Abstract
Chlorine dioxide gas and vaporous hydrogen peroxide sterilant have been used in the cleanup of building interiors contaminated with spores of Bacillus anthracis. A systematic study, in collaboration with the U.S. Environmental Protection Agency, was jointly undertaken by the U.S. Army-Edgewood Chemical Biological Center to determine the sporicidal efficacies of these two fumigants on six building structural materials: carpet, ceiling tile, unpainted cinder block, painted I-beam steel, painted wallboard, and unpainted pinewood. Critical issues related to high-throughput sample processing and spore recovery from porous and nonporous surfaces included (i) the extraction of spores from complex building materials, (ii) the effects of titer challenge levels on fumigant efficacy, and (iii) the impact of bioburden inclusion on spore recovery from surfaces and spore inactivation. Small pieces (1.3 by 1.3 cm of carpet, ceiling tile, wallboard, I-beam steel, and pinewood and 2.5 by 1.3 cm for cinder block) of the materials were inoculated with an aliquot of 50 μl containing the target number (1 × 106, 1 × 107, or 1 × 108) of avirulent spores of B. anthracis NNR1Δ1. The aliquot was dried overnight in a biosafety cabinet, and the spores were extracted by a combination of a 10-min sonication and a 2-min vortexing using 0.5% buffered peptone water as the recovery medium. No statistically significant drop in the kill efficacies of the fumigants was observed when the spore challenge level was increased from 6 log units to 8 log units, even though a general trend toward inhibition of fumigant efficacy was evident. The organic burden (0 to 5%) in the spore inoculum resulted in a statistically significant drop in spore recovery (at the 2 or 5% level). The effect on spore killing was a function of the organic bioburden amount and the material type. In summary, a high-throughput quantitative method was developed for determining the efficacies of fumigants, and the spore recoveries from five porous materials and one nonporous material ranged between 20 and 80%.
Biological terrorism has become a major concern in the United States since the anthrax spore-tainted letters in the fall of 2001 resulted in contamination and closure of the U.S. Postal Service Curseen-Morris Processing and Distribution Center (Brentwood Post Office), the Hart Senate Office Building, and the American Media Inc. office building in Boca Raton, FL. The contamination of infrastructure posed an unprecedented challenge of decontaminating over 20,000,000 cubic feet (∼1 million sq. ft.) of combined building interior space (6). The incident required concerted action from the government of the United States and the private sector to develop technologies for building interior cleanup. A number of liquid (29) and gaseous (3) products were granted crisis exemptions under the Federal Insecticide, Fungicide, and Rodenticide Act by the U.S. Environmental Protection Agency (EPA) for use as sterilants against Bacillus anthracis spores, but their application and efficacy in the context of large three-dimensional spaces and complex building material surfaces were not fully understood. No products were (or currently are) registered for use in such applications, involving large volumes and complex (porous and nonporous) structural building materials.
In early 2005, a systematic study of laboratory-scale decontamination of five porous surfaces (carpet, ceiling tile, cinder block, painted wallboard, and unpainted wood) and one nonporous surface (painted I-beam steel) was initiated by the U.S. EPA in collaboration with the U.S. Army Edgewood Chemical Biological Center (ECBC). The overall objective of this collaborative study was to systematically investigate the abilities of fumigants to effectively decontaminate building materials contaminated with anthrax spores. This unprecedented systematic investigation involved the determination of efficacy (or log reduction in the number of viable spores) as a function of fumigant technology, technology operating parameters (e.g., fumigant concentration and exposure time), environmental conditions (temperature and relative humidity [RH]), and building material types. The magnitude and scope of this study required that new methods be developed to incorporate the use of complex materials in sporicidal efficacy testing and the processing of an unprecedented number of complex samples.
Current standardized sporicidal test methods include the Association of Official Analytical Chemists (AOAC International) sporicidal activity of disinfectant test (AOAC Official Method 966.04) (4) and the American Society for Testing and Materials (ASTM) 2414-05 (3) and quantitative carrier test (QCT) (2). All of these methods are based on testing hard-surface carrier-based spores, which are submerged in a disinfectant for a desired contact time, followed by the addition of a neutralizer and enumeration of viable spores recovered from the carrier. Almost all standard test methods for liquid disinfectants use small coupons, e.g., 5- by 5-mm squares or 1-cm discs, on which 1 million to 10 million (6 to 7 log) spores are inoculated. While AOAC Official Method 966.04 is qualitative, the other two test methods are quantitative and provide log reduction estimates. Currently, demonstration of a >6-log-unit inactivation of B. anthracis or an appropriate surrogate spore (e.g., Bacillus subtilis) using a quantitative test method, such as QCT, which is also known as ASTM 2197-02, or the three-step method (TSM), also known as ASTM 2414-05, by a decontaminant is a requirement for product registration as a sporicidal agent against spores of B. anthracis Ames (18).
