the in situ conditions of the alimentary canal of O. multiporus (a species that is very large in terms of weight, length, and thickness) are optimal for complete denitrification (i.e., the reduction of nitrate or nitrite primarily to N2). N2O concentrations are highest in the anoxic core of earthworms that emit N2O, indicating that the production of N2O is optimal in the center of the alimentary canal (14, 39). The radius of O. multiporus individuals was approximately five times greater than that of the other worms evaluated, which might increase the likelihood that N2O is further reduced to N2 before it radially leaves the alimentary canal. Alternatively, the theoretical capacity of the gut biota of O. multiporus to reduce nitrate might be linked primarily to dissimilatory nitrate reducers (i.e., anaerobes that reduce nitrate and nitrite to ammonium [37]). Dissimilatory nitrate reducers can constitute a major portion of the cultured nitrate reducers isolated from alimentary canal contents of earthworms (3, 15). However, the evidence to date indicates that denitrifiers rather than dissimilatory nitrate reducers are the primary source of N2O in the alimentary canal (13-15, 17, 25, 39), a finding that is consistent with the nearly identical capacities of alimentary canals from O. multiporus and L. rubellus to produce N2O when they are exposed to nitrite and acetylene (Fig. 3). Thus, denitrifiers and a source of reductant for denitrification were present in the alimentary canal of O. multiporus. The forest soil from which O. multiporus was obtained contained more available nitrate than the pasture soil from which L. rubellus was obtained (Table 1), and the ratio of available nitrate to readily utilizable organic compounds in the alimentary canal might have favored complete denitrification in O. multiporus. Likewise, the nearly neutral pH of the forest soil would theoretically favor complete denitrification (acidity inhibits N2O reductase [37, 41]), suggesting that soil denitrifiers ingested by O. multiporus might have been more poised to reduce nitrate to N2 rather than to N2O. Commercially obtained specimens of Lumbricus terrestris initially were negative for emission of N2O but later emitted N2O after they were maintained for 2.5 days in garden soil (25). Thus, it is likely that several factors (including body geometry, the in situ status of the nitrate-reducing biota in the alimentary canal, and the chemical parameters of the ingested soil) accounted for the inability of O. multiporus to emit N2O in the absence of external effectors of denitrification.
Microbial trophic links in the alimentary canal.
One square meter of soil can contain up to nearly 1 liter of earthworm gut (3), suggesting that this soil-processing gut ecosystem can be important to the ecology of soil microorganisms. This niche (i.e., the alimentary canal of the earthworm) in aerated soil (i) is anoxic (14, 39), (ii) is rich in intestinal mucus (24, 38) that contains millimolar concentrations of sugars (14, 39), (iii) contains readily utilizable amino acids (3, 14), and (iv) thus offers a unique transient habitat for ingested soil microbial biomes (3). Alimentary canal contents of European earthworms contain substantially higher numbers of cultured anaerobes (i.e., microbes capable of survival under anaerobic conditions) than soils inhabited by earthworms (3, 15, 16). Indeed, fermentation occurs along the entire alimentary canal of L. terrestris and yields significant amounts of organic acids that might be oxidized by denitrifiers for the in situ reduction of nitrate and nitrite to nitrogenous gases (39). Fermentation in the alimentary canal can also yield H2 as an in vivo emission product, which might be a source of reductants for soil microorganisms (39). The occurrence of millimolar concentrations of sugars and organic acids in the alimentary canal contents of New Zealand earthworms (Table 2) corroborates these findings and demonstrates that in situ fermentation is a universal trait of the earthworm alimentary canal. The differences in the amounts of sugars and organic acids detected in alimentary canal contents of O. multiporus and L. rubellus suggest that the in situ microbial activities and/or assimilation tendencies of these worm species were not the same.
Denitrifiers in the alimentary canal of earthworms in New Zealand.
It has been proposed that denitrifiers in the alimentary canal of European earthworms are derived from ingested soil (3, 4). This proposal is based on evidence obtained by cultivation, gut simulation, and molecular analyses (12, 14, 15). nosZ sequences detected in the alimentary canals of L. rubellus and O. multiporus were similar to sequences retrieved from soil and were distantly related to sequences of uncultured soil bacteria and genera common in soils (i.e., Bradyrhizobium, Azospirillum, Rhodopseudomonas, Rhodospirillum, Pseudomonas, Oligotropha, and Sinorhizobium) (Fig. 4). These results are therefore consistent with those obtained previously; i.e., they indicate that the primary origin of denitrifiers in New Zealand earthworms is ingested soil and support the hypothesis that ingested denitrifiers are linked to the earthworm's capacity to emit N2O.
