Abstract
We examined the ability of the metal-reducing bacteria Geobacter metallireducens GS-15 and Shewanella oneidensis MR-1 to reduce Pu(VI) and Pu(V). Cell suspensions of both bacteria reduced oxidized Pu [a mixture of Pu(VI) and Pu(V)] to Pu(IV). The rate of plutonium reduction was similar to the rate of U(VI) reduction obtained under similar conditions for each bacteria. The rates of Pu(VI) and U(VI) reduction by cell suspensions of S. oneidensis were slightly higher than the rates observed with G. metallireducens. The reduced form of Pu was characterized as aggregates of nanoparticulates of Pu(IV). Transmission electron microscopy images of the solids obtained from the cultures after the reduction of Pu(VI) and Pu(V) by S. oneidensis show that the Pu precipitates have a crystalline structure. The nanoparticulates of Pu(IV) were precipitated on the surface of or within the cell walls of the bacteria. The production of Pu(III) was not observed, which indicates that Pu(IV) was the stable form of reduced Pu under these experimental conditions. Experiments examining the ability of these bacteria to use Pu(VI) as a terminal electron acceptor for growth were inconclusive. A slight increase in cell density was observed for both G. metallireducens and S. oneidensis when Pu(VI) was provided as the sole electron acceptor; however, Pu(VI) concentrations decreased similarly in both the experimental and control cultures.
Effective bioremediation and waste management strategies at nuclear sites require an understanding of the fundamental biogeochemical processes that control the mobility of actinides. Microorganisms can influence the chemical speciation, valence state, and distribution of actinides in subsurface environments (2, 8, 12, 14). Dissimilatory metal-reducing bacteria (DMRB), which derive energy by respiring oxidized metals (Fe and Mn in nature), may play a particularly important role in the mobility of actinides, since the oxidized forms of many radionuclides are more mobile than their reduced forms. Remedial strategies have been proposed to biomineralize radionuclides via direct reduction by DMRB or indirectly by DMRB by-products (9-11). Several DMRB have been shown to conserve energy for anaerobic growth via the reduction of U(VI) (9-11, 14).
Plutonium redox chemistry is more complex than that of most other actinides. Under environmental conditions, plutonium can exist in the III, IV, V, and VI oxidation states, and multiple oxidation states can coexist simultaneously (4, 5). The oxidized species of plutonium [Pu(V) and Pu(VI)] generally are much more soluble than the reduced species (4). Predicting the influence DMRB have on plutonium biogeochemistry is complicated by the fact that both Pu(III) and Pu(IV) are possible products of biological reduction. Also, the presence of chelating ligands can greatly influence the oxidation state formed during reduction as well as the reduction rate. The reduction of oxidized Pu species to Pu(IV) is desired, because it is highly insoluble and not very mobile. However, in the presence of complexing ligands and under reducing conditions the production of Pu(III) is favored, and Pu(III) complexes can be quite soluble (2). The conditions leading to the reduction of Pu(V) and Pu(VI) need to be understood and controlled so that they do not lead to the production of Pu(III), if the biological reduction of Pu(V) or Pu(VI) is to be used as an effective remediation strategy.
There is little information available concerning the influence DMRB have on plutonium biogeochemistry. Few previous studies have reported the biological reduction of Pu(IV) to Pu(III) (2, 7, 16). During the earlier experiments (16), the solubilization of PuO2 increased approximately ∼40% in solutions with DMRB. In solutions with DMRB and nitrilotriacetic acid (NTA), approximately 90% of the available Pu was solubilized, but the production of Pu(III) was not observed in any of the cultures, either with or without NTA added (16). The enhanced solubility of Pu was attributed to Pu(IV) reduction, the solubilization of resultant Pu(III), and the reoxidation of Pu(III) to Pu(IV) with the NTA complexation of Pu(III). Since Pu(III) was not observed, the biological reduction of Pu(IV) was inferred from the data (16). The biological reduction of Pu(IV) to Pu(III) was first conclusively documented with the production of Pu(III) in monocultures of G. metallireducens GS-15 and S. oneidensis MR-1 both with and without the addition of a chelating agent (EDTA) (2). In experiments without EDTA, the aqueous concentration of Pu(III) in DMRB cultures was very low (<0.05 mM Pu) (2). The aqueous concentration of Pu(III) increased to approximately 60 to 80% (0.3 to 0.4 mM Pu) of the total Pu(IV) when EDTA was added to the cultures (2). To our knowledge, there are no published studies documenting the biological reduction of Pu(V) or Pu(VI) to either Pu(IV) or Pu(III). However, based on thermodynamics calculations, the reduction of Pu(V) and Pu(VI) by DMRB should be possible and yield greater energy for the bacteria than Pu(IV) reduction (2).
The study presented here was designed first to assess the ability of G. metallireducens GS-15 and S. oneidensis MR-1 to reduce Pu(V) and Pu(VI) in monocultures under cell resting and growth conditions. Second, the aqueous and solid phases produced during the experiments were analyzed to determine the extent of biological reduction [i.e., to Pu(IV) or Pu(III)].
MATERIALS AND METHODS
Reagents.
