Abstract
Beauveria bassiana is an economically important insect-pathogenic fungus which is widely used as a biocontrol agent to control a variety of insect pests. However, its insecticide efficacy in the field is often influenced by adverse environmental factors. Thus, understanding the genetic regulatory processes involved in the response to environmental stress would facilitate engineering and production of a more efficient biocontrol agent. Here, a mitogen-activated protein kinase (MAPK)-encoding gene, Bbhog1, was isolated from B. bassiana and shown to encode a functional homolog of yeast HIGH-OSMOLARITY GLYCEROL 1 (HOG1). A Bbhog1 null mutation was generated in B. bassiana by targeted gene replacement, and the resulting mutants were more sensitive to hyperosmotic stress, high temperature, and oxidative stress than the wild-type controls. These results demonstrate the conserved function of HOG1 MAPKs in the regulation of abiotic stress responses. Interestingly, ΔBbhog1 mutants exhibited greatly reduced pathogenicity, most likely due to a decrease in spore viability, a reduced ability to attach to insect cuticle, and a reduction in appressorium formation. The transcript levels of two hydrophobin-encoding genes, hyd1 and hyd2, were dramatically decreased in a ΔBbhog1 mutant, suggesting that Bbhog1 may regulate the expression of the gene associated with hydrophobicity or adherence.
Mycoinsecticides are important insect pest control agents (10, 19, 41). Unlike entomopathogenic bacteria and viruses that invade insects through their digestive tracks, fungal pathogens penetrate the host integument and are considered the only group of microbial biocontrol agents active against sucking-type insect pests (21, 49). However, low killing speed and sensitivity to adverse environment factors such as desiccation, high temperature, and UV radiation limit the widespread use of entomopathogenic fungi (6, 22, 40). Thus, an understanding of the regulatory processes involved in response to environment stress is essential for commercial development and improvement of these biocontrol fungi.
Mitogen-activated protein kinases (MAPKs), a family of serine-threonine protein kinases, are widespread in eukaryotic cells and play crucial roles in transduction of a variety of extracellular signals and regulation of various development and differentiation processes (37, 45). MAPKs are usually activated by MAPK kinases, which are in turn activated by MAPK kinase kinases. These MAPK kinase kinase-MAPK kinase-MAPK cascades are conserved in eukaryotic cells and have been studied extensively in many organisms (45). In Saccharomyces cerevisiae, at least five MAPK pathways have been identified. These pathways are designated the FUS3, KSS1, HOG1, SLT2, and SMK1 MAPK cascades, and they are involved in mating, filamentous growth, the high-osmolarity response, cell integrity, and ascospore formation, respectively (20). Recent studies showed that homologs of HOG1 are involved in responses to osmotic stress (12, 29, 44, 58), oxidative stress (28, 47), heat shock (28), and tolerance to a phenylpyrrole fungicide (12, 29, 58) in several other fungi. For instance, deletion of HOG1-encoding genes in Candida albicans (44), Neurospora crassa (58), Magnaporthe grisea (12), and Colletotrichum lagenarium (29) causes a defect in adaptation to high-osmolarity conditions. A hog1-silenced strain of Trichoderma harzianum was highly sensitive to osmotic stress and showed intermediate levels of sensitivity to oxidative stress (11). HOG1 MAP kinase is activated by high osmolarity, oxidative stress, and UV stress in Debaryomyces hansenii (47). In Aspergillus nidulans, a SakA protein kinase related to HOG1 has been implicated in stress signal transduction, sexual development, and spore survival (28). Inactivation of HOG1 orthologs in M. grisea, N. crassa, and C. lagenarium results in increased resistance to the phenylpyrrole fungicide fludioxonil (12, 29, 58). A T. harzianum strain overexpressing the hog1(F315S) allele was highly resistant to the calcineurin inhibitor cyclosporine A, which suggests that there are links between the two pathways (11). In addition, HOG1 orthologs participating in fungal pathogen-host interactions have been demonstrated to be present in the plant pathogen Mycosphaerella graminicola and the mycoparasitic fungus T. harzianum. Deletion of the HOG1 kinase gene in M. graminicola results in mutants that are nonpathogenic to the host plant (35). In T. harzianum, either hyperactivation [with the hog1(F315S) allele] or silencing (using RNA interference) of a HOG1 protein leads to strongly reduced antagonistic activity against some plant fungal pathogens (11). These results suggested that HOG1 MAPKs might provide a clue for understanding the regulatory mechanism that responds to environmental stresses in fungi and fungal pathogenesis. However, whether HOG1-type MAPKs in entomopathogenic fungi are involved in the response to environmental stress and pathogenesis is far from clear.