The bulk of nosZ sequences obtained were affiliated with the genus Bradyrhizobium. The specificity of the nosZ primers used (nosZ-F-1181 and nosZ-R-1880) is likely the main reason for the preponderance of Bradyrhizobium-affiliated sequences, since these primers tend to target genera affiliated with Bradyrhizobium (26). The use of different nosZ primers (nosZ661F and nosZ1773R [29]; PsNosZ175F and PsNosZ1144R [12]) resulted in an alternative diversity of nosZ from European earthworms (12). However, consistent with the current study, the denitrifers in the alimentary canal that were revealed in the previous study were also phylogenetically related to soil denitrifying genera, including Bradyrhizobium, Pseudomonas, Rhodopseudomonas, and Sinorhizobium, as well as Dechloromonas and Flavobacterium (12).
Conclusions.
The present study suggests that the capacity of earthworms to emit nitrogenous gases is not geographically restricted and also corroborates previous findings that link the emissions to ingested soil denitrifiers in the alimentary canal. Furthermore, this study increases the information about this capacity to Megascolecidae. Comparative evaluation of other species of Megascolecidae might determine if the observations made with O. multiporus are representative of this taxonomic family. More complete resolution of the in situ parameters and in situ status of the microbial biome of the alimentary canal of O. multiporus might yield information that explains why this native Megascolecidae species had a lower capacity to emit N2O than the Lumbricidae species examined in this and previous studies.
Acknowledgments
We thank Masha Minor and Oliver Schmidt (Massey University) for assistance with locating, identifying, and collecting earthworms, Bryan Treloar (AgResearch Ltd.) for assistance with the high-performance liquid chromatography analysis, and Jagrati Singh (Landcare Research) for assistance with the gas chromatography analysis.
Support for this study was provided by the Deutsche Forschungsgemeinschaft (grant DR310/4-1), the University of Bayreuth, and Massey University.
Footnotes
Published ahead of print on 3 April 2009.
REFERENCES
- 1.Bertora, C., P. C. J. van Vliet, E. W. J. Hummelink, and J. W. van Groenigen. 2007. Do earthworms increase N2O emissions in ploughed grassland? Soil Biol. Biochem. 39:632-640. [Google Scholar]
- 2.Borken, W., S. Grundel, and F. Beese. 2000. Potential contribution of Lumbricus terrestris L. to carbon dioxide, methane and nitrous oxide fluxes from a forest soil. Biol. Fertil. Soils 32:142-148. [Google Scholar]
- 3.Drake, H. L., and M. A. Horn. 2007. As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes. Annu. Rev. Microbiol. 61:169-189. [DOI] [PubMed] [Google Scholar]
- 4.Drake, H. L., and M. A. Horn. 2006. Earthworms as a transient heaven for terrestrial denitrifying microbes: a review. Eng. Life Sci. 6:261-265. [Google Scholar]
- 5.Edwards, C. A., and P. J. Bohlen. 1996. Biology and ecology of earthworms, 3rd ed. Chapman & Hall, London, United Kingdom.
- 6.Egert, M., S. Marhan, B. Wagner, S. Scheu, and M. W. Friedrich. 2004. Molecular profiling of 16S rRNA genes reveals diet-related differences of microbial communities in soil, gut, and casts of Lumbricus terrestris L. (Oligochaeta: Lumbricidae). FEMS Microbiol. Ecol. 48:187-197. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich, G. G., D. F. Goerlitz, J. H. Bourell, G. V. Eisen, and E. M. Godsy. 1981. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl. Environ. Microbiol. 42:878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein, J. 1985. Confidence limits on phylogenies—an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 9.Furlong, M. A., D. R. Singleton, D. C. Coleman, and W. B. Whitman. 2002. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 68:1265-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths, R. I., A. S. Whiteley, A. G. O'Donnell, and M. J. Bailey. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl. Environ. Microbiol. 66:5488-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedley, C. B., S. Saggar, and K. R. Tate. 2006. Procedure for fast simultaneous analysis of the greenhouse gases: methane, carbon dioxide, and nitrous oxide in air samples. Commun. Soil Sci. Plant Anal. 37:1501-1510. [Google Scholar]
- 12.Horn, M. A., H. L. Drake, and A. Schramm. 2006. Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar. Appl. Environ. Microbiol. 72:1019-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn, M. A., R. Mertel, M. Gehre, M. Kastner, and H. L. Drake. 2006. In vivo emission of dinitrogen by earthworms via denitrifying bacteria in the gut. Appl. Environ. Microbiol. 72:1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn, M. A., A. Schramm, and H. L. Drake. 2003. The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms. Appl. Environ. Microbiol. 69:1662-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ihssen, J., M. A. Horn, C. Matthies, A. Gößner, A. Schramm, and H. L. Drake. 2003. N2O-producing microorganisms in the gut of the earthworm Aporrectodea caliginosa are indicative of ingested soil bacteria. Appl. Environ. Microbiol. 69:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karsten, G. R., and H. L. Drake. 1995. Comparative assessment of the aerobic and anaerobic microfloras of earthworm guts and forest soils. Appl. Environ. Microbiol. 61:1039-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karsten, G. R., and H. L. Drake. 1997. Denitrifying bacteria in the earthworm gastrointestinal tract and in vivo emission of nitrous oxide (N2O) by earthworms. Appl. Environ. Microbiol. 63:1878-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar, S., M. Nei, J. Dudley, and K. Tamura. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Küsel, K., and H. L. Drake. 1995. Effects of environmental parameters on the formation and turnover of acetate by forest soils. Appl. Environ. Microbiol. 61:3667-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavelle, P., D. Bignell, M. Lepage, V. Wolters, P. Roger, P. Ineson, O. W. Heal, and S. Dhillion. 1997. Soil function in a changing world: the role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 33:159-193. [Google Scholar]
- 21.Lee, K. E. 1959. The earthworm fauna of New Zealand, vol. 130. R. E. Owen, Government Printer, Wellington, New Zealand.