All solutions were prepared with deionized-distilled water. Plutonium(IV) stock solutions were prepared as described elsewhere (2). Briefly, plutonium metal was dissolved in chilled 6 M HCl, which was purified using anion-exchange chromatography. Pu(VI) stock solutions were prepared by bubbling ozone through purified Pu(IV) solutions in HCl. The concentration and purity of the Pu(VI) stock solutions were assessed using visible-near-infrared (Vis-NIR) spectra of a 1.0 M HClO4 solution. Pu(VI) gives an intense and distinctive absorbance band at 830 nm (ɛ = 550 liters mol−1 cm−1) (6). The concentrated Pu(VI) stock in HCl then was diluted to an appropriate concentration, and the pH was adjusted to approximately pH 3 with 1.0 M NaOH just prior to addition to the experimental cultures. Upon addition to the experimental cultures at neutral pH, the Pu(VI) began to reduce slowly to Pu(V). The Pu available for biological reduction therefore was a mixture of Pu(VI) and Pu(V), which will be referred to as Pu(VI)/(V) here.
Bacterial strain and culture conditions.
G. metallireducens GS-15 and S. oneidensis MR-1 were obtained from the American Type Culture Collection. A detailed description of the growth medium used for maintaining cultures and growing cells for experiments can be found elsewhere (2). Briefly, the growth medium composition per liter was 3.4 g NaOH, 12.25 g Fe(III)-citrate anhydrous, 0.25 g NH4Cl, 1.08 g glycerol-2-PO4, 0.025 g KCl, 10.46 g morpholinepropanesulfonic acid (MOPS), 10.0 ml Wolfe's minerals, 10 ml Thauer's vitamins, and 10 mM acetate (G. metallireducens) or 10 mM lactate (S. oneidensis). Oxygen was removed from the medium by bubbling ultra-high-purity argon gas through the solution for approximately 1 h. The solutions then were sealed in serum bottles and autoclaved. The bacteria were grown anaerobically at 30°C in 50-ml serum bottles or 1.0-liter airtight culture flasks maintained under constant shaking at 100 rpm in the dark. Fully grown cultures were transferred anaerobically to centrifuge tubes, and the cells were pelleted by centrifugation at 5,000 rpm. The cells were washed two times with fresh anoxic MOPS buffer and then were resuspended in the same buffer at pH 7.0. The cell density of the stock culture used to prepare the cell suspensions and to inoculate the experimental cultures was determined using a calibration curve relating cell density to optical absorbance, which was determined by direct counts. The optical density of cells grown in ferric citrate solutions was obtained by first washing the cells with 100 mM MOPS and then resuspending the cells in 100 mM MOPS.
Enzymatic reduction of Pu(VI) by G. metallireducens and S. oneidensis.
Cell suspensions with cell densities of 5 × 108 cells/ml at pH 7.0 were used for all cell suspension experiments, and either 10 mM acetate (G. metallireducens) or 10 mM lactate (S. oneidensis) was used as the electron donor. All experimental cultures with Pu were incubated at 30°C in the dark with constant shaking at 100 rpm. Cell suspension experiments were conducted using 20-ml sealed serum bottles with an initial fluid volume 10.5 ml, which consisted of 10 ml medium and 0.50 ml Pu stock. The initial plutonium concentration for the cell suspension experiments was 0.50 mM Pu(VI). In addition to the experimental cultures, each cell suspension experiment also had controls consisting of no cells, no electron donor, and heat-killed cells with Pu(VI) and electron donor. All control and experimental cultures were conducted in triplicate. Total aqueous Pu concentrations in the cultures were determined by liquid scintillation counting (LSC) analysis. The aqueous concentrations of Pu(III), Pu(IV), Pu(V), and Pu(VI) were monitored with Vis-NIR spectroscopy. Triplicate samples were collected for LSC from each triplicate culture, and one culture of each triplicate was sampled for Vis-NIR spectroscopy for each time point. Approximately 0.5 ml of culture was collected for LSC analysis, and an additional ∼1 ml was collected for Vis-NIR spectroscopy. Samples were collected using sterile syringes with metal needles (purged with sterile Ar) and immediately filtered through a 0.22-μm polytetrafluoroethylene filter (Millex SLLG013SL) into a vessel containing 15 μl of concentrated HCl. Manipulations of cultures containing Pu were done within a radiological fume hood, which was open to the atmosphere.
The reduction of U(VI) by cell suspensions of G. metallireducens and S. oneidensis, which has been well documented in the literature (14, 18, 19), also was conducted for comparison with the Pu(VI) experiments. The conditions for the U(VI) cell suspension experiments were identical to those described above for the Pu(VI) cell suspension experiments, with the exceptions that the initial concentration of U(VI) was 2.0 mM and 10 mM HCO3− was added to the cultures. Also, during the S. oneidensis experiment, the U(VI) control without cells was compromised at the start of the experiment and was not utilized.
Analysis of solid phases produced during reduction of Pu.
In addition to the aqueous analysis, samples of the solid phase that developed during the cell suspension experiments were collected for diffuse reflectance spectroscopy (DR; Pu experiments only) at the end of these experiments. The solid-phase samples were collected in an inert (N2) atmosphere glove bag using syringes with metal needles. For the DR analysis, the solid phase was deposited on a 0.22-μm polycarbonate filter (Osmonics K02CP01300) using a syringe filter holder. The samples were allowed to dry in the glove bag before being loaded in DR sample holders. Samples containing only cells without added Pu also were collected for DR analysis as a control. A Pu(IV) control consisting of solids precipitated by increasing the pH of an acidic Pu(IV) stock to near-neutral pH also was collected for DR analysis.
