Abstract
Electricity generation from wheat straw hydrolysate and the microbial ecology of electricity-producing microbial communities developed in two-chamber microbial fuel cells (MFCs) were investigated. The power density reached 123 mW/m2 with an initial hydrolysate concentration of 1,000 mg chemical oxygen demand (COD)/liter, while coulombic efficiencies ranged from 37.1 to 15.5%, corresponding to the initial hydrolysate concentrations of 250 to 2,000 mg COD/liter. The suspended bacteria found were different from the bacteria immobilized in the biofilm, and they played different roles in electricity generation from the hydrolysate. The bacteria in the biofilm were consortia with sequences similar to those of Bacteroidetes (40% of sequences), Alphaproteobacteria (20%), Bacillus (20%), Deltaproteobacteria (10%), and Gammaproteobacteria (10%), while the suspended consortia were predominately Bacillus (22.2%). The results of this study can contribute to improving understanding of and optimizing electricity generation in microbial fuel cells.
Wheat straw is one of the most abundant renewable resources. According to the Food and Agriculture Organization of the United Nations, approximately 1.9 × 109 tons of wheat straw annually are produced worldwide, accompanied by 6.2 × 108 tons of wheat production. Wheat straw is composed of 35 to 45% cellulose and 20 to 30% hemicelluloses with a relatively low lignin content (<20%) (42). The hemicellulose fraction of the straw is easily hydrolyzed to its constituent sugars by a hydrothermal treatment process, forming a carbohydrate-enriched liquid hydrolysate (46). Chemical and biological approaches to sustainable energy production from the liquefied hydrolysates to energy carriers, such as methane, ethanol, and H2, have been developed. However, many of these approaches encounter technical and economical hurdles (10, 12, 15, 16). An alternative strategy is direct conversion of wheat straw biomass to electrical energy in microbial fuel cells (MFCs).
MFCs are bioelectrochemical reactors in which microorganisms mediate the direct conversion of chemical energy stored in organic matter or bulk biomass into electrical energy (12, 15, 16, 40). Various substrates, such as simple carbohydrates, low-molecular-weight organic acids, starch, amino acids, chitin, cellulose, domestic wastewater, food-processing wastewater, recycled paper wastewater, and marine sediment organic matter, have been successfully utilized for power generation in MFCs (16-18, 27, 30, 33). To understand the microbial constraints on various fuel-powered MFCs, microbial communities have been characterized by several groups. Microbial communities from various systems are very different and often diverse, ranging from well-known metal- and anode-reducing bacteria to unknown exoelectrogens (1, 20, 21). It has been found that parameters such as the substrates used as fuels and the inocula used for starting up the MFCs can influence the anode bacterial communities in an MFC, which subsequently influence the efficiency of the MFCs (3, 14, 22, 38, 44). Different pure substrates, such as acetate, glucose, and lactate, were used as fuel to compare the microbial communities that developed in the MFCs. Regardless of the different substrates, all anode communities contained sequences closely affiliated with Geobacter sulfurreducens (>99% similarity) and an uncultured bacterium clone belonging to the family Bacteroidaceae (99% similarity). Firmicutes were only found in glucose-fed MFCs (20). Microbial-community analyses of MFCs powered with complex substrates have also been performed by several researchers, and their results were very diverse. The microbial community in starch wastewater-powered MFC was dominated by unidentified bacteria (35.9%), followed by Betaproteobacteria (25.0%), Alphaproteobacteria (20.1%), and the Cytophaga/Flexibacter/Bacteroides group (19.0%) (21). The anode-attached consortia in a cellulose-powered MFC were related to Clostridium spp., while Comamonas spp. were abundant in the suspended consortia (13). Although many studies have reported the microbial compositions of MFCs, it is still unclear which microbial communities develop as a function of the external parameters.
Wheat straw biomass constitutes a large source for bioenergy production and shows promising prospects for electricity generation in MFCs. Therefore, wheat straw biomass was used to study the microbial communities that develop during the operation of an MFC in order to better understand the microbial electrochemical roles and potentially improve MFC performance.
The objectives of this study were to (i) test wheat straw hydrolysate as a potential fuel in an MFC for electricity generation and (ii) study the microbial composition and evolution of electricity-producing communities in a two-chamber MFC system. Phylogenetic-diversity analysis of the enriched consortia was conducted to verify the presence of hydrolytic and respiratory anaerobes that could couple hydrolysate oxidation with proton reduction in the anode chamber. This is the first report of exploiting microbial communities for direct conversion of wheat straw hydrolysate to electrical energy in an MFC.
MATERIALS AND METHODS
Pretreatment of wheat straw.
The wheat straw was pretreated by liquefaction in three steps, as described by Thomsen et al. (45). The pretreatment resulted in a predominately cellulose-containing solid fraction and a liquid fraction, called the hydrolysate. The composition of the hydrolysate is shown in Table 1. Before use, the hydrolysate was diluted to the desired chemical oxygen demand (COD) concentration (250 to 2,000 mg COD/liter, as indicated) using wastewater or a buffered nutrient medium (31). The concentrations of furfurals and phenols therefore ranged from 0.01 mM to 0.12 mM in this study. It was previously reported that furan derivatives and phenolic compounds did not affect electricity generation from glucose even at concentrations of 10 mM (7). The pH of the diluted solutions was adjusted to 7.0 with 0.1 N NaOH.
TABLE 1.