Key information on three critical issues was lacking at the start of this study. First, optimal spore extraction protocols that could be scaled to process over 200 samples per run (or day) were lacking. Second, the appropriate spore challenge level for fumigation studies was unknown, even though a range between 5 and 8 log spores/coupon has been used in a number of recent disinfection studies (12, 13, 14, 16, and 17). Finally, it was not known if protein serum (an organic burden is included in standard procedures, such as AOAC Official Method 966.04) should be included in the testing performed with the fumigants. The specific objectives of this study, therefore, were to (i) develop scalable coupon-processing/spore extraction protocols from six building materials that would result in recovery of >20% of the spores inoculated per coupon, (ii) investigate the effects of three spore challenge levels on spore extraction and the efficacy of chlorine dioxide (CD) gas and vaporous hydrogen peroxide (VHP), and, finally, (iii) investigate the effect of organic burden inclusion on spore recovery and sterilization using CD gas.
MATERIALS AND METHODS
The fumigation studies planned under the joint program between the U.S. Army ECBC and the U.S. EPA's National Homeland Security Research Center presented the daunting challenge of processing over 200 coupons per test (e.g., time course study), including both the test and the control coupons. As a prelude to initiating a laboratory-scale systematic study of this magnitude (between October 2004 and July 2007) of the sporicidal efficacies of two fumigants, CD gas and VHP, critical issues pertaining to titer challenge levels, bioburden, efficient spore extraction from complex building surfaces, exposure of 180 inoculated coupons, and a timely withdrawal of fumigated coupons over a period of 0.5 to 36 h had to be addressed. This method development effort in preparation for the fumigation studies included determining (i) the effects of titer challenge levels on the efficacy of CD gas and VHP, (ii) the effect of the bioburden on the recovery and sporicidal action of CD, and (iii) improved spore recovery from complex building interior surfaces. A custom-designed and -built chamber was developed for the fumigation study. A special sampling port with five compartments, each equipped with doors on the inside (facing the test chamber) and the outside (facing the analyst), had to be designed for ease of withdrawal of the coupons at each of the sampling times. Each compartment was vented independently to the exhaust under negative pressure for safe exposure and handling of the test coupons.
Building material source and coupon preparation and handling.
Bulk quantities of four of the six building interior materials were procured from a high-volume retail store (Home Depot, Bel Air, MD). Cinder blocks were procured from York Building Materials (Aberdeen, MD). The structural steel was procured from Specialized Metals (Coral Springs, FL). With the exception of cinder block (2 to 2.5 by 1.3 cm), 1.3- by 1.3-cm coupons were cut from the interior of the bulk materials. The wallboard coupons were painted with Speed Wall Interior PVA Primer (Glidden; procured from Home Depot, Bel Air, MD), followed by a topcoat of Evermore interior latex paint (Glidden; procured from Home Depot, Bel Air, MD). The I-beam coupons were painted with TT-P-636 red oxide primer (Colorado Paint no. 35-147). The dried painted I-beam steel coupons were rinsed in 70% ethanol, washed thoroughly in distilled water, and completely dried before use. The cinder block coupons were washed in deionized water (to remove loose residues resulting from the cutting process) and completely dried before use. All coupons were sterilized in 15-cm glass petri dishes by autoclaving them for 45 min, using a dry cycle, prior to use.
Bacterial strain, culture, and spore preparation.
A plasmid-free strain of B. anthracis (NNR1Δ1; received from USAMRIID, Fort Detrick, MD; derived from NNR1 [9]) was used in this study. Lack of both plasmids was confirmed by PCR amplification of tox and cap loci (results not shown). Broth cultures were grown in tryptic soy broth, and titer enumerations were performed using tryptic soy agar (TSA) plates after incubation for 22 ± 2 h at 37°C.
An aliquot (1 ml) of overnight-grown broth culture was spread on Lab-Lemko agar plates and incubated at 37°C for 10 ± 4 days. The medium (per liter) contained 23 g Lab-Lemko agar (Oxoid Ltd., Hampshire, United Kingdom), 2 g tryptone (Fisher Scientific, Fair Lawn, NJ), 3 g Bacto yeast extract (Becton Dickinson, Sparks, MD), 2 g Bacto agar (Becton Dickinson, Sparks, MD), and 10 mg MgCl2 (Sigma Chemical Co., St. Louis, MO). Spores were gently dislodged from the plate using a sterile cell spreader and collected in a sterile centrifuge bottle. The spores were triple washed with sterile distilled water before a 30-min treatment in 70% ethanol, followed by a 30-min heat treatment at 65 ± 2°C to rid the sample of nonsporulated vegetative cells. The presence of a ≥85 to 90% spore population following a spore stain and microscopic analysis was an acceptable criterion for the spore quality (14). The spore titer in the suspension was enumerated by making a 10-fold serial dilution in 0.5% Bacto buffered peptone water (BPW) (Becton Dickinson, Sparks, MD) and plating an aliquot of 0.1 ml on triplicate TSA plates for the appropriate dilutions between 10−5 and 10−8. The spore stock was diluted to a 4 × 109/ml titer, and a 1-ml suspension was aliquoted in sterile Eppendorf tubes. For long-term storage and minimal loss of viability, the spores were pelleted, 0.6 ml of the supernatant was discarded, and the tubes were stored in a −20°C freezer.