Here, we characterized a HOG1-related MAPK gene, designated Bbhog1, in Beauveria bassiana, an important entomopathogenic fungus which targets a variety of insect pests, including agricultural pests and vectors of human pathogens (5, 8, 32). Our data showed that Bbhog1 regulates responses to stress stimuli. Interestingly, disruption of Bbhog1 resulted in a significant reduction in virulence and impairment of characteristics associated with pathogenicity.
MATERIALS AND METHODS
Fungal and bacterial strains.
B. bassiana single-spore isolate Bb0062 has been described previously (17). Cultures were grown on Sabouraud's dextrose agar (Difco Laboratories, Detroit, MI) supplemented with 1% (wt/vol) yeast extract for 14 days at 26°C with a cycle consisting of 15 h of light and 9 h of darkness. Escherichia coli DH5α was employed for DNA manipulations and transformations. Agrobacterium tumefaciens AGL-1 was used for fungal transformations.
Identification of the HOG1 MAPK-encoding gene.
Fungal genomic DNA was prepared as described previously (39), and total RNA was isolated from B. bassiana using the method of Wan and Wilkins (53). RQ1 RNase-free DNase (Promega) was used to remove DNA contamination in RNA samples. To PCR amplify a fragment of the HOG1 gene from B. bassiana, degenerate primers Bh-F and Bh-R (Table 1) were designed based on alignments of C. lagenarium OSC1 (29), N. crassa OS-2 (58), M. grisea OSM1 (12), and S. cerevisiae HOG1 (7). PCR was performed using ExTaq DNA polymerase (TaKaRa). The resulting PCR product was shown to be a fragment of a HOG1-related MAPK gene. The complete gene was subsequently cloned by PCR walking using the Y-shaped adaptor-dependent extension (YADE) method as described previously (14). The primers used for PCR walking are shown in Table 1. All PCR products were cloned into the A-T cloning vector pGEM-T Easy (Promega) according to manufacturer's instructions and subsequently sequenced. Designated Bbhog1, the complete gene was resolved using the PCR and YADE products based on overlapping regions. Primers (Table 1) were designed to clone the cDNA sequence of this gene using the 3′ full random amplification of cDNA ends (RACE) core set (TaKaRa).
TABLE 1.