- 22.Lee, K. E. 1985. Earthworms. Their ecology and relationships with soils and land use. Academic Press, Sydney, Australia.
- 23.Makeschin, F. 1997. Earthworms (Lumbricidae: Oligochaeta): important promoters of soil development and soil fertility, p. 173-223. In G. Benckiser (ed.), Fauna in soil ecosystems. Marcel Dekker Inc., New York, NY.
- 24.Martin, A., J. Cortez, I. Barois, and P. Lavelle. 1987. The production of intestinal mucus by earthworms—a key process in their interactions with the soil microflora. Rev. Ecol. Biol. Sol 24:549-558. [Google Scholar]
- 25.Matthies, C., A. Griesshammer, M. Schmittroth, and H. L. Drake. 1999. Evidence for involvement of gut-associated denitrifying bacteria in emission of nitrous oxide (N2O) by earthworms obtained from garden and forest soils. Appl. Environ. Microbiol. 65:3599-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rich, J. J., R. S. Heichen, P. J. Bottomley, K. Cromack, Jr., and D. D. Myrold. 2003. Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Appl. Environ. Microbiol. 69:5974-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizhiya, E., C. Bertora, P. C. J. van Vliet, P. J. Kuikman, J. H. Faber, and J. W. van Groenigen. 2007. Earthworm activity as a determinant for N2O emission from crop residue. Soil Biol. Biochem. 39:2058-2069. [Google Scholar]
- 28.Saitou, N., and M. Nei. 1987. The neighbor-joining method—a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 29.Scala, D. J., and L. J. Kerkhof. 1999. Diversity of nitrous oxide reductase (nosZ) genes in continental shelf sediments. Appl. Environ. Microbiol. 65:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer, M. 2000. Brohmer, Fauna von Deutschland: ein Bestimmungsbuch unserer heimischen Tierwelt, 20th ed. Quelle & Meyer Verlag, Wiebelsheim, Germany.
- 31.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singleton, D. R., P. F. Hendrix, D. C. Coleman, and W. B. Whitman. 2003. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae; Oligochaeta). Soil Biol. Biochem. 35:1547-1555. [Google Scholar]
- 33.Springett, J. A. 1992. Distribution of lumbricid earthworms in New Zealand. Soil Biol. Biochem. 24:1377-1381. [Google Scholar]
- 34.Springett, J. A., and R. A. J. Gray. 1998. Burrowing behaviour of the New Zealand indigenous earthworm Octochaetus multiporus (Megascolecidae: Oligochaeta). N. Z. J. Ecol. 22:95-97. [Google Scholar]
- 35.Stackebrandt, E., and J. Evers. 2006. Taxonomic parameters revisited: tarnished gold standards. Microbiol. Today 33:152-155. [Google Scholar]
- 36.Stackebrandt, E., and B. M. Goebel. 1994. A place for DNA-DNA reassociation and 16S ribosomal RNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 37.Tiedje, J. M. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium, p. 179-243. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. John Wiley and Sons, New York, NY.
- 38.Trigo, D., I. Barois, M. H. Garvin, E. Huerta, S. Irisson, and P. Lavelle. 1999. Mutualism between earthworms and soil microflora. Pedobiologia 43:866-873. [Google Scholar]
- 39.Wüst, P. K., M. A. Horn, and H. L. Drake. 2009. In situ hydrogen and nitrous oxide as indicators of concomitant fermentation and denitrification in the alimentary canal of the earthworm Lumbricus terrestris. Appl. Environ. Microbiol. 75:1852-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshinari, T., and R. Knowles. 1976. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem. Biophys. Res. Commun. 69:705-710. [DOI] [PubMed] [Google Scholar]
- 41.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]