Composition of wheat straw hydrolysatea
| Component | Content |
|---|---|
| pH | 4.87 ± 0.1 |
| TS (%) | 4.40 ± 0.01 |
| VS (%) | 3.40 ± 0.01 |
| Ash content (%) | 1 ± 0.01 |
| SS (g/liter) | 0.155 ± 0.05 |
| VSS (mg/liter) | 0.32 ± 0.1 |
| COD (g/liter) | 37.95 ± 1.31 |
| SCOD (g/liter) | 32.05 ± 2.22 |
| VFA (g/liter) | 0.68 ± 0.14 |
| Ethanol (g/liter) | ND |
| Total nitrogen (g/liter) | 0.20 ± 0.01 |
| Ammonia (g/liter) | 0.03 ± 0.01 |
| Protein (g/liter) | 1.09 ± 0.03 |
| Lipids (%) | 0.24 |
| Furfurals (g/liter) | 0.25 ± 0.04 |
| HMF (g/liter) | 0.14 ± 0.02 |
| Phenols (g/liter) | 0.142 |
| Lignin (g/liter) | ND |
| Arabinose (g/liter) | 1.3 |
| Xylose (g/liter) | 9.27 |
| Glucose (g/liter) | 2.94 |
ND, nondetectable; TS, total solids; VS, volatile solids; SS, suspended solid; VSS, volatile suspended solid; SCOD, soluble COD; VFA, volatile fatty acid; HMF, 5-hydroxymethyl furfural.
MFC construction and operation.
H-type two-chamber MFCs were constructed as described by Oh et al. (36). The anode and cathode chambers were separated by a proton exchange membrane (diameter, 15 mm; Nafion 117; DuPont Co.), and the total volume and the working volume of each chamber were 300 and 250 ml, respectively. The anode and cathode electrodes (3 by 7 cm; 42 cm2) were made of Toray carbon paper (TGPH-120; Etek). The distance between the electrodes was approximately 10 cm. Electrical connections and pretreatment of electrodes were as previously described (36).
Wastewater collected from a primary clarifier (Lyngby Wastewater Treatment Plant, Copenhagen, Denmark) was first amended with hydrolysate and then used as the inoculum and fuel in the anode. Following inoculation and stable power generation, the wastewater medium was replaced with a vitamin-amended nutrient medium as described previously (31). The system was considered to be ready for stable electricity production when the maximum voltage output of one batch cycle was reproducible after the reactor was filled with fresh medium at least twice. The medium in the reactor was replaced when the voltage dropped below 50 mV (resistance, 1,000 Ω). The cathode chamber was filled with 50 mM ferricyanide solution [K3Fe(CN)6 in 50 mM phosphate buffer (NaH2PO4·H2O, 4.22 g liter−1; Na2HPO4·H2O, 2.75 g liter−1) adjusted to pH 7.0 with 1 N NaOH] as the catholyte. The cathodic solution was continuously stirred at 300 rpm using a magnetic stirring bar to ensure effective mixing.
The initial pH of all solutions was adjusted to 7.0. MFCs were operated at room temperature (20°C). All electrode transfers and inoculation procedures were conducted in an anaerobic glove box (Coy Scientific Products).
Electrochemical measurements and calculations.
The voltage (V) across an external resistor in the MFC circuit was monitored at 30-min intervals using a multimeter connected to a personal computer. The current (I), power (P = IV), and coulombic efficiency (CE) were calculated as previously described, with the power density normalized to the projected surface area of the anode (15).
Chemical analysis.
The COD, pH, ammonia, and total solids (volatile solids) of the wheat straw hydrolysate were analyzed by standard methods as described previously (18). Sugar concentrations (Sigma-Aldrich, Germany) were analyzed by high-performance liquid chromatography (Agilent 1100), and volatile organic acids were measured by gas chromatography (Agilent 6890) as previously described (27). Before high-performance liquid chromatography and gas chromatography analysis, samples were first filtered through 0.2-μm Millipore membrane. Hydrogen and methane in the headspace were analyzed using a gas chromatograph (MicroLab, Arhus, Denmark) equipped with a thermal conductivity detector and a stainless steel column packed with Porapak Q (50/80 mesh). Nitrogen was used as the carrier gas.
SEM.
Bacteria attached to the electrodes were visualized using a scanning electron microscope (SEM). Electrodes were removed from the chambers, rinsed with a sterile medium, and immersed in 5% formaldehyde overnight to fix the samples. Then, the samples were dehydrated stepwise in a graded series of water/ethanol solutions (25, 50, 70, 85, 95, and 100%) and then dried. The electrode samples were mounted onto copper specimen mounts with contact adhesive. The samples were then sputter coated in a Polaron E-5100 sputter coater using a gold-palladium target and observed in an FEI (Germany) Quanta 200 FEG SEM. The SEM images were captured digitally (47).
Community analysis.