Oligonucleotides used in this study
| Category and oligonucleotide | Sequence (5′-3′)a | Remarks |
|---|---|---|
| PCR-based cloning of a fragment of Bbhog1 | ||
| BH-F | TCGGCATGGGNGCNTTYGGN | |
| BH-R | TCGTCGGTNGGRTCRTGRTA | |
| YADE-based PCR walking | ||
| Bh3X | AGATATAGCGATCGATCTGA | Used to clone the 3′ fragment obtained with BH-F and BH-R |
| Bh3Z | ATCGATCTGATGGAGCGCAT | |
| Bh5X | GTCGGAGATGCTTGAGCAAT | Used to clone the 5′ fragment obtained with BH-F and BH-R |
| Bh5X | AGGGCTTCATGATCTTCTTG | |
| Cloning cDNA of Bbhog1 using 3′ RACE | ||
| BH-3 | TTGCCCCTCACAATGGCCGA | |
| Bbhog1 disruption vector construction | ||
| L1 | GAATTCATTCACCCTTTGCGT | |
| L2 | AAAGTCGCAAATCTTGAGAT | |
| R1 | GGACTAGTCCCTTGAGTCATAGGT | |
| R2 | TCCCCCGGGGGAAGTAATACCCTTTCAGAGCA | |
| Disruption vector and disruptant confirmation | ||
| B2 | TTAGATCTCGGTGACGGGCA | 3′ end sequence of Bar |
| G1 | ATGAGTAAAGGAGAAGAACT | 5′ end sequence of GFP |
| R2′ | AGATATTGGAGCAGATTGGT | 5′ flanking sequence of R2 |
| RT-PCR | ||
| Hog1 RT-1 | TACTCTACGATTCGTCAAGT | Used for RT-PCR analysis of Bbhog1 |
| Hog1 RT-2 | TGCTGGAACAGAGCCGTCTT | |
| Hyd1-1 | ATCTACTGCTGCAACGAGAA | Used for real-time RT-PCR analysis of hyd1 |
| Hyd1-2 | TACTGGATAAGACTGCCAAT | |
| Hyd2-1 | AGTGTCAAGACTGGCGACAT | Used for real-time RT-PCR analysis of hyd2 |
| Hyd2-2 | ATCCGAGGACGGTGATGGGA | |
| Cdep1-1 | TGCACCGTCGGAGCTACCGA | Used for real-time RT-PCR analysis of cdep1 |
| Cedp1-2 | AGTTGACGGTGCCTGAAGGA | |
| Bbchit1-1 | TACCTGGACAATGGTCGTCT | Used for real-time RT-PCR analysis of Bbchit1 |
| Bbchit1-2 | TGGAGCCGTCCCAGTTCAGA | |
| 18S rRNA-1 | CGGGTAACGGAGGGTTAGG | Used for real-time RT-PCR analysis of 18S rRNA |
| 18S rRNA-2 | AGTACACGCGGTGAGGCGG | |
| β-tubulin 1 | TACTCTACGATTCGTCAAGT | Used for RT-PCR and real-time RT-PCR analysis of β-tubulin |
| β-tubulin 2 | TGCTGGAACAGAGCCGTCTT | |
| Bgpd-1 | TCGACCTGACTGCTCGTCTT | Used for RT-PCR and real-time RT-PCR analysis of Bgpd |
| Bgpd-2 | TAGGAGATAAGGTCCAGGA | |
| Actin-1 | TTGGTGCGAAACTTCAGCGTCTAGTC | Used for real-time RT-PCR analysis of actin |
| Actin-2 | TCCAGCAAATGTGGATCTCCAAGCAG |
N = A, G, C, or T; Y = C or T; R = A or G. Underlining in the R1 and R2 sequences indicates introduced SpeI and SmaI sites, respectively.
Disruption of Bbhog1.
Plasmid pK2-BarGFP containing the herbicide resistance gene bar and the enhanced green fluorescent protein gene egfp was generated by inserting a Bar::GFP fusion gene (27) into EcoRI and HindIII sites of the binary vector pPK2 (34) in which an hph cassette was deleted. The 3′ end of Bbhog1 (3′ hog1) was cloned by performing PCR with primers R1 and R2 (Table 1) using ExTaq DNA polymerase (TaKaRa). The resulting PCR product was digested with SpeI and SmaI and inserted into HindIII and XbaI sites of pK2-BarGFP which was blunt ended using T4 DNA polymerase at a HindIII site, to form pK2-BarGFP:3′hog1. The 5′ end of Bbhog1 (5′ hog1) was amplified with primers L1 and L2 with ExTaq DNA polymerase (TaKaRa). The PCR product was cloned into pGEM-T Easy (Promega) to form pGEM-5′hog1. Then the 5′ end of Bbhog1 was excised from pGEM-5′hog1 with EcoRI and inserted into the unique EcoRI site of pK2-BarGFP:3′hog1. The resulting gene replacement vector was confirmed by performing PCR with primers L1 and B2 (5′ end sequence of Bar) (Table 1) to be a 3.5-kb fragment and designated pBGΔhog1, and then it was mobilized into A. tumefaciens AGL-1 for fungal transformation (17).