As required, two or four frozen tubes were thawed and 0.6 ml sterile distilled water was added to each tube. The contents were then pooled in a sterile tube, and a measured volume of sterile distilled water was added to prepare a working stock with a titer of 4 × 108/ml. Additional working stocks of other titers were prepared in a similar manner as needed to achieve the desired challenge level (spores/coupon). Equal volumes of the appropriate working stock and 1% sterile fetal bovine serum solution were mixed to obtain a spore suspension with a 2 × 108/ml titer containing 0.5% serum protein. Spore suspensions containing other amounts of fetal serum were prepared by mixing equal volumes of a working stock and a solution containing 2, 4, or 10% protein serum. All working stocks and spore suspensions were stored at 4°C in sterile tubes and used within 30 days of preparation.
Coupon inoculations.
Sterile coupons were inoculated with an aliquot of 50 μl of the appropriate spore suspension. Spore inoculation was performed as one 50-μl spot to each coupon. The suspension was allowed to dry in a biosafety level 2 hood for 16 ± 2 h before use. The inoculated coupons were used within 24 h of inoculation.
Spore extraction from coupons.
Control and/or fumigated coupons were transferred into 10 ml BPW in a 50-ml disposable sterile tube. The effects of different surfactants (Tween 20, Tween 80, and Triton X-100) on spore recovery from complex building materials were investigated. No statistically significant difference in spore recovery was observed among different surfactants (results not shown), and 0.05% Tween 80 was included in all subsequent experiments. The tubes were then sonicated for 10 min and vortexed for 2 min to dislodge the spores from the coupon surface. Sonication was done using a tabletop Bransonic cleaner (40-kHz frequency; Branson Ultrasonic, Danbury, CT). The ceiling tile and wallboard were found to lose their integrity, resulting in generation of particulate debris; this degeneration necessitated the use of wide-orifice pipette tips for aliquoting small sample volumes. Following extraction, 10-fold dilutions were performed as needed, 0.1-ml aliquots were spread in triplicate on TSA plates (spread plating), and the plates were incubated for 18 to 24 h at 37°C. For fumigated test samples containing very low numbers of viable spores, 1.0-ml aliquots were transferred in triplicate (3 ml total) to each of the three petri plates, followed by addition of 20 to 25 ml of liquefied TSA (prewarmed to 55°C) to each plate, and mixed by gentle rotational swirling (pour plating). Following 2 to 3 h of solidification at room temperature, the plates were inverted and incubated at 37°C. CFU were read with a QCount counter (Spiral Biotech, Norwood, MA) after 2 and 6 days of incubation.
CD gas generator, VHP generator, and coupon fumigation.
The Cloridox-GMP generator (ClorDiSys, Inc., Lebanon, NJ) used for generating CD gas was equipped for real-time monitoring of key process parameters, i.e., pressure, temperature, RH, and gas concentration. The generator produced CD gas by passing chlorine gas over a series of three cartridges containing solid sodium hypochlorite to generate (theoretically) a 20-liter/min flow rate at 40,000 ppm of volume (ppmv) of CD gas. This flow was introduced into the custom-designed fumigation chamber (discussed below) as required to achieve and maintain the target CD concentration in the chamber throughout the sterilization cycle. The CD gas in the fumigation chamber was measured in real time by a spectrophotometer on the Cloridox-GMP system, and the concentration of CD was controlled by feedback via a programmable logic controller valve. Typically, a sterilization cycle with the desired CD concentration was programmed with four phases: (i) preconditioning (RH ramps to 75% ± 5%) and conditioning (30 min at 75% ± 5% RH), (ii) charging (CD concentration ramps to the set point, ranging between 1.4 and 10.0 mg/liter or 500 to 3,600 ppmv), (iii) exposure or sterilization (holding time during which the RH was maintained at 75% ± 5% and the CD concentration at the desired set point), and (iv) aeration (the CD gas was removed from the chamber to permit safe opening of the chamber door).
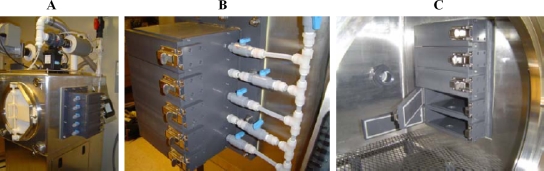
Test coupons and uninoculated control coupons of all six building materials were placed in 15-mm petri plates. The petri plates were then placed on the floor of the 8-ft3 stainless steel fumigation (or test) chamber, which was equipped with five sampling ports (Fig. 1) for withdrawal of fumigated samples exposed for different times without altering the process parameters. This test chamber was custom designed and built to accommodate the time course studies that were conducted following the development of the method discussed in this paper.
FIG. 1.
Fumigation chamber with five sampling ports. (A) The fumigation chamber with the sampling port from the outside. (B) The sampling port with five compartments individually aerated to the exhaust. (C) The sampling port is equipped with doors, which open on the inside of the fumigation chamber.