The anode electrodes were removed from the MFCs and rinsed with sterile distilled water to remove attached debris at the end of each stage. The attached biofilm was then scraped from the 2-cm2 carbon anode using a sterilized scalpel, and suspended microorganisms in the anode chamber were also collected by centrifuging 2 ml of the suspended solution at 13,000 × g for 10 min at 4°C. Genomic DNA was extracted directly using the Qiaamp DNA Stool Mini Kit (Qiagen catalog no. 51504) according to the manufacturer's instructions. The extracted DNA was first amplified using the universal primers 27f (5′-AGA GTT TGA TCM TGG CTC AG-3′) and 1492r (5′-TAC GGY TAC TTG TTA CGA CTT-3′), and then the products were amplified again with the primer set 357f, containing a GC clamp (5′-CGC CCG CCG CGC GGC GGC GGG GCG GGG GCA CGG GGG GCC TAC GGG AGG CAG CAG-3′), and 518r (5′-ATT ACC GCG GCT GCT GG-3′). The PCR amplifications were performed with a thermocycler (Eppendorf) as described by Muyzer et al. (35). The PCR products (25 μl) were separated using 6% (wt/vol) polyacrylamide gels with a denaturant gradient between 40% and 60%. The Dcode Universal Mutation Detection System (Bio-Rad) was used for denaturant gradient gel electrophoresis (DGGE), which was first run in 0.5× Tris-acetate-EDTA buffer at 120 V for 30 min and subsequently at 60 V for 14 h (60°C). After electrophoresis, the gels were stained using SYBR gold (Bio-Medicine) for 40 min and destained in 0.5× Tris-acetate-EDTA buffer (pH 8.0) before the DNA bands were observed with a Gel-Doc image analyzer (Bio-Rad Laboratories). The similarities between lanes from DGGE were analyzed with Quantity One Software (Bio-Rad Laboratories). Bands of interest were cut and excised from the gel and sent for sequencing (MWG, Germany). The sequences were subjected to Basic Local Alignment Search Tool (BLAST) and Ribosomal Database Project analysis. Phylogeny was determined with the Ribosomal Database Project's classifier and Sequmatch.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences have been deposited in the GenBank database and are available under accession numbers FJ222393 to FJ222403.
RESULTS AND DISCUSSION
Voltage generation using wheat straw hydrolysate.
Following inoculation and stable power generation (29 mW/m2) during the period of enrichment (approximately 15 days) with wastewater medium, the anodic solution was replaced with the hydrolysate-modified nutrient medium. In the first transfer, an initial maximum power density of 13.6 mW/m2 (0.24 V), with a fixed 1,000-Ω resistor, was achieved (Fig. 1). An abiotic control did not generate any electricity (data not shown). After two additional loadings (third transfer), a stable increase of electricity generation from the hydrolysate (1,000 mg COD/liter) was produced without a lag phase, and a stable power density of 79.6 mW/m2 (0.58 V) could be maintained for 12 days. The disappearance of the lag phase for initiation of electricity production, along with the successive transfers of the electrode to new media, suggests that electrons were transferred directly to the electrode from the bacteria attached to the anode. When the electrode was transferred to new medium for the fourth time, a similar power density was immediately obtained, implying that the biofilm formed on the electrode had reached equilibrium. The stable period for power generation with hydrolysate was normally longer than that with other substrates, such as xylose, at the same COD concentration with the same reactor (18). The longer stable period for power generation, which might have been due to the humic acid content of the hydrolysate, showed that hydrolysate was a more suitable fuel for MFCs than xylose.
FIG. 1.
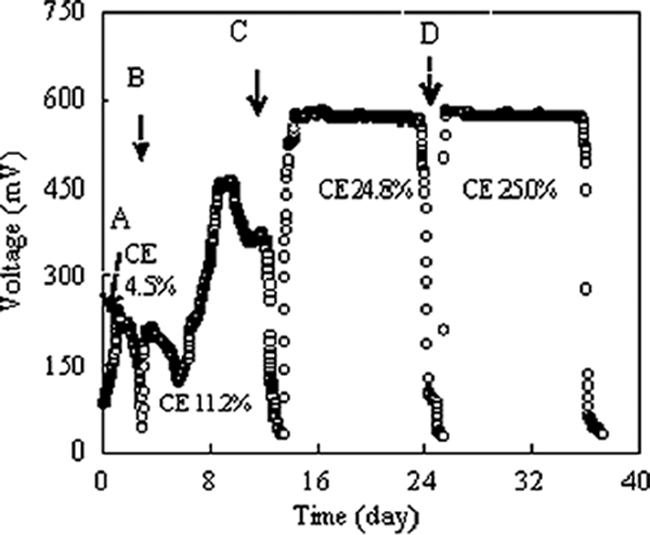
Voltage output as a function of time from the initial hydrolysate concentration of 1,000 mg COD/liter. A, first transfer; B, second transfer; C, third transfer; D, fourth transfer. The CEs of the transfers (A to D) were 4.5%, 11.2%, 24.8%, and 25.0%, respectively.
Power outputs and CEs with different concentrations of hydrolysate.
The power generated in the MFC was monitored at hydrolysate concentrations ranging from 250 to 2,000 mg COD/liter (Fig. 2). The maximum power density increased from 65 to 124 mW/m2 with the initial hydrolysate concentrations from 250 to 2,000 mg COD/liter. Power generation was saturated at hydrolysate concentrations higher than 1,000 mg COD/liter. The open-circuit voltage in the MFC was 0.73 V, and the internal resistance evaluated by the polarization slope method was about 220 Ω. The maximum power output obtained with wheat straw hydrolysate was much higher than those obtained with xylose (38 mW/m2) and glucose (43 mW/m2), which were the major constituents of the hydrolysates in two-chamber MFCs (18, 20). The higher power output might be mainly due to the broad organic composition of the hydrolysate, which can easily be utilized by different communities for electricity generation (29, 34). However, this power was lower than the 810 mW/m2 obtained from corn stover biomass pretreated by steam explosion in a membrane-free single-chamber MFC (46). The lower power density obtained could be due to the higher internal resistance caused by the presence of a membrane and the longer distance between the anode and cathode electrodes in this study (8, 11, 23). The relationship between the hydrolysate concentration and the CE is also shown in Fig. 2. When the initial substrate concentration increased from 250 to 2,000 mg COD/liter, the CE decreased from 37.1 to 15.5%. The differences in CE at different hydrolysate concentrations indicated that some electrons had been consumed by other mechanisms than power generation. With the lower CE achieved in this study, the COD loss was presumably due to biomass generation, incomplete biodegradation of the substrate, hydrogen production, methanogenesis, aerobic degradation, and neutral metabolites diffusing to the cathode chamber (16, 20, 36). Methane and hydrogen were not detected in the headspace in any of the experiments, indicating that methanogenesis and hydrogen production had been effectively inhibited in the system (data not shown). The absence of methane or hydrogen formation indicated that the low CE was not due to the formation of hydrogen and methane.