Growth of G. metallireducens and S. oneidensis with Pu(VI) as the sole terminal electron acceptor.
Growth experiments were conducted at pH 7.0 using the growth media described above without Fe(III)-citrate, with an initial Pu(VI) concentration of 4.0 mM, and with an initial cell density of 2 × 107 cells/ml. Due to material limitations and potential toxicological effects (15), we could provide only enough Pu(VI) to allow for modest cell growth, so it was necessary to use a higher-than-normal initial cell density for the growth experiments. The growth medium was amended with 2.0 mM bicarbonate in an effort to prevent Pu(V) or Pu(VI) precipitation as a result of its interactions with the growth medium components. Pu(VI) was added to the serum vials as a carbonate stock at pH 9. The Pu(VI)-carbonate stock was prepared by combining equimolar solutions of NaCO3 and Pu(VI) stock together while bubbling them with CO2. The pH of the cultures at the end of the experiment was between 7 and 7.5. The total volume of the cultures was 5.5 ml, which consisted of 5 ml medium and 0.5 ml Pu(VI)-carbonate stock. The evolution of Pu(VI) during the course of the experiment was monitored spectrophotometrically and by LSC. Ultra small volume UV-Vis-NIR cuvettes (200 μl) were used (only for the growth experiments) to minimize the volume collected for each sample (total sample volume, ∼0.5 ml). Calibration curves relating cell densities of G. metallireducens and S. oneidensis to protein content measured using the Bradford assay (3) were established to measure growth and were used in addition to direct cell counts to quantify cell densities during the experiments. The cultures were sampled as described above. Samples for the growth experiments were processed differently than were samples for the cell suspension experiments and were immediately centrifuged at 10,000 rpm for 10 min and then analyzed without filtration. In addition to the experimental cultures conducted in triplicate, each growth experiment also had single controls consisting of no cells, no electron donor, and no added Pu(VI).
RESULTS
Direct reduction of Pu(VI)/(V) by G. metallireducens and S. oneidensis in cell suspension.
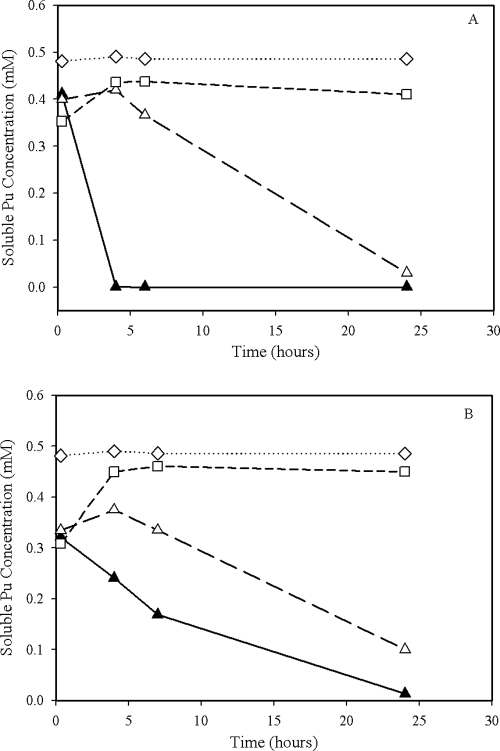
Cell suspension cultures containing living cells of G. metallireducens and S. oneidensis with electron donor added show the most rapid decrease in total soluble Pu concentrations relative to that of the control cultures (Fig. 1). However, the concentration of soluble Pu in the control cultures without an added electron donor also decreased by nearly the same magnitude as the cultures with an electron donor added. Metal reduction in cultures without an added electron donors is not uncommon and has been observed by others (17). The rate of reduction was high for both bacteria; however, the reduction rates of Pu(V/VI) by S. oneidensis were slightly higher than those by G. metallireducens (Fig. 1). For both G. metallireducens and S. oneidensis experiments, there was an initial decrease in the Pu concentrations in the cultures with dead cells, which subsequently increased to near the starting Pu concentration. There were no significant changes in Pu concentrations in the no-cell control. The concentration of soluble Pu as determined by scintillation counting likely is reflecting the combined concentrations of both Pu(V) and Pu(VI).
FIG. 1.
Direct reduction of Pu(VI) by cell suspensions of S. oneidensis (A) and G. metallireducens (B). Conditions: cell density, 5 × 108 cells/ml suspended in 100 mM MOPS at pH 7.4, 0.50 mM Pu(VI), 30°C. The concentrations of the electron donor were 10.0 mM lactate for S. oneidensis (A) and 10.0 mM acetate for G. metallireducens (B). Symbols: ▴, living cells with the electron donor; Δ, living cells with no electron donor; ⋄, no cells control; □, heat-killed-cell control.