The VHP generator, M100SX (laboratory scale), capable of sterilizing a space of 2 to 70 cubic feet, was procured from Steris Corporation (Mentor, OH). The VHP was generated by passing warm Vaporox (35% formulated hydrogen peroxide) over a vaporizer (hot plate), which converted liquid hydrogen peroxide solution into vapor form. A typical programmed cycle involved the following phases: (i) dehumidification of the chamber (RH was brought to 30 to 35%); (ii) conditioning, in which the VHP concentration ramped to a set point (e.g., 300 ppmv, used in this study); (iii) sterilization (the VHP concentration was maintained for the holding time); and (iv) aeration (the fumigant was removed from the chamber). VHP is highly unstable in a compressed-gas cylinder, so the sterilant is not available commercially. Hence, the VHP concentration was monitored using an electroluminescence-based Draeger sensor (Draeger Safety Inc, Sugarland, TX) calibrated with 10 ppm of sulfur dioxide gas in accordance with the manufacturer's manual (8). During calibration, the hydrogen peroxide sensor stores a defined sulfur dioxide cross-sensitivity in the internal memory, and the cross-calibration may give an error of ±20% for VHP (8). The chamber discussed above was used for both CD and VHP fumigation experiments.
Spore enumeration, log reduction, and recovery estimation.
Typically, the plates with between 10 and 300 CFU (averaged from three replicate plates) were included in the enumeration calculations. Samples with CFU values outside this range were replated at appropriate dilutions. Since only a small fraction of the recovered sample volume (0.3 ml out of the 10-ml total sample volume) was analyzed by spread plating, samples with 0 CFU on spread plates (from 10−1 dilution tube) were further analyzed by screening a one-third fraction of the sample volume by pour plating (1 ml/plate on three plates). An extensive attempt in this study was therefore made to account for the presence of as few as one to five viable spores in such samples, i.e., low numbers of viable spores. The log values for the total number of CFU per coupon were determined for each of the five replicate coupons of each material type in the sterilization cycle. The average and standard deviation were computed from five replicate log values. Samples with 0 CFU on all three plates per coupon were assigned a value of 1 CFU in order to obtain a log value of 0. Log reduction (log reduction = log CFU control − log CFU fumigated) was computed by subtracting the log CFU of fumigated samples from that of the control samples. Titer controls (BPW samples spiked with the same volume and number of spores), positive controls (building material coupons inoculated, dried, and extracted), and test samples (inoculated and fumigated) were all treated similarly, i.e., sonicated and vortexed. Ten-minute sonication and 2-min vortexing treatments of spores had no adverse effect on spore viability (results not shown). Titer controls were performed with each fumigation experiment. Percent recoveries were computed from the titer control values and the mean CFU values recovered from the coupons after they were dried and processed. Spore recovery was estimated as a percentage of the spore numbers recovered relative to the number inoculated per coupon.
For all data, a Wilcoxon rank sum test was used to determine if the statistically significant differences were due to variations in the response variables. This is a nonparametric test and thus does not require that the data come from any distributional shape. The exact form of the test was used due to the small sample sizes involved. The magnitudes of the differences between the experimental conditions were estimated using the Hodges-Lehman estimators (11).
RESULTS
STEM.
Standardized test methods, such as ASTM E 2414-5 (TSM) (3) or QCT-2 (quantitative disc carrier test) (2), for determining the efficacies of disinfectants typically were designed to process 12 to 18 samples a day and rely on the use of very thin, small (0.1- by 0.5- by 0.5-cm or 0.1- by 1- by 1-cm) carriers (14, 16); multiple washings; and/or multiple transfers. The three-dimensional structure of the building interior surfaces selected for this study necessitated the use of larger coupons in order to account for the lack of homogeneity of the test materials. Coupons with dimensions of 1.3 by 1.3 cm and various thicknesses were used for this effort. Due to the greater size, thickness, and complexity of the materials and the number of samples per test, a single-tube extraction method (STEM) was developed in the study. The STEM made use of presterilized disposable 50-ml tubes for which physical treatments (sonication and vortexing) to dislodge spores from the surface were all performed in the same tube to reduce the logistical burden of generating multiple tubes for analysis (as is the case with standardized test methods). The use of a high-capacity vortexer (Glas-Col; Q Glass Co., Inc., Towaw, NJ) for simultaneous vigorous mixing of 48 tubes, the use of petri plates for holding a large number of coupons (36 in all), and the designing of a novel sampling port for withdrawal of multiple plates without altering the process parameters were essential for successful completion of this unprecedented level of study. In general, the spore recovery from complex building materials using the method described above ranged between 25 and 80% (Table 1).
TABLE 1.
Average spore recovery from different material surfacesa
| Coupon type | Mean % recovery ± SD |
|---|---|
| Carpet | 61 ± 25 |
| Ceiling tile | 78 ± 18 |
| Concrete | 32 ± 11 |
| Steel | 24 ± 15 |
| Wallboard | 50 ± 28 |
| Wood | 26 ± 5 |
These results are based on averages computed from a set of four experiments, each with five replicate coupons. The spore recovery estimations were computed as described in Materials and Methods.