FIG. 2.
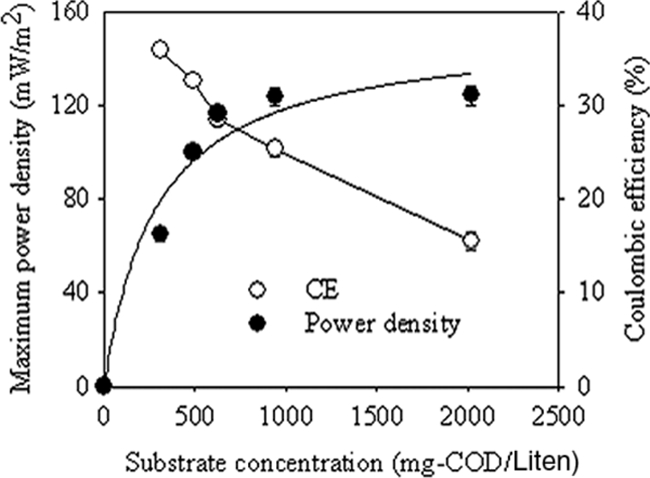
Maximum power density and CE as functions of the initial substrate concentration (the error bars represent standard deviations based on averages obtained during the highest power output in three or more separate batch experiments).
Substrate degradation and intermediate accumulation.
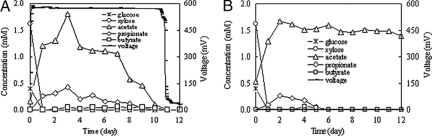
In order to assess the roles of biofilm and suspended bacteria involved in electricity generation, substrate degradation and intermediate accumulation were analyzed in two MFCs operated in different modes. Initially, an MFC was operated in a normal closed-circuit mode. Once there was stable power generation over three batches, the anode chamber was refilled with new medium, while 20 ml of old, suspended solution was transferred to a new MFC with a new anode electrode that was not coated with biofilm to examine substrate degradation and intermediate accumulation (each MFC was fed with 1,000 mg COD/liter hydrolysate). These two MFCs showed the same substrate degradation. The disappearance of xylose and glucose was rapid in both reactors, which resulted in fast formation of acetate (1.8 mM) and other volatile organic acids (less than 0.5 mM) (Fig. 3). However, the time courses of intermediates were distinctly different, especially regarding acetate (Fig. 3). The concentration of acetate in the MFC with the anode electrode coated with biofilm decreased with electricity production (Fig. 3A). In comparison, the concentration of acetate in the MFC with the old, suspended solution was nearly unchanged during the following 10 days of operation (no electricity production) (Fig. 3B). The longer time (more than 12 days) than the normal 3 to 4 days required to produce electricity after the first inoculation might have been due to the different microbial compositions in the inocula used to start up the MFC in this study. Several studies have reported the effect of the inoculum on power production and analyzed the corresponding compositions of electrochemically active biofilms in MFCs (8, 14, 22, 25, 43). There was a far greater diversity of exoelectrogens in these biofilms than was previously suspected, and the community composition to some extent depended on the inoculum (14, 22).
FIG. 3.
Substrate degradation and intermediate accumulation as functions of time with (A) or without (B) an anode electrode coated with biofilm.
In regard to fermentation, both the suspended bacteria and the biofilm could ferment simple sugars in the hydrolysate to organic acids or alcohols. However, compared to the suspended bacteria, the biofilm might have contained more diverse communities consisting of both electrochemically active bacteria and fermentative bacteria and, consequently, not only fermented simple sugars, but also utilized these fermentation by-products for power generation. While the fermentation process has obviously been observed, the direct utilization of hydrolysate by exoelectrogenic bacteria in general has not been well examined in MFC studies. A previous study showed that Rhodoferax ferrireducens can directly oxidize glucose, fructose, sucrose, and xylose to CO2 with Fe(III) serving as the sole electron acceptor (9). Thus, the possibility that some simple sugars in the hydrolysate might be also utilized directly and completely by some bacteria in the biofilm for electricity production without fermentation cannot be excluded.
Morphological features of biofilm organisms in the hydrolysate-fed MFC.
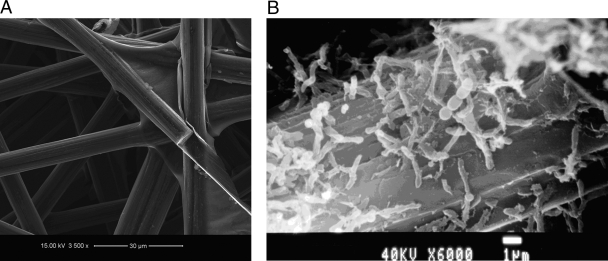
In order to examine the morphology of the anode biofilm, biofilm samples were taken after 36 days of operation with electricity generation and subjected to SEM analysis (Fig. 4). Bacteria of different sizes and shapes were scattered around the electrode, associated with a biofilm formed on its surface. A diverse bacterial community was enriched during this process, and microscopic observation showed an increase in the microbial population and a microbial biofilm attached to the electrode surface with loosely associated microbial clumps (Fig. 4B). Previous studies have proposed that these microbial clumps consist of bacteria that can ferment fuel, such as glucose, into simple fermentation products (19, 22, 26).
FIG. 4.
Morphological features of biofilm attached to an electrode. (A) Carbon paper before use. (B) Electrode after electricity generation from hydrolysate.