Bbhog1 disruption mutants were initially selected on the basis of herbicide (glufosinate ammonium) resistance and expression of green fluorescent protein. PCR performed with the primer pairs G1/R2 and G1/R2′ (Table 1), Southern blotting, reverse transcription-PCR (RT-PCR), and Western blotting were used to confirm disruption of Bbhog1 in B. bassiana transformants.
Blotting analysis.
Southern gel blot analysis was performed with about 25 μg DNA digested with appropriate restriction enzymes for each sample. The DNA probes were labeled with [α-32P]dCTP using a labeling kit (Amersham).
Fungal proteins were extracted as described previously (28). For Western blot analysis, 20 μg proteins was transferred from a sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis gel to a polyvinylidene difluoride membrane (Roche), and immunoblotting and blot development were carried out according to the instructions provided with an Opti-4CN Western blot kit (Bio-Rad). Proteins related to HOG1 were detected using anti-Hog1p antibody y-215 (Santa Cruz Biotech, United States).
Quantification of spore yield.
Twenty-milliliter portions of 1:4-diluted Sabouraud's dextrose agar supplemented with 1% (wt/vol) yeast extract or Czapek agar containing 0.5% (wt/vol) peptone (CZP agar) were tempered to 45°C, mixed with 50 μl of conidial suspensions at a concentration of 1 × 107 conidia ml−1, and poured into 90-mm-diameter petri dishes. After incubation at 26°C using a cycle consisting of 15 h of light and 9 h of darkness for a total of 15 days, conidia were collected in 0.05% (vol/vol) Tween 80 and filtered through four layers of lens-cleaning tissue (Hangzhou Special Paper Industry Co., Ltd., People's Republic of China) to remove mycelial debris. The number of conidia produced was determined by counting the conidia with a hemocytometer using a Motic microscope (E220G). The experiment was repeated four times with three replicates for each strain.
Identification of compatible solutes for B. bassiana.
Two grams (fresh weight) of mycelia harvested from 48-h liquid cultures was inoculated into 100 ml Sabouraud's dextrose broth containing 1% (wt/vol) yeast extract (pH 7.0) (SDY broth) supplemented with 0 or 0.4 M NaCl and incubated at 26°C on a rotary shaker (180 rpm) for 24 h. Mycelia were harvested, frozen in liquid nitrogen, and freeze-dried under a vacuum for 3 to 5 days for carbohydrate extraction using a method described previously (12). A 0.5-g (dry weight) portion of mycelia was used to extract carbohydrates after addition of 20 μg ribitol as the internal standard by a previously described method (12). The mannitol, trehalose, erythritol, arabitol, and glycerol contents of mycelia were determined using a gas-liquid chromatography method described previously (25). The experiment was repeated three times.
Bioassay.
Apterous adults of Myzus persicae (≤2 days after the last ecdysis) reared on cabbage in the greenhouse were prepared by using a previously described method (51) and used for a bioassay. A fungal conidial suspension was diluted to obtain suspensions containing 5 × 107 and 1 × 107 conidia ml−1 using 0.05% (vol/vol) Tween 80. For inoculation, the aphids on each cabbage leaf (replicates were used for each treatment) were exposed to a spray consisting of 1 ml of a conidial suspension using a Potter precision laboratory spray tower (Burkard Manufacturing Co., Ltd., United Kingdom) and then transferred to an HPG-280H artificial climate cell (Harbin Donglian Electronic & Technology Development Co. Ltd. of China) kept at 22 to 24°C. Control aphids were treated with water plus 0.05% (vol/vol) Tween 80. For each treatment there were three replicates, each with 30 to 40 aphids, and the experiments were repeated three times. Aphid mortality was examined daily and corrected using Abbott's formula: PT = [(PO − PC)/(100 − PC)] × 100, where PO is the observed mortality and PC is the control mortality (1).