All standardized test methods developed to date are limited to using either glass or steel as a test material and the use of liquid disinfectants as sporicidal chemicals. A test was run to compare the STEM method with the TSM, and the results were comparable (<10% difference in terms of spore recovery and sporicidal efficacy [data not shown]).
In summary, a new quantitative test method (STEM) for determining the efficacies of fumigants was developed. The method can be scaled to process a large number of samples. A spore challenge level of 1 × 107 per coupon was selected based on >5-log spore kill on various coupons. Inclusion of up to 0.5% serum in the spore suspension was shown to have a negligible effect on the spore recovery and kill efficacies of the two fumigants. The results of the testing used to develop the method to fit the needs of large-scale decontaminant efficacy evaluations are presented here.
Sporicidal efficacies of CD gas and VHP vapor as a function of spore number.
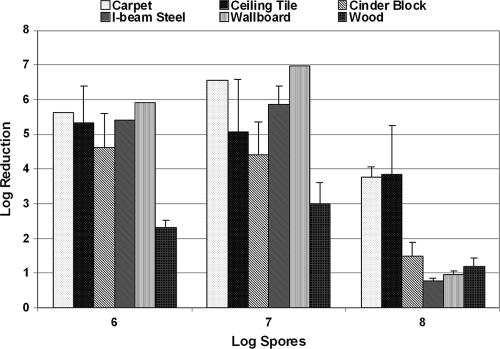
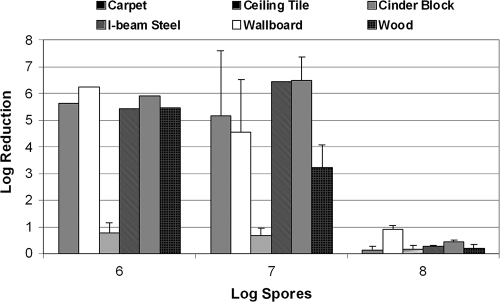
The spore challenge levels used for testing the efficacies of liquid sporicidal disinfectants have ranged between 1 × 105 and 1 × 108 in the literature (12, 14). In order to determine the appropriate spore load for the fumigation study, coupons of building interior surfaces were inoculated with spores at three challenge levels: 1 × 106, 1 × 107, and 1 × 108, prepared from avirulent B. anthracis NNR1Δ1. The test coupons were then exposed to CD gas (∼3,600 ppmv [10 mg/liter] for 3 h for a dose, or a CT [concentration × time product] of 10,800 ppmv·h) in the fumigation chamber. As seen in Fig. 2, the log reduction values ranged between 2.5 (wood) and 6.6 (ceiling tile) log units at the 1 × 106 challenge level. No significant drop in log reduction was observed at 1 × 107. However, a decrease in log reduction (or the sporicidal efficacy of CD gas) was observed for all six surface types at the 1 × 108-spore challenge level. Further analysis of the data showed no statistically significant difference in the CD efficacy of spore killing on different materials at the three spore-loading levels at a >95% confidence level (P = 0.05). Statistically significant differences were evident for CD efficacies at different loading levels (6 log units versus 8 log units) among carpet, cinder block, I-beam steel, wallboard, and wood at a >90% confidence level, albeit with greater risk of false positives.
FIG. 2.
Sporicidal efficacy of CD gas (9,000-ppmv·h CT) as a function of spore challenge levels. Five replicate coupon types were exposed to 3,600 ppmv of CD gas for 3 h. Spores were extracted and enumerated. Log reductions were computed as described in Materials and Methods. The error bars indicate standard deviations.
Figure 3 summarizes the results of a similar experiment performed to assess the sporicidal action of VHP (290 ppmv for 3 h for a dose, or a CT of 870 ppmv·h) as affected by the spore challenge levels. As observed with CD gas, a range in log reduction values (0.8 for cinder block to 6.1 for ceiling tile) was observed, depending on the material type, at both inoculation levels of 1 × 106 and 1 × 107 spores without a significant impact of the challenge titer. The log reductions, as with CD gas, were observed to be a strong function of the material type. However, a significant decrease in the effectiveness of VHP was observed when the spore number per coupon was increased to 1 × 108. This change in log reduction was far more pronounced for VHP than for CD gas. Further analysis of the data showed a lack of statistically significant difference in VHP efficacies of spore killing on different materials at the three spore-loading levels at a >95% confidence level (P = 0.05%). Statistically significant differences were evident for VHP efficacies at different loading levels (6 log units versus 8 log units) among carpet, cinder block, wallboard, and wood at a >90% confidence level, albeit with greater risk of false positives.
FIG. 3.
Efficacy of VHP (870-ppmv·h CT) as a function of spore challenge levels. Five replicate coupons were exposed to 290 ppmv of VHP for 180 min, and spores were extracted. The log reductions were computed as described in Materials and Methods. The error bars indicate standard deviations.