DGGE analysis of the microbial community in the MFC.
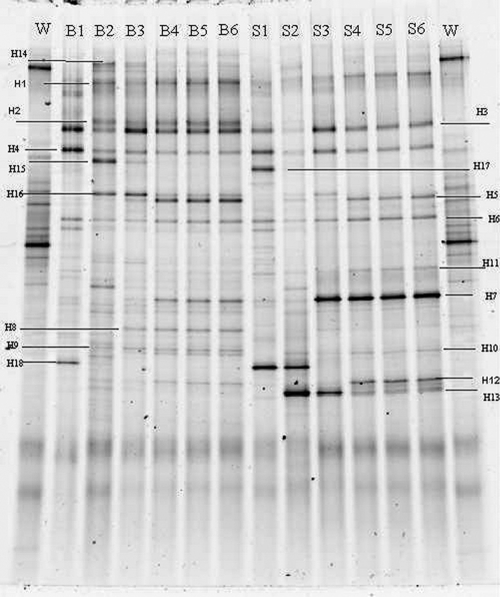
Changes in microbial communities during MFC operation were analyzed by DGGE. The DGGE profiles of biofilm and suspended microorganisms sampled from the MFC at the end of each batch (as shown in Fig. 1) are summarized in Fig. 5. Based on the migration distance, intensities, and similarities between the lanes on the DGGE gel, the banding patterns of the biofilm showed great differences in the first three batch running periods (lanes B1 to B3). The similarities between the lanes were less than 50%. The pattern became stable after the fourth batch and remained unchanged during the following 2 months (lanes B4 to B6). The similarities between the lanes after the third running time were more than 85%. During this period, the intensities of some bands became stronger (e.g., bands H1, H5, H7, H8, and H12), while some bands became weaker and even disappeared (e.g., bands H4, H13, H14, H15, and H16). The same trend was also found in the suspended bacterial community. It is clear that the bacterial populations changed with time, and electrochemically active bacteria might have been enriched, as indicated by the increase in the power density observed during the successive tests, as shown in Fig. 1. The major bands in the biofilm were different from those in the suspension, indicating that different microbial communities developed in the biofilm and the suspended consortia in MFCs powered by hydrolysates. Most of the bacteria in the original inoculum were absent in the biofilm and suspension, which indicates that a specialized inoculum is needed for successful electrogenesis in MFCs. This is the first report confirming the enrichment of electrochemically active communities using hydrolysate-powered MFCs.
FIG. 5.
Anode bacterial community profiles revealed by DGGE. W, wastewater; B, biofilm; S, suspension; H, representative bands.
Phylogenetic diversity revealed by cloning and sequencing.
In order to provide greater insight into the microbial ecology and diversity, bacterial 16S rRNA gene libraries were examined separately for the biofilm and suspended communities (Table 2). Based on the 16S rRNA gene library analysis, we found that the microbial community in the biofilm of the hydrolysate-enriched two-chamber MFC was dominated by Bacteroidetes (40% of sequences), followed by 20% Alphaproteobacteria, 20% Bacillus, 10% Deltaproteobacteria, and 10% Gammaproteobacteria. Kim et al. (22) also observed that Bacteroidetes and Gammaproteobacteria phylotypes were present at higher numbers within libraries from the bacterial clumps and electrode biofilm than in other parts of the fuel cell and suggested that Gammaproteobacteria might be involved in current generation. In another study, Alphaproteobacteria dominated a community with a river sediment inoculum enriched with a low concentration of glucose and glutamate (39). Deltaproteobacteria have been identified as the major bacterial clones in MFCs enriched with acetate (26), and they were believed to be responsible for direct electron transfer to the electrode. In this study, the sequences related to Deltaproteobacteria (10% of the sequences) were detected in the biofilm, which also confirmed the involvement of Deltaproteobacteria in electricity generation. However, the proportion of Deltaproteobacteria in the current study was much lower than that in the literature (70%) for an acetate enrichment study (3). The different medium composition might result in a different bacterial community. Another possibility was that our system might have had too high a redox potential for anaerobic Geobacteraceae, due to the oxygen diffusion mentioned above. Shewanella species, previously reported to transfer electrons to the surfaces of electrodes via electron-transferring proteins, were not found in either the biofilm or the suspended bacterial samples (2, 5, 41).
TABLE 2.
DGGE 16S rRNA gene band identifications
| Band | Accession no. | Class or genusa | GenBank closest match | Identity(%)b | Isolation source |
|---|---|---|---|---|---|
| H1 | FJ222393 | Bacteroidetes | D. wimpennyi ANFA2; AY643492 | 99 | An electrochemically active Fe(III)-reducing bacterium isolated from a microbial fuel cell without a mediator |
| H2 | FJ222394 | Gammaproteobacteria | Acinetobacter sp. strain PD4; EF412969 | 99 | Phenol-degrading bacteria |
| H3 | FJ222395 | Bacteroidetes | Uncultured bacterium clone 45#2; AY491548 | 100 | Microbial fuel cell enriched with acetate; electrochemically active bacterium |
| H4 | FJ222396 | Alphaproteobacteria | Uncultured alphaproteobacterium clone BIsi40; AJ318175 | 100 | Waste gas biofilter |
| H5 | FJ222397 | Bacteroidetes | Uncultured Bacteroidetes bacterium clone MB6A-bac2; DQ205192 | 91 | Enrichment culture from an acidic peat bog |
| H6 | FJ222398 | Bacteroidetes | D. capnocytophagoides; U41355 | 98 | Cutaneous abscess |
| H7/H11 | FJ222399 | Bacillus | Uncultured actinobacterium clone AS42; EU283368 | 98 | Activated sludge from membrane bioreactor |
| H8 | FJ222400 | Deltaproteobacteria | G. metallireducens GS-15; CP000148 | 98 | Complete sequence of G. metallireducens GS-15 |
| H9/H10 | FJ222401 | Bacillus | Uncultured bacterium clone PL; DQ191232 | 97/98 | Up-flow anaerobic sludge bed reactor treating peach lye canning effluent |
| H12 | FJ222402 | Alphaproteobacteria | Bradyrhizobium sp. strain KO3D; AB367687 | 99 | Root nodule of the legume Pueraria montana |
| H13 | FJ222403 | Betaproteobacteria | Azospira oryzae strain N1; DQ863512 | 98 | Bioreactor |
The phylotypes were assigned to phyla based on Ribosomal Database Project II taxonomy classifications.