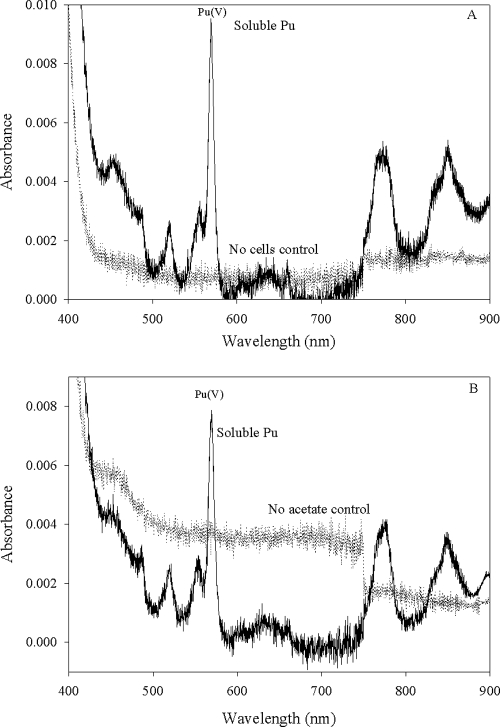
The distribution of the oxidation states in solution was determined spectrophotometrically by recording the Vis-NIR spectra of the filtered samples (Fig. 2). Aqueous Pu species typically have specific absorbance bands that can be used to determine the oxidation state distribution in solution. This characteristic is particularly useful for identifying Pu(VI) species, which usually have higher molar absorptivities than other Pu oxidation states. The absorbance peak at 830 nm represents the aqueo-Pu(VI) species, peaks at 813 and 850 nm represent the carbonate and hydroxo complexes, respectively, and the peak at 569 nm represents Pu(V) (6). Under the conditions of these experiments, Pu(V) was favored, and the Pu(VI) added at the beginning of the experiment slowly reduced to Pu(V). By the end of the experiment, most of the added Pu(VI) was reduced to Pu(V) in the controls without living cells (Fig. 2). No aqueous Pu absorbance peaks were observable at the end of the experiment in the cultures containing living cells (Fig. 2). Unreduced Pu(VI) and Pu(V) in the solutions without living cells remained soluble throughout the experiment. The presence of Pu(III) (characterized by an absorbance peak at 601 nm) was not apparent from the absorbance spectra of any cultures or controls.
FIG. 2.
Vis-NIR spectra of experimental cultures recorded at the end of the experiments. (A) Spectra of filtered S. oneidensis cell suspension with a no-cell control (solid line) showing a peak characteristic Pu(V) at 570 nm and a flat line (dotted line) for the experimental culture with living cells and electron donor. (B) Spectra of filtered G. metallireducens cell suspension with cultures at the end of the experiment showing the spectra of the solution with living cells and acetate (dotted line) and the control with no acetate showing the presence of Pu(V/VI) (solid line). Results for different control treatments were selected to show similar behaviors in both types of controls and to minimize confusion by limiting the number of spectra displayed on a single figure.
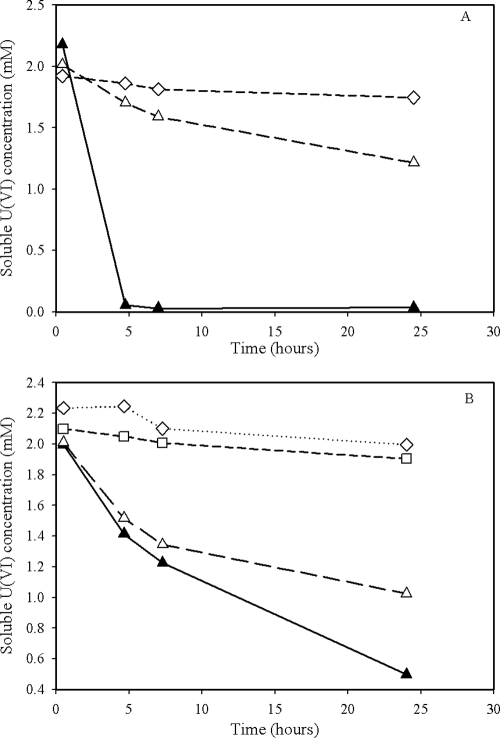
The results of U(VI) reduction by G. metallireducens and S. oneidensis reported previously in the literature (14) were reproduced here under the same experimental conditions as those used for Pu(VI) reduction. The data in Fig. 3 show the results of U(VI) anaerobic reduction under non-growth conditions. The rates of Pu(V/VI) and U(VI) reduction were similar for the two bacteria. However, the reduction of Pu(V/VI) and U(VI) by G. metallireducens are slightly slower than those of Pu(V/VI) and U(VI) reduction by S. oneidensis (Fig. 1 and 3).
FIG. 3.
Direct reduction of U(VI) under non-growth condition by S. oneidensis (A) and G. metallireducens (B). Conditions: cell density, 5 × 108 cells/ml, 10 mM lactate or acetate, pH = 7.4, 2.0 mM U(VI), 30°C. Symbols: ▴, living cells with the electron donor; Δ, living cells with no electron donor; ⋄, no-cell control; □, heat-killed-cell control.
Characterization of Pu(V/VI) bioreduction products.