Determination of conidial germination, adherence, appressorium formation, and hyphal body differentiation.
One hundred microliters of a conidial suspension containing 1 × 107 spores ml−1 was spread onto CZP agar plates and incubated at 26°C. After 8 h, the percentage of germinated conidia was assessed hourly. Three hundred conidia were examined for each time, and all experiments were repeated on three different dates. A conidium was considered to have germinated if the length of its germ tube was greater than its width.
Conidial adherence to cicada hind wings was investigated by using a previously described method (55). Appressoria were induced on cicada hind wings using a method described previously (54), and the frequency of appressorium formation was determined by counting the number of appressoria that developed from 300 germinated conidia after 22 h. All experiments were repeated three times.
Hyphal body differentiation was investigated by injecting conidia into insects. Third instars of Pieris brassicae larvae were inoculated with 2 μl of a conidial suspension containing 5 × 107 spores ml−1 and bled at 12-h intervals to observe hyphal bodies with a microscope.
RT-PCR.
Two micrograms of total RNA was reverse transcribed using an oligo(dT)-primed cDNA synthesis kit (MBI Fermentas), and the first-strand cDNA was amplified with the Bbhog1, Bgpd, and β-tubulin primers listed in Table 1. The expression of these three genes was investigated during growth in CZP broth supplemented with 0.4 M NaCl for 0, 10, and 20 min.
Real-time RT-PCR analysis.
First-strand cDNA was synthesized using oligo(dT) primers according to the manufacturer's instructions for a cDNA synthesis kit (MBI Fermentas). Five micrograms of total RNA was used for cDNA synthesis, and the cDNA was subsequently diluted with nuclease-free water to obtain a concentration of 10 ng μl−1. One microliter of diluted cDNA was used as a template for subsequent PCR amplification using a quantitative real-time PCR kit (Bio-Rad). The transcript levels of two hydrophobin-encoding genes, hyd1 and hyd2 (9), the Pr1 protease gene cdep1 (18), and the chitinase gene Bbchit1 (14) were analyzed using real-time RT-PCR. The stability of expression of four potential reference genes, the 18S rRNA, β-tubulin, actin, and Bgpd genes, was evaluated using the wild type and Bbhog1 disruption mutants grown in insect cuticle or chitin medium (9) and a method described previously (52). Three of the four reference genes with the most stable gene expression were selected to normalize levels of gene transcription using geNORM (52). Primer sequences for the eight genes used are shown in Table 1.
The transcript levels of hyd1 and hyd2 were ascertained using insect cuticle media as described previously (9). Conidia harvested from plates were inoculated into 1:4-diluted Sabouraud's dextrose broth containing 1% (wt/vol) cicada cuticle at a final concentration of 5 × 105 conidia ml−1 and were grown for 3 days at 26°C with aeration, after which the total RNA was extracted and subjected to real-time RT-PCR analysis.
The levels of expression of cdep1 and Bbchit1 in insect cuticle broth (basal salt medium supplemented with 0.5% [wt/vol] cicada cuticle as the sole carbon and nitrogen source) were also assessed. Two grams of washed mycelia was transferred from SDY medium into cicada cuticle broth and grown for an additional 12, 24, 36, 48, 60, and 72 h. Total RNA was extracted and subjected to real-time RT-PCR analysis.
Data analysis.
Variation among the treatments in a given experiment was differentiated by using one-way analysis of variance, and the significance between treatments was tested using the lease significant difference or the Dunnett T3 method. All statistical analyses were carried out with the SPSS 8.0 program.
Nucleotide sequence accession numbers.
Data reported here have been deposited in the GenBank database under the following accession numbers: AY572854 for Bbhog1 genomic DNA, EF452344 for hyd1 mRNA, EF520285 for hyd2 mRNA, AY040532 for cdep1 genomic DNA, AY145440 for Bbchit1 genomic DNA, and AY679162 for Bgpd genomic DNA.