The values represent the similarities between the associated DGGE band sequence and the closest-match sequence from GenBank.
BLAST analysis of the gene sequence from band H1 showed 99% identity to Dysgonomonas wimpennyi ANFA2, which is an electrochemically active Fe(III)-reducing bacterium isolated from an MFC without a mediator (unpublished data). There was no conventional taxonomic description, precluding any further discussion of its physiological significance. The gene sequence from band H3 showed 100% similarity to uncultured bacterium 45 no. 2, which is an electrochemically active bacterium enriched with acetate in an MFC reported by Lee et al. (26). The gene sequence from band H2 showed 99% sequence similarity to Acinetobacter sp. strain PD4, some of which can degrade phenol. Acinetobacter spp. can also be enriched in formate-powered MFCs (38). The gene sequence from band H5 showed 98% similarity to Dysgonomonas capnocytophagoides, which was also enriched with acetate in a previous study (26). These results indicated that some bacterial species can be enriched in both acetate- and hydrolysate-powered MFCs. Previous studies suggested that members of the Geobacteraceae, Desulfuromonadaceae, and Desulfobulbaceae, related to Fe(III)-reducing bacteria and sulfate-reducing bacteria, could use MFC electrodes as terminal electron acceptors for anaerobic respiration. Several members of these bacterial families, such as G. sulfurreducens, Geobacter metallireducens, and Geopsychrobacter electrodiphilus, have been successfully used in pure culture to generate electricity, which clearly showed that Fe(III)-reducing bacteria could be enriched in MFCs (4, 14, 22). In this study, G. metallireducens GS-15 was detected only in the sequence of band H8 from biofilm, indicating that bacteria from band H8 might be involved in electricity generation.
The suspended community, dominated by Bacteroidetes (44.4% of sequences), Alphaproteobacteria (22.2%), Bacillus (22.2%), and Betaproteobacteria (11.2%), showed a composition different from that of the biofilm. Deltaproteobacteria and Gammaproteobacteria, believed to be responsible for the direct electron transfer to an electrode, were not found in the suspended community. Betaproteobacteria have been reported as the major class in microbial biofilms in rivers (6). Since band H7 was shown to be a major band in the DGGE patterns of all the suspension samples, the predominant species recovered from the suspended bacteria in the anode chamber appeared to be phylogenetically related (97% similarity) to the genus Bacillus of the Firmicutes. Previous studies have also reported the presence of Firmicutes in the anode-colonizing community of MFCs fed with artificial wastewater containing glucose and glutamate (27%) (37), in acetate-enriched MFCs inoculated with marine sediments (>20%) (4), and in activated sludge (>6%) (26). It was believed that Firmicutes might have played the role of converting fermentable substrates (e.g., glucose) into simple molecules and scavenging oxygen, due to their aerotolerant nature, for power producers and methanogens, a potential example of commensalism (13). This hypothesis was supported by the observation that suspended bacteria from the anode of the MFC produced more reduced metabolites and could not further utilize these metabolites when placed in a new MFC without an anode biofilm.
Comparing the 16S rRNA gene libraries in relation to energy generation, Bacteroidetes predominated in the biofilm during operation, indicating that the diversity of iron-reducing and potentially relevant microbes for electricity production might extend beyond the commonly studied Shewanella and Geobacter species. In general, the microbial diversity found in this study was greater than that found in other MFC studies (20, 26, 32), which were dominated by Shewanella and Geobacter species. This distinction was probably attributable to the complex composition of the hydrolysate and the wide range of nonselective intermediates and metabolites derived from hydrolysate degradation.
Conclusions.
The present study demonstrated that stable power could be generated from wheat straw hydrolysate, which is the most abundant component of plant biomass readily available as a waste material in many parts of the world. The power density reached 123 mW/m2 with an initial hydrolysate concentration of 1,000 mg COD/liter, while CEs ranged from 37.1 to 15.5%, corresponding to the initial hydrolysate concentrations of 250 to 2,000 mg COD/liter. This is the first study showing that biofilm and suspended microbial communities play different roles in electricity generation from wheat straw hydrolysate. The results imply a potential substrate utilization process in which suspended bacteria and biofilm ferment the complex fuel into simple fermentation products, which can subsequently be utilized by anode electrochemical bacteria to generate electricity. Although suspended cultures have been used for MFC inoculation (28), the suspended culture could not produce electricity from hydrolysate in this study. Mainly fermentation was taking place in this suspended culture. The lack of exoelectrophilic bacteria in the suspended culture was probably due to unfavorable conditions for the growth and proliferation of these cultures. The growth of exoelectrophilic bacteria is probably promoted in an immobilized state on the electrode matrix. Furthermore, a lower stirring rate in the anode chamber probably prevented the immobilized exoelectrophilic cultures from detaching from the biofilm. However, in another study, inoculum scraped from the anode electrode biofilm was found to be effective in electricity generation, underlining the importance of bacterial immobilization on the anode electrode for power generation (24). Further examination will be needed to better clarify the factors affecting the detachment of biofilm in a hydrolysate culture. The microbial community in the anode biofilm was dominated by Bacteroidetes, while the suspended bacteria were dominated by Firmicutes. Understanding how the microbial community develops and changes over time with wheat straw hydrolysate will assist in the optimization of MFC technology. Further study of the biological factors driving these systems and improvement of MFC design and configuration are required to achieve a technology with practical applications in agricultural and industrial hydrolysate waste utilization.