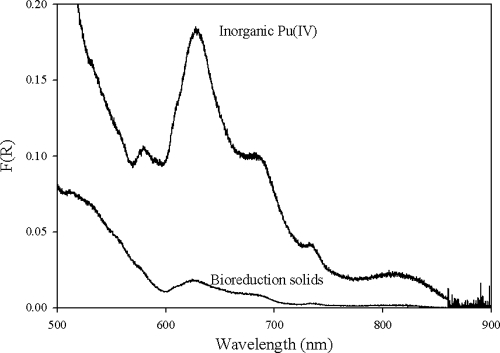
After the cell suspension experiments with living cells, a green precipitate was visible at the bottom of the serum vials. This material, containing both Pu precipitates and cells, was collected for characterization using DR. The DR spectrum of the precipitates obtained after the incubation of 0.5 mM Pu(VI) with cell suspensions of S. oneidensis is shown in Fig. 4, along with a spectrum of freshly precipitated inorganic Pu(IV) obtained by Pu(IV) precipitation at near-neutral pH. DR spectrum collected from a G. metallireducens experiment was not distinguishable from that of the S. oneidensis spectrum. Although the spectrum of bioreduction solids has a much lower concentration of Pu solid with less pronounced peaks than the inorganic Pu(IV) spectrum, the spectra in Fig. 4 are very similar and strongly suggest that the reduction product from our experiments contained a Pu(IV) solid phase.
FIG. 4.
Defuse reflectance spectra of inorganic Pu(IV) obtained by the precipitation of Pu(IV) at near-neutral pH and bioreduction solids obtained by incubation of 0.5 mM Pu(IV) with a cell suspension of S. oneidensis. F(R) is the remission function, which is proportional to the absorption coefficient.
Utilization of Pu(V/VI) as the sole electron acceptor by G. metallireducens and S. oneidensis to support growth.
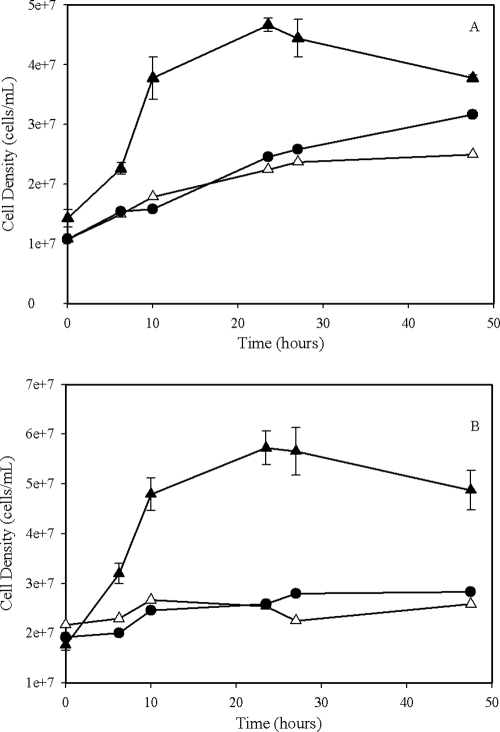
Cell growth and soluble plutonium concentrations were monitored for 48 h. A substantial cell density increase was observed in the cultures containing living cells with the electron donor and plutonium (Fig. 5). The control cultures with no electron donor or no Pu added show only a slight or no increase in cell density (Fig. 5).
FIG. 5.
Growth of G. metallireducens (A) and S. oneidensis (B) using Pu(V/VI) as the sole electron acceptor. Conditions: starting cell density, 2 × 107 cells/ml, 10 mM lactate or acetate, pH 7.4, 4.0 mM Pu(VI), 30°C, with shaking at 100 rpm. Symbols: ▴, living cells with the electron donor; Δ, living cells with no electron donor; •, no-Pu control. Error bars represent the standard deviations calculated using results from triplicate samples. The no-Pu control for both cultures was not done in triplicate.
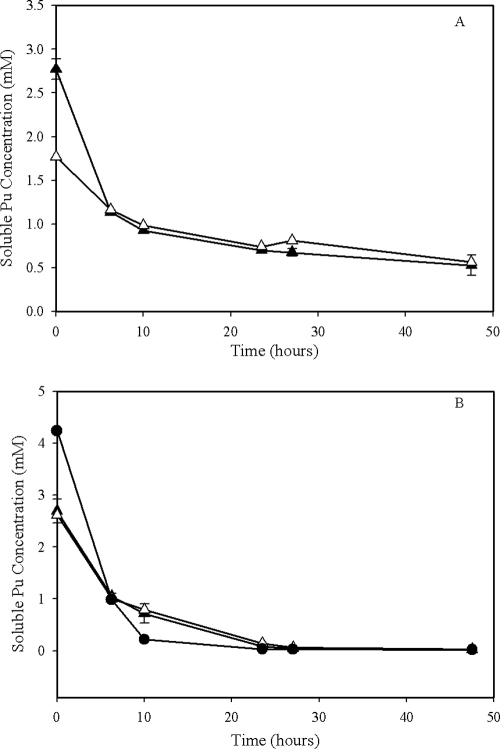
Soluble Pu concentrations rapidly decreased to near zero in the cultures with living cells, but they also decreased similarly in both of the controls without cells and without an electron donor (Fig. 6). A repeat of the experiments produced similar results even after lowering the initial concentration of Pu(VI) to 2 or 1 mM. Attempts to sequentially remove the various chemical components from the growth medium also failed to yield a solution capable of sustaining soluble Pu(VI) concentrations that were large enough to support measurable growth. Aqueous Pu(VI) was present in the cultures containing S. oneidensis in the samples collected approximately 24 h after Pu addition (data not presented), but no aqueous Pu species were detectable in any of the cultures after 48 h. Unfortunately, the oxidation state of the precipitate in these experiments was not determined. Subsequent experiments were conducted to try to identify a growth solution that would maintain Pu(VI) in solution by eliminating individual components of the growth medium to no avail. In fact, a solution of >1 mM Pu(VI) in 100 mM MOPS caused Pu(VI) to precipitate.