RESULTS
Cloning of the Bbhog1 gene.
A HOG1-like MAPK gene, designated Bbhog1, was cloned from B. bassiana by performing PCR with degenerate primers (Table 1) and YADE (14). Based on the putative coding sequence identified using BLASTX, additional primers (described in Materials and Methods) were designed to clone the cDNA using 3′ RACE. The cDNA fragment contained a 1,077-bp open reading frame which encodes a 358-amino-acid protein with a predicted molecular mass of 49.9 kDa and a deduced pI of 8.7. Eight introns were identified in the DNA sequence by comparison to the cDNA sequence. The predicted Bbhog1 contained the conserved TGY activation loop at positions 171 to 173 found in the stress-activated protein kinase subgroup of MAPKs (42). The amino acid sequence of Bbhog1 showed 96%, 94%, 94%, and 80% identity to C. lagenarium OSC1 (29), M. grisea OSM1 (12), N. crassa OS-2 (58), and S. cerevisiae HOG1 (7), respectively. Southern analysis indicated that a single copy of Bbhog1 is present in B. bassiana (data not shown).
Disruption of Bbhog1 leads to sensitivity to high osmolarity, oxidative stress, and high temperature and resistance to the fungicide fludioxonil.
To investigate the roles of Bbhog1, a gene disruption strategy was used. The gene replacement vector pBGΔhog1 was constructed to delete 677 bp of the Bbhog1 coding region and to replace it with a 4.2-kb Bar::GFP fusion gene cassette (see Fig. S1A in the supplemental material). The disruption of Bbhog1 was first screened by PCR with both primer pair G1/R2 and primer pair G1/R2′ (Table 1) as a 2.8-kb fragment (data not shown) and then confirmed by Southern blotting (see Fig. S1B in the supplemental material), RT-PCR (see Fig. S1C in the supplemental material), and Western blotting (see Fig. S1D in the supplemental material). Transformants were selected randomly for further study.
On standard medium (CZP medium), no obvious variation in the growth rate (Fig. 1A) or hyphal morphology (Fig. 1B) was observed in ΔBbhog1 mutants. However, the mutants did not form obvious daylight rings resulting in rhythmic sporulation like the wild-type strain (Fig. 1A), which was confirmed by a significant reduction in conidiation on agar plates. The numbers of conidia produced by ΔBbhog1 mutants on 1:4-diluted Sabouraud's dextrose agar supplemented with 1% (wt/vol) yeast extract (3.3 × 105 ± 0.6 × 105 conidia mm−2) and CZP agar (1.0 × 105 ± 0.1 × 105 conidia mm−2) plates were 75.6% (df = 6; t = 11.33; P < 0.001) and 90.5% (df = 6; t = 22.69; P < 0.001) less than the numbers of conidia produced by the wild-type strain, respectively.
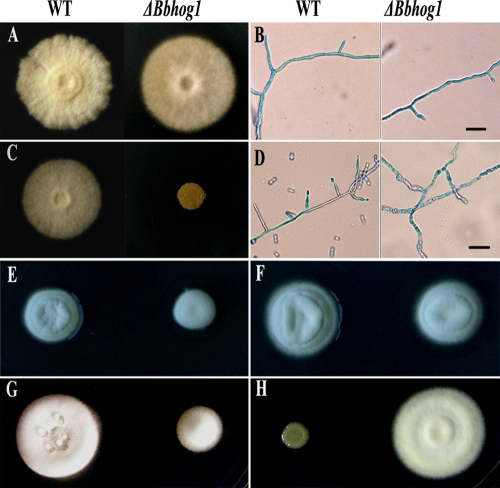
FIG. 1.