Acknowledgments
We thank Sompong O-Thong and Dimitar Karakashev for advice on molecular biotechnology work and also thank Hector Garcia for his help with analytical measurements.
This study was funded by a Danida research fellowship and the Danish Research Agency, Ministry of Science Technology and Innovation (2104-05-0003).
Footnotes
Published ahead of print on 17 April 2009.
REFERENCES
- 1.Aelterman, P., K. Rabaey, H. T. Pham, N. Boon, and W. Verstraete. 2006. Continuous electricity generation at high voltages and currents using stacked microbial fuel cells. Environ. Sci. Technol. 40:3388-3394. [DOI] [PubMed] [Google Scholar]
- 2.Biffinger, J. C., J. Pietron, R. Ray, B. Little, and B. R. Ringeisen. 2007. A biofilm enhanced miniature microbial fuel cell using Shewanella oneidensis DSP10 and oxygen reduction cathodes. Biosens. Bioelectron. 22:1672-1679. [DOI] [PubMed] [Google Scholar]
- 3.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 4.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bretschger, O., A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B. H. Kim, J. K. Fredrickson, and K. H. Nealson. 2007. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 73:7003-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brümmer, I. H. M., W. Fehr, and I. Wagner-Döbler. 2000. Biofilm community structure in polluted rivers: abundance of dominant phylogenetic groups over a complete annual cycle. Appl. Environ. Microbiol. 66:3078-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catal, T., Y. Fan, K. Li, H. Bermek, and H. Liu. 2008. Effects of furan derivatives and phenolic compounds on electricity generation in microbial fuel cells. J. Power Sources 180:162-166. [Google Scholar]
- 8.Catal, T., K. Li, H. Bermek, and H. Liu. 2008. Electricity production from twelve monosaccharides using microbial fuel cells. J. Power Sources 175:196-200. [Google Scholar]
- 9.Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. [DOI] [PubMed] [Google Scholar]
- 10.Datar, R., J. Huang, P. C. Maness, A. Mohagheghi, S. Czernik, and E. Chornet. 2007. Hydrogen production from the fermentation of corn stover biomass pretreated with a steam explosion process. Int. J. Hydrogen Energy 32:932-939. [Google Scholar]
- 11.Fan, Y., H. Hua, and H. Liu. 2007. Enhanced coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources 171:348-354. [Google Scholar]
- 12.Fan, Y., Y. Zhang, S. Zhang, H. Hou, and B. Ren. 2006. Efficient conversion of wheat straw wastes into biohydrogen gas by cow dung compost. Bioresour. Technol. 97:500-505. [DOI] [PubMed] [Google Scholar]
- 13.Hamid R.-Y., A. D. Christy, B. A. Dehority, M. Morrison, Z. Yu, and O. H. Tuovinen. 2007. Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol. Bioeng. 97:1398-1407. [DOI] [PubMed] [Google Scholar]
- 14.Holmes, D. E., D. R. Bond, R. A. O'Neil, C. E. Reimers, L. R. Tender, and D. R. Lovley. 2004. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 48:178-190. [DOI] [PubMed] [Google Scholar]
- 15.Huang, L., and I. Angelidaki. 2008. Effect of humic acids on electricity generation integrated with xylose degradation in microbial fuel cells. Biotechnol. Bioeng. 100:413-422. [DOI] [PubMed] [Google Scholar]
- 16.Huang, L., and B. E. Logan. 2008. Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl. Microbiol. Biotechnol. 80:349-355. [DOI] [PubMed] [Google Scholar]
- 17.Huang, L., and B. E. Logan. 2008. Electricity production from xylose in fed-batch and continuous-flow microbial fuel cells. Appl. Microbiol. Biotechnol. 80:655-664. [DOI] [PubMed] [Google Scholar]
- 18.Huang, L., R. J. Zeng, and I. Angelidaki. 2008. Electricity production from xylose using a mediator-less microbial fuel cell. Bioresour. Technol. 99:4178-4184. [DOI] [PubMed] [Google Scholar]
- 19.Jong, B. C., B. H. Kim, I. S. Chang, P. W. Liew, Y. F. Choo, and G. S. Kang. 2006. Enrichment, performance, and microbial diversity of a thermophilic mediatorless microbial fuel cell. Environ. Sci. Technol. 40:6449-6454. [DOI] [PubMed] [Google Scholar]
- 20.Jung, S., and J. M. Regan. 2007. Comparison of anode bacterial communities and performance in microbial fuel cells with different electron donors. Appl. Microbiol. Biotechnol. 77:393-402. [DOI] [PubMed] [Google Scholar]
- 21.Kim, B. H., H. S. Park, H. J. Kim, G. T. Kim, I. S. Chang, J. Lee, and N. T. Phung. 2004. Enrichment of microbial community generating electricity using a fuel-cell-type electrochemical cell. Appl. Microbiol. Biotechnol. 63:672-681. [DOI] [PubMed] [Google Scholar]
- 22.Kim, G. T., G. Webster, J. W. Wimpenny, B. H. Kim, H. J. Kim, and A. J. Weightman. 2006. Bacterial community structure, compartmentalization and activity in a microbial fuel cell. J. Appl. Microbiol. 101:698-710. [DOI] [PubMed] [Google Scholar]