FIG. 6.
Changes in soluble Pu concentration recorded during S. oneidensis MR-1 (A) and G. metallireducens GS-15 (B) growth on Pu(VI) as the sole electron acceptor. Conditions were the same as those described for Fig. 5. Symbols: ▴, living cells with the electron donor; Δ, living cells with no electron donor; •, no-cell control.
DISCUSSION
Plutonium speciation and accessibility of Pu(V) and Pu(VI) species to biological reduction.
The oxidized forms of plutonium [Pu(VI) and Pu(V)] are soluble and fairly stable under aerobic conditions, but the two oxidation states form very different complexes in aqueous solutions. In acidic media (pH < 4) and in the absence of complexants, Pu(VI) is present mainly as an aqueo ion (13). At higher pHs (pH > 4), hydrolysis dominates the complexation of Pu(VI), and mono- and bis-hydroxo species form (13), and these species were likely the dominant Pu(VI) species in our system. However, under most environmental conditions, the speciation of Pu(VI) is dominated by the formation of polycarbonate complexes [Pu(VI)(CO3)n−(2n− 2)] (13). The speciation of Pu(V) in solution is slightly different from that of Pu(VI). Pu(V) does not hydrolyze until a very high pH (pH > 8) and is weakly complexed by inorganic anions (13), which suggests that Pu(V)O2+ is the most likely Pu(V) species present in our system.
All of the potential aqueous Pu(VI) and Pu(V) species in our system appear to be accessible for bacterial reduction, because their reduction potentials are within the range of redox potentials accessible to metal-reducing bacteria (Table 1) (2). The data in Table 1 also indicate that the reduction of Pu(VI) and Pu(V) species to Pu(IV) are energetically more favorable than their reduction to Pu(III). These data indicate that the biological reduction of Pu(VI) and Pu(V) is more likely to produce Pu(IV) than Pu(III) species.
TABLE 1.
Formal reduction potentials of the predominant Pu speciesa
| Redox system | Species | Reduction reaction | E0′ (V) | E (V) |
|---|---|---|---|---|
| Pu(VI)/Pu(V) | PuO22+ | PuO22+ + e− = PuO2+ | 0.90 | |
| Pu(VI)/Pu(IV) | PuO22+ | PuO22+ + 2e− = PuO2(s) | 1.275 | |
| PuO2(OH)+ | PuO2(OH)+ + H+ + 2e− = PuO2(s)+H2O | 0.994 | 0.817 | |
| PuO2(OH)2 | PuO2(OH)2 + 2H+ + 2e− = PuO2(s) + 2H2O | 0.767 | 0.590 | |
| PuO2CO3 | PuO2CO3 + H+ + 2e− = PuO2(s) + HCO3− | 1.138 | 0.962 | |
| PuO2(CO3)22− | PuO2(CO3)22− + 2H+ + 2e− = PuO2(s) + 2HCO3− | 1.016 | 0.839 | |
| PuO2(CO3)34− | PuO2(CO3)34− + 3H+ + 2e− = PuO2(s) + 3HCO3− | 0.781 | 0.604 | |
| Pu(VI)/Pu(III) | PuO22+ | PuO22+ + 4H+ +3e− = Pu3+ + 2H2O | 0.379 | |
| PuO2(OH)+ | PuO2(OH)+ + 5H+ + 3e− = Pu3+ + 3H2O | 0.271 | ||
| PuO2(OH)2 | PuO2(OH)2 + 6H+ + 3e− = Pu3+ + 4H2O | −0.017 | ||
| PuO2CO3 | PuO2CO3 + 5H+ + 3e− = Pu3+ + HCO3− + 2H2O | 0.367 | ||
| PuO2(CO3)22− | PuO2(CO3)22− + 6H+ + 3e− = Pu3+ + 2HCO3− + | 0.285 | ||
| PuO2(CO3)34− | 2H2O | 0.129 | ||
| PuO2(CO3)34− + 7H+ + 3e− = Pu3+ + 3HCO3− + | ||||
| Pu(V)/Pu(IV) | PuO2+ | 2H2O | 1.11 | 0.756 |
| Pu(V)/Pu(III) | PuO2+ | PuO2+ + e− + 2H2O = Pu(OH)4(s) | −0.369 | 0.006 |
| Pu(IV)/Pu(III) | Pu(OH)4 | PuO2+ +4H+ +2e− = Pu3+ + 2H2O | −0.67 | −0.316 |
| Pu(OH)4(s) + 4H+ + e− = Pu3+ + 4H2O |
The reduction potential E0′ was calculated at pH 7 with 1 mM carbonate. The reduction potential E is calculated under the same conditions by fixing the concentration of the soluble species to 1 × 10−6 M. All calculations were made using the thermodynamic data recommended by the OECD Nuclear Energy Agency review (13).
Reduction of Pu(V/VI) species by metal-reducing bacteria.