Based on these results from the trial CD gas and VHP tests, a spore challenge level of 1 × 107 per coupon was selected for the detailed fumigation studies. This spore challenge level was determined to be appropriate to demonstrate a 6-log-unit kill efficacy, generally considered acceptable as a performance criterion for registration of sporicidal products for B. anthracis spores under the Federal Insecticide, Fungicide, and Rodenticide Act (18). In addition, related studies (12, 14) of the sporicidal efficacies of liquid disinfectants and VHP have used 7 log units and 8 log units (distributed as 10 droplets over a greater surface area), respectively.
Effect of bioburden on spore killing by CD gas and spore recovery.
All standardized test methods for liquid disinfectant efficacy testing (e.g., AOAC Official Method 966.04 and ASTM 2197-02), with the specific exception of ASTM 2414-05, use a spore suspension containing an organic burden (a serum solution to mimic the organic burden). In the context of building cleanup, neither the nature nor the amount of inorganic or organic burden has been characterized. In the present study, the effect of serum inclusion on spore recovery and kill efficacy was studied in an attempt to understand the effect of the bioburden on the sporicidal action of the fumigant. Previous efficacy studies with liquid disinfectants have included various organic burdens ranging between 0 and 5% bovine serum in the spore preparation (1, 3, 12, 13). The protein serum represents the organic burden typically expected in disinfection of medical devices used in dental or medical operating rooms (7, 15). However, spores on environmental surfaces, including building interiors, are expected to be in contact with diverse categories of inorganic and/or organic burden. A consensus is lacking with regard to the nature and amount of bioburden that should be included with the test inoculant in laboratory-scale studies to evaluate the efficacies of fumigants and gaseous products. In this series of experiments, fetal bovine serum was included as representative of organic bioburden, and its effect on spore recovery and CD gas efficacy was investigated.
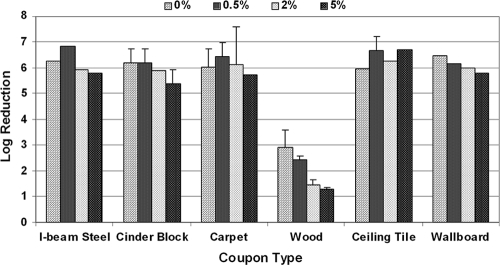
All six coupon types were inoculated with 1 × 107 spores containing 0, 0.5, 2, or 5% (volume of total spore suspension) serum and fumigated with CD gas (3,600 ppmv for 3 h for a dose, or a CT of 10,800 ppmv·h). As seen in the results summarized in Fig. 4, no significant drop in the effectiveness of CD gas on log reduction was observed for carpet and ceiling tile surfaces for inclusion of serum up to 5% with the spore suspension. A slight drop in the kill efficacy was observed for I-beam steel, cinder block, and wallboard as the serum concentration was increased to 5%. Interestingly, the most significant drop in kill efficacy for CD gas was observed for the pine wood surface when the serum concentration was increased to 5%. Further analysis of the data showed that at a >95% confidence level (P = 0.05), statistically significant differences were observed in CD efficacy for increased bioburden on all six materials between 0.5% and 2%, 0.5% and 5%, and 2% and 5%. However, the addition of bioburden from 0 to 0.5% resulted in statistically significant differences for only four of the building materials (carpet, ceiling tile, I-beam steel, and wallboard).
FIG. 4.
Effect of fetal bovine serum on the efficacy of CD gas (9,000-ppmv·h CT). Five replicate coupons were exposed to 3,600 ppmv of CD gas for 3 h, and spores were extracted. The log reductions were computed as described in Materials and Methods. The error bars indicate standard deviations.
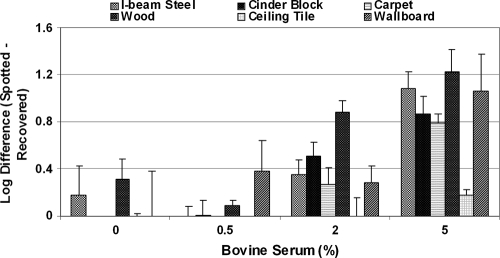
The effect of an increasing organic burden on spore recovery is summarized in Fig. 5. The lowest spore number was recovered from pine wood, a highly porous surface. The spore recovery dropped by 0.2 to 1 log unit when the serum concentration was increased to 5% from all but the wood surface (>2-log-unit drop). Further analysis of the data showed that at the >95% confidence level, spore recoveries from all materials were statistically significantly different when the bioburden was increased from 0 or 0.5% to 5%. Statistically significant differences in spore recovery were also observed for all materials (except ceiling tile) when the bioburden level was increased from 2 to 5%. Spore recoveries were statistically significantly different for ceiling tile, I-beam steel, and wood (0 and 0.5% serum) and for carpet, cinder block, and wood (0 and 2% serum).
FIG. 5.
Effect of bioburden inclusion on spore recovery from building surfaces using CD gas fumigant. Spore suspensions were prepared with different serum concentrations. Five replicate coupons were inoculated with an aliquot of 50 ml spore suspension and dried as described in Materials and Methods. The log difference was computed by subtracting the log CFU recovered from the coupons after drying and log CFU estimated in the aliquot spotted on coupons The error bars indicate standard deviations.