- 23.Kim, J. R., S. H. Jung, J. M. Regan, and B. E. Logan. 2007. Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour. Technol. 98:2568-2577. [DOI] [PubMed] [Google Scholar]
- 24.Kim, J. R., B. Min, and B. E. Logan. 2005. Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl. Microbiol. Biotechnol. 68:23-30. [DOI] [PubMed] [Google Scholar]
- 25.Lee, H. S., P. Parameswaran, A. Kato-Marcus, C. Torres, and B. E. Rittmann. 2008. Evaluation of energy-conversion efficiencies in microbial fuel cells (MFCs) utilizing fermentable and non-fermentable substrates. Water Res. 42:1501-1510. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J., N. T. Phung, I. S. Chang, B. H. Kim, and H. C. Sung. 2003. Use of acetate for enrichment of electrochemically active microorganisms and their 16S rDNA analyses. FEMS Microbiol. Lett. 223:185-191. [DOI] [PubMed] [Google Scholar]
- 27.Liu, H., S. Cheng, and B. E. Logan. 2005. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 39:658-662. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Z., H. Li, J. Liu, and Z. Su. 2008. Effects of inoculation strategy and cultivation approach on the performance of microbial fuel cell using marine sediment as bio-matrix. J. Appl. Microbiol. 104:1163-1170. [DOI] [PubMed] [Google Scholar]
- 29.Logan, B. E., B. Hamelers, R. Rozendal, U. Schroder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete, and K. Rabaey. 2006. Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 40:5181-5192. [DOI] [PubMed] [Google Scholar]
- 30.Logan, B. E., C. Murano, K. Scott, N. D. Gray, and I. M. Head. 2005. Electricity generation from cysteine in a microbial fuel cell. Water Res. 39:1576-1584. [DOI] [PubMed] [Google Scholar]
- 31.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathis, B. J., C. W. Marshall, C. E. Milliken, R. S. Makkar, S. E. Creager, and H. D. May. 2008. Electricity generation by thermophilic microorganisms from marine sediment. Appl. Microbiol. Biotechnol. 78:147-155. [DOI] [PubMed] [Google Scholar]
- 33.Min, B., and I. Angelidaki. 2008. Innovative microbial fuel cell for electricity production from anaerobic reactors. J. Power Sources 180:641-647. [Google Scholar]
- 34.Min, B., S. Cheng, and B. E. Logan. 2005. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 39:1675-1686. [DOI] [PubMed] [Google Scholar]
- 35.Muyzer, G., E. C. De Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh, S., B. Min, and B. E. Logan. 2004. Cathode performance as a factor in electricity generation in microbial fuel cells. Environ. Sci. Technol. 38:4900-4904. [DOI] [PubMed] [Google Scholar]
- 37.Park, H. I., K. H. Nealson, and B. H. Kim. 2006. Electrochemically active bacteria (EAB) and mediator-less microbial fuel cells. J. Microbiol. Biotechnol. 16:163-177. [Google Scholar]
- 38.Phuc, T. H., T. Beomseok, and I. S. Chang. 2008. Performance and bacterial consortium of microbial fuel cell fed with formate. Energy Fuels 22:164-168. [Google Scholar]
- 39.Phung, N. T., J. Lee, K. H. Kang, I. S. Chang, G. M. Gadd, and B. H. Kim. 2004. Analysis of microbial diversity in oligotrophic microbial fuel cells using 16S rDNA sequences. FEMS Microbiol. Lett. 233:77-82. [DOI] [PubMed] [Google Scholar]
- 40.Rezaei, F., T. L. Richard, R. A. Brennan, and B. E. Logan. 2007. Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environ. Sci. Technol. 41:4053-4058. [DOI] [PubMed] [Google Scholar]
- 41.Ringeisen, B. R., E. Henderson, P. K. Wu, J. Pietron, R. Ray, B. Little, J. C. Biffinger, and J. M. Jones-Meehan. 2006. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ. Sci. Technol. 40:2629-2634. [DOI] [PubMed] [Google Scholar]
- 42.Saha, B. C., L. B. Iten, M. A. Cotta, and Y. V. Wu. 2005. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem. 40:3693-3700. [DOI] [PubMed] [Google Scholar]
- 43.Sukkasem, C., S. T. Xua, S. Park, P. Boonsawang, and H. Liu. 2008. Effect of nitrate on the performance of single chamber air cathode microbial fuel cells. Water Res. 42:4743-4750. [DOI] [PubMed] [Google Scholar]
- 44.Tender, L. M., C. E. Reimers, H. A. Stecher, D. E. Holmes, D. R. Bond, D. A. Lowy, K. Pilobello, S. J. Fertig, and D. R. Lovley. 2002. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 20:821-835. [DOI] [PubMed] [Google Scholar]
- 45.Thomsen, M. H., A. Thygesen, and A. B. Thomsen. 2008. Hydrothermal treatment of wheat straw at pilot plant scale using a three-step reactor system aiming at high hemicellulose recovery, high cellulose digestibility and low lignin hydrolysis. Bioresour. Technol. 99:4221-4228. [DOI] [PubMed] [Google Scholar]
- 46.Yi, Z., P. Maness, and B. E. Logan. 2006. Electricity production from steam-exploded corn stover biomass. Energy Fuels 20:1716-1721. [Google Scholar]
- 47.Zhang, E., W. Xu, G. Diao, and C. Shuang. 2006. Electricity generation from acetate and glucose by sedimentary bacterium attached to electrode in microbial-anode fuel cells. J. Power Sources 161:820-825. [Google Scholar]