During the experiments, aqueous Pu concentrations decreased rapidly in the cultures with living cells. The DR analysis of the resulting Pu solids indicates that the resulting solids contain Pu(IV). In these systems, the initial Pu(VI) was partially reduced to Pu(V) by nonbiological processes, which started as soon as the Pu(VI) was added to the cultures. Aqueous Pu in the controls without living cells remained soluble throughout the experiments as a combination of Pu(V) and Pu(VI). These data indicate that G. metallireducens and S. oneidensis are capable of the direct enzymatic reduction of aqueous oxidized Pu(V/VI). Given the redox chemistry of Pu(VI) in these systems, it was not possible to resolve the biological reduction of Pu(VI) and Pu(V) independently. The rates of Pu(V/VI) reduction under cell suspension conditions are comparable to the rates of U(VI) reduction and are higher than that observed for Np(V) (12).
There are no previously published studies that we are aware of that document the enzymatic reduction of Pu(V) or Pu(VI) by G. metallireducens, S. oneidensis, or any other bacteria. However, there are previously published studies that report the biological reduction of Pu(IV) to Pu(III) (2, 7, 16). In the earlier study (16), Pu(III) was not observed directly, and biological reduction was inferred as a result of an enhanced solubility of Pu(IV) with the addition of NTA to cultures containing metal-reducing bacteria. The Pu(IV) bioreduction study conducted by the authors of this paper was conducted under conditions similar to those for the data presented here for Pu(V/VI) bioreduction (2). During the previous Pu(IV) reduction experiments, the production of aqueous Pu(III) was observed at very low concentrations (<0.05 mM) in the absence of chelating agents (2). In the presence of chelating agents, both G. metallireducens and S. oneidensis are able to reduce Pu(IV) solids rapidly to Pu(III) (2).
The cell suspensions of both G. metallireducens and S. oneidensis were able to reduce Pu(VI) and Pu(V) species to Pu(IV), but Pu(III) was not observed in any of the cultures, indicating that further reduction to Pu(IV) did not occur. This observation appears to contradict previous findings (2, 7, 16). However, based on the redox chemistry of Pu (Table 1), there is a greater potential energy yield from Pu(V/VI)-Pu(IV) reduction than from either Pu(V/VI)-Pu(III) or Pu(IV)-Pu(III) reduction. Therefore, in these cultures Pu(V/VI)-Pu(IV) biological reduction is likely to be more favorable than the reduction of Pu(IV) compounds. It also is possible that the reactivity of the biogenic Pu(IV) solid produced through bioreduction is different from both the crystalline PuO2(solid) used for calculations in Table 1 and the inorganically produced Pu(IV) solid used in the previous study, which would preclude further reduction to Pu(III). It also is possible that in these experiments the available nutrients were exhausted and/or the cells were not viable for a sufficient length of time to allow further reduction to Pu(III). We currently are investigating the structure and physicochemical properties of biogenic PuO2, and we plan to examine its accessibility to biological reduction in future experiments.
In our recent examination of Pu(IV) reduction by G. metallireducens and S. oneidensis, we found that the addition of EDTA enhanced the rate and yield of Pu(IV) reduction (2). In the current study, we were unable to introduce ligands in the system to test if they would promote the reduction of Pu(V) and Pu(IV) to Pu(III), because the addition of EDTA, NTA, or any chelators with hard oxygen donor groups would promote the rapid abiotic reduction of Pu(V) and Pu(VI) to Pu(IV). For example, if EDTA is added to cultures containing living cells with Pu(V) and Pu(VI), it will rapidly complex Pu(V) and Pu(VI) and favor their reduction to Pu(IV)-EDTA (1). The Pu(IV)-EDTA then would be rapidly reduced to Pu(III)-EDTA under cell suspension conditions (2).
Aqueous Pu concentrations in cell suspension cultures containing dead cells showed an initial decrease of about 20% for both G. metallireducens and S. oneidensis experiments. This observation was repeatable and consistent. One possible explanation of these data is that Pu(VI) added to the cultures adsorbs onto components of the cell, eventually reduces to the more stable Pu(V), and then desorbs back into solution. The experiments reported here were not designed to assess adsorption processes; however, future experiments should be directed to evaluating the sorption characteristics of Pu(VI) to natural materials.
Although it is clear from our data that cell suspensions of S. oneidensis and G. metallireducens reduce oxidized Pu(V/VI) enzymatically to Pu(IV), it is not clear whether these microorganisms can use oxidized Pu species as the sole terminal acceptor to support their growth. The cell density data suggest that cultures of G. metallireducens and S. oneidensis were able to utilize Pu(V/VI) as the sole electron acceptor to support their growth. However, we were unable to document the production of Pu(IV) in the growth experiment cultures and therefore cannot conclusively state that these organisms are able to use oxidized Pu species to support their growth. The decrease in soluble Pu concentrations in the cultures with living cells may be due to the enzymatic reduction of Pu coupled with bacterial growth, but similar decreases in Pu solubility in the controls could not be attributed to the enzymatic reduction of Pu. We were unable to determine if the reduction in soluble Pu concentrations observed in the control cultures was due to the precipitation of Pu(VI) or the abiological reduction of Pu(VI). Based on our experience with Pu(VI) in these solutions, the most likely explanation is that we exceeded the solubility of Pu(VI), and it precipitated out of solution as a Pu(VI) solid. Experiments on the ability of these organisms to utilize solid forms of Pu(VI) were beyond the scope of this study, and more work needs to be done in this area.