Disruption of Bbhog1 influences the growth of B. bassiana. Wild-type strain Bb0062 (WT) and the Bbhog1 disruption mutant (ΔBbhog1) were incubated on CZP agar plates at 26°C for 10 days (A) or in CZP broth at 26°C on a rotary shaker (180 rpm) for 12 h (B) (control). The test strains were incubated on CZP agar plates supplemented with 0.4 M NaCl at 26°C for 10 days (C), in CZP broth containing 0.4 M NaCl at 26°C on a rotary shaker (180 rpm) for 12 h (D), on CZP agar plates supplemented (E) or not supplemented (F) with 0.4 M NaCl at 32°C for 10 days, or on CZP agar plates containing 20 mM H2O2 (G) or 2.5 μg ml−1 fludioxonil (H) at 26°C for 10 days. Scale bars in panels B and D, 20 μm.
Under hyperosmotic stress conditions, the growth of the mutants was dramatically reduced compared to that of the wild-type strain (Fig. 1C). Hyperosmotic stress also led to a distinct morphological change in the mutant hyphae, which became swollen and vacuolated (Fig. 1D). The reduction in radial growth of the mutants was also found to be consistent with the biomass formation during osmotic stress in shake flasks (see Fig. S2 in the supplemental material). Similar reductions in growth were observed when ΔBbhog1 mutants were subjected to chronic hyperosmotic stress by exposure to concentrations of glycerol or KCl generating identical osmotic potentials (data not shown). The sensitivity to osmotic stress of ΔBbhog1 mutants was alleviated at a higher temperature (32°C) (Fig. 1E), like that of S. cerevisiae (48). Furthermore, disruption of Bbhog1 increased the sensitivities to high temperature and oxidative stress. At 32°C or in medium containing 20 mM H2O2, ΔBbhog1 mutants showed a significant reduction in growth compared to the wild-type strain (Fig. 1F and G). In addition, ΔBbhog1 mutants exhibited remarkable resistance to the fungicide fludioxonil (Fig. 1H), demonstrating that Bbhog1 has the same function in regulating sensitivity to fludioxonil as HOG1 MAPKs in other fungal species.
Disruption of Bbhog1 alters erythritol and arabitol accumulation.
To determine the effect of the ΔBbhog1 mutation on the cellular response to hyperosmotic stress, we prepared mycelial extracts from wild-type and ΔBbhog1 mutant cultures under isosmotic and hyperosmotic conditions and measured mannitol, trehalose, erythritol, arabitol, and glycerol concentrations using the gas-liquid chromatography method (25).
Disruption of Bbhog1 in B. bassiana did not interfere with the accumulation of mannitol and glycerol in mycelium. Under normal conditions, B. bassiana accumulated mannitol as a major carbohydrate in the mycelium (400.11 ± 6.71 μmol g−1 [dry weight] mycelium). During hyperosmotic stress, which was imposed by incubating fungal mycelium in 0.4 M NaCl, the level of mannitol increased by 30.0% (Fig. 2A). Compared to the mannitol contents of the wild-type strain, no significant changes in the mannitol contents of the ΔBbhog1 mutant were observed under normal conditions or under hyperosmotic stress conditions (Fig. 2A). Smaller amounts of glycerol were present both in the wild-type strain and in the ΔBbhog1 mutant, and the amounts increased by 50.5% and 61.8% during osmotic stress, respectively (Fig. 2B). The trehalose accumulation patterns were similar in the wild-type and ΔBbhog1 strains, although the levels were decreased during hyperosmotic stress (data not shown).
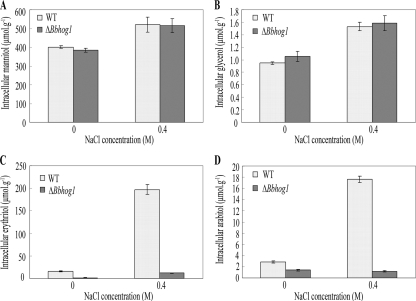
FIG. 2.
Compatible solute production in mycelia of the wild-type strain and the ΔBbhog1 mutant. Mycelia were