Because of a negligible effect of 0.5% serum on the sporicidal efficacy of CD gas and the spore recovery results, a low level of protein serum, 0.5%, was included with the spore preparation used in future fumigation studies to increase the sporicidal challenge for the fumigant (unpublished data). Further work is required to determine the nature (organic or inorganic) and the amount of burden that should be included in laboratory- or building-scale studies. It may be reiterated here that in many of the published studies of disinfectant testing, no bioburden was included (12, 13, 17).
The results summarized here were obtained using a spore suspension dried on various surfaces. In contrast to the use of aerosolized spores for determining the efficacies of swabs and wipes for spore recovery (5, 10), recovery of dried spores on environmental surfaces is even more challenging. This challenge is likely due to the increased adhesion of spores to the material surface and/or penetration of the spores within the pores of complex building materials.
Finally, the mean spore recovery from different material surfaces ranged between 24 and 78% of the inoculated spores (Table 1). Generally, >50% average spore recoveries were obtained for carpet, ceiling tile, and wallboard. Spore recoveries from cinder block, I-beam steel, and wood were low (24 to 32%). At a >95% significance level, a paired t test showed statistically significant spore recoveries from all except carpet-wallboard, cinder block-wallboard, cinder block-wood, cinder block-steel, and steel-wood. There was no apparent correlation between spore recovery and the nature of the coupon material, i.e., porous or nonporous. The varying effectiveness of physical treatments, i.e., sonication and vortexing, used to dislodge the spores from various coupons could have determined the extents of spore recovery from different materials.
DISCUSSION
A number of published studies have compared the efficacies of different liquid disinfectants on relatively simple, smooth surfaces, such as glass and steel. The efficacies of fumigants on building materials are relatively unknown. A systematic decontamination program was jointly launched by the U.S. EPA and the U.S. Army to focus on optimizing the efficacies of fumigants on building interior surfaces. Critical issues pertaining to the spore challenge levels and bioburden with respect to two fumigant sterilants, CD gas and VHP, were addressed in this study. Significant progress was made toward high-throughput sample processing and overcoming the challenges to performing future kinetics studies with these fumigants (unpublished data). In brief, the use of small coupons (1.3 by 1.3 cm, easily processed in a single tube) and a STEM, along with the design and use of a test chamber with novel sampling ports, permitted the processing of over 200 samples a day. A significant drop in the efficacies of both sterilants, CD gas and VHP, was observed when 1 × 108-spore challenge levels were used. However, a significant difference in efficacy was not observed between 1 × 106-and 1 × 107-spore challenge levels per coupon. Inclusion of 0.5% fetal bovine serum (as an organic burden) with the spore preparation was found to have a negligible effect on either spore recovery or the efficacy of CD gas. However, higher bioburden concentrations did significantly impact spore recovery from positive control coupons. In general, the mean spore recovery from different material surfaces ranged between 24 and 78% of the inoculated spores (Table 1). There was no apparent correlation between spore recovery and the nature of the coupon material, i.e., porous or nonporous. The effectiveness of physical treatments, i.e., sonication and vortexing, used to dislodge the spores from various coupons may determine the extent of spore recovery.
Since only one strain (avirulent B. anthracis) was used in the present study, the relevance of the results obtained with STEM in the context of other strains or surrogates must be addressed. Recent work by Sagripanti et al. (14) clearly established similar or comparable sensitivities of spores from B. anthracis strains and other commonly used simulants, such as B. subtilis, Bacillus cereus, Bacillus thuringiensis, Bacillus atrophaeus, and Bacillus megaterium, for a number of different disinfectants, e.g., Clorox, Decon Green, and Sandia DF 100 and DF 200. Even though the results presented here were derived from the use of only one strain of B. anthracis, we have confirmed high spore recovery and fumigant efficacy with the STEM protocol with spores from three other simulants of B. anthracis, i.e., B. subtilis, B. atrophaeus, B. thuringiensis, and Geobacillus stearothermophilus (results not shown).
In conclusion, a high-throughput quantitative test method was developed for processing a large sample set (200 coupons). The efficacies of the two sterilants, CD gas and VHP, were not statistically significantly different when three different spore-loading levels were used in separate experiments. A spore challenge level of 1 × 107 per coupon was selected for efficacy studies (unpublished data). Interestingly, inclusion of increasing amounts of serum as a bioburden to 2% or 5% resulted in decreased efficacy of CD gas and decreased spore recovery from building materials. However, inclusion of 0.5% bioburden concentrations affected spore recovery from only three out of six materials, i.e., ceiling tile, I-beam steel, and wood from positive control coupons.
Acknowledgments
We thank Phil Koga, U.S. Army ECBC, for his comments on the development of the quality assurance project plan for this effort. We also acknowledge Luther A. Smith and Melissa Nysewander of Alion Science and Technology for their statistical support provided under an independent vehicle with the U.S. EPA.
The U.S. EPA through its Office of Research and Development funded and collaborated with the Department of the Army, ECBC, in the research described here under Interagency Agreement DW-21-93991701.