Acknowledgments
The preparation of this paper and research reported herein were supported by the Environmental Remediation and Sciences Program (ERSP), Office of Biological and Environmental Research, U.S. Department of Energy.
Footnotes
Published ahead of print on 10 April 2009.
REFERENCES
- 1.AlMahamid, I., K. A. Becraft, N. L. Hakem, R. C. Gatti, and H. Nitsche. 1996. Stability of various plutonium valence states in the presence of NTA and EDTA. Radiochim. Acta 74:129-134. [Google Scholar]
- 2.Boukhalfa, H., G. A. Icopini, S. D. Reilly, and M. P. Neu. 2007. Plutonium(IV) reduction by the metal-reducing bacteria Geobacter metallireducens GS15 and Shewanella oneidensis MR1. Appl. Environ. Microbiol. 73:5897-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Choppin, G. 2003. Actinide speciation in the environment. Radiochim. Acta 91:645-649. [Google Scholar]
- 5.Choppin, G. R., A. H. Bond, and P. M. Hromadka. 1997. Redox speciation of plutonium. J. Radioanal. Nucl. Chem. 219:203-210. [Google Scholar]
- 6.Cohen, D. 1961. The absorption spectra of plutonium ions in perchloric acid solution. J. Inorg. Nucl. Chem. 18:211-218. [Google Scholar]
- 7.Francis, A. J., C. J. Dodge, and J. B. Gillow. 2008. Reductive dissolution of Pu(IV) by Clostridium sp. under anaerobic conditions. Environ. Sci. Technol. 42:2355-2360. [DOI] [PubMed] [Google Scholar]
- 8.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, M. C. Duff, Y. A. Gorby, S. M. W. Li, and K. M. Krupka. 2000. Reduction of U(VI) in goethite (alpha-FeOOH) suspensions by a dissimilatory metal-reducing bacterium. Geochim. Cosmochim. Acta 64:3085-3098. [Google Scholar]
- 9.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, C. X. Liu, M. C. Duff, D. B. Hunter, and A. Dohnalkova. 2002. Influence of Mn oxides on the reduction of uranium(VI) by the metal-reducing bacterium Shewanella putrefaciens. Geochim. Cosmochim. Acta 66:3247-3262. [Google Scholar]
- 10.Ganesh, R., K. G. Robinson, G. D. Reed, and G. S. Sayler. 1997. Reduction of hexavalent uranium from organic complexes by sulfate- and iron-reducing bacteria. Appl. Environ. Microbiol. 63:4385-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorby, Y. A., and D. R. Lovley. 1992. Enzymatic uranium precipitation. Environ. Sci. Technol. 26:205-207. [Google Scholar]
- 12.Icopini, G., H. Boukhalfa, and M. P. Neu. 2007. Biological reduction of Np(V) and Np(V)-citrate by metal reducing bacteria. Environ. Sci. Technol. 41:2764-2769. [DOI] [PubMed] [Google Scholar]
- 13.Lemire, R. J., J. Fuger, H. Nitsche, P. Potter, M. H. Rand, J. Rydberg, a. Spahiuk, J. C. Sullivan, W. J. Ullman, P. Vitorge, and H. Wanner. 2001. Chemical thermodynamics of neptunium and plutonium, vol. 4. Elsevier, Issy-les-Moulineaux, France.
- 14.Lovley, D. R., E. J. P. Phillips, Y. A. Gorby, and E. R. Landa. 1991. Microbial reduction of uranium. Nature (London) 350:413-416. [Google Scholar]
- 15.Ruggiero, C. E., H. Boukhalfa, J. H. Forsythe, J. G. Lack, L. E. Hersman, and M. P. Neu. 2005. Actinide and metal toxicity to prospective bioremediation bacteria. Environ. Microbiol. 7:88-97. [DOI] [PubMed] [Google Scholar]
- 16.Rusin, P. A., L. Quintana, J. R. Brainard, B. A. Strietel Meier, C. D. Tait, S. A. Ekberg, P. D. Palmer, T. W. Newton, and D. L. Clark. 1994. Solubilization of plutonium hydrous oxide by iron-reducing bacteria. Environ. Sci. Technol. 28:1686-1690. [DOI] [PubMed] [Google Scholar]
- 17.Sani, R. K., B. M. Peyton, W. A. Smith, W. A. Apel, and J. N. Petersen. 2002. Dissimilatory reduction of Cr(VI), Fe(III), and U(VI) by Cellulomonas isolates. Appl. Microbiol. Biotechnol. 60:192-199. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki, Y., S. D. Kelly, K. M. Kemner, and J. F. Banfield. 2002. Radionuclide contamination—nanometre-size products of uranium bioreduction. Nature 419:134. [DOI] [PubMed] [Google Scholar]
- 19.Truex, M. J., B. M. Peyton, N. B. Valentine, and Y. A. Gorby. 1997. Kinetics of U(VI) reduction by a dissimilatory Fe(III)-reducing bacterium under non-growth conditions. Biotechnol. Bioeng. 55:490-496. [DOI] [PubMed] [Google Scholar]