This study has been subjected to an administrative review but does not necessarily reflect the views of the U.S. EPA. No official endorsement should be inferred. The U.S. EPA does not endorse the purchase or sale of any commercial products or services.
Footnotes
Published ahead of print on 3 April 2009.
REFERENCES
- 1.American Society for Testing and Materials. 2000. Standard quantitative carrier test method to evaluate the bactericidal, fungicidal, mycobactericidal, and sporicidal potencies of liquid chemical germicides. Standard test method E-2111-05. American Society for Testing and Materials, West Conshohocken, PA.
- 2.American Society for Testing and Materials. 2002. Standard quantitative disk carrier test method. American Society for Testing and Materials, West Conshohocken, PA.
- 3.American Society for Testing and Materials. 2005. Quantitative sporicidal three-step method (TSM) to determine sporicidal efficacy of liquids, liquid sprays, and vapor or gases on contaminated carrier surfaces. Standard test method E-2414-05. American Society for Testing and Materials, West Conshohocken, PA.
- 4.Association of Official Analytical Chemists. 2006. Official methods of analysis, 18th ed. AOAC International, Gaithersburg, MD.
- 5.Brown, G. S., R. G. Betty, J. E. Brockman, D. A. Lucero, C. A. Souza, K. S. Walsh, R. M. Boucher, M. Tezak, M. C. Wilson, and T. Rudolph. 2007. Evaluation of a wipe surface sample method for collection of Bacillus spores from nonporous surfaces. Appl. Environ. Microbiol. 73:706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canter, D. A. 2005. Addressing residual risk issues at anthrax cleanup. How clean is safe? J. Toxicol. Environ. Health Anal. 68:1017-1032. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, R. P., R. A. Robison, D. F. Robinson, B. J. Ploegger, R. W. Leavitt, and H. L. Bodily. 1989. Antimicrobial activity of environmental surface disinfectants in the absence and presence of bioburden. J. Am. Dent. Assoc. 119:493-505. [DOI] [PubMed] [Google Scholar]
- 8.Dräger Safety AG. 2008. DrägerSensor H2O2 JC-68 09 070 data sheet. http://www.draeger.com/ST/internet/pdf/Master/En/gt/9023385_h2o2_hc_d_e.pdf. Dräger Safety AG, Lübeck, Germany.
- 9.Green, B. D., L. Battisti, T. M. Koehler, C. B. Thorne, and B. E. Ivins. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun. 49:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodges, L. R., L. J. Rose, A. Peterson, J. Noble-Wang, and M. J. Arduino. 2006. Evaluation of macrofoam swab protocol for the recovery of Bacillus anthracis spores from a steel surface. Appl. Environ. Microbiol. 72:4429-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollander, M., and D. A. Wolfe. 1999. Nonparametric statistical methods, p. 787-791. Wiley, New York, NY.
- 12.Rogers, J. V., C. L. K. Sabourin, Y. W. Choi, W. R. Rudnicki, D. C. Riggs, M. L. Taylor, and J. Chang. 2005. Decontamination assessment of Bacillus anthracis, B. subtilis, and Geobacillus stearothermophilus spores on indoor surfaces using a hydrogen peroxide gas generator. J. Appl. Microbiol. 99:739-748. [DOI] [PubMed] [Google Scholar]
- 13.Sagripanti, J.-L., and A. Bonifacino. 1997. Effects of salt and serum on sporicidal activity of liquid disinfectants. J. AOAC Int. 80:1198-1207. [PubMed] [Google Scholar]
- 14.Sagripanti, J.-L., M. Carrera, J. Insalaco, M. Ziemski, J. Rogers, and R. Zandomeni. 2007. Virulent spores of Bacillus anthracis and other Bacillus species deposited on solid surfaces have similar sensitivity to chemical decontaminants. J. Appl. Microbiol. 102:11-21. [DOI] [PubMed] [Google Scholar]
- 15.Saunders, T. R., V. L. Guillory, S. T. Gregoire, M. Pimsler, and M. S. Mitchell. 1998. The effect of bio-burden on in-depth disinfection of denture base acrylic resin. J. Calif. Dent. Assoc. 26:846-850. [PubMed] [Google Scholar]
- 16.Springthorpe, V. S., and S. A. Sattar. 2005. Carrier tests to assess microbial activities of chemical disinfectants for use on medical devices and environmental surfaces. J. AOAC Int. 88:182-201. [PubMed] [Google Scholar]
- 17.Tomasino, S. F., and M. A. Hamilton. 2007. Comparative evaluation of two quantitative test methods for determining the efficacy of liquid sporicides and sterilants on a hard surface. J. AOAC Int. 90:456-464. [PubMed] [Google Scholar]
- 18.U.S. Environmental Protection Agency. 2007. A set of scientific issues being considered by the environmental protection agency regarding: guidance on test methods for demonstrating the efficacy of antimicrobial products for inactivating Bacillus anthracis spores on environmental surfaces. FIFRA SAP meeting no. 2007-50. http://www.epa.gov/scipoly/sap/meetings/2007/071707_mtg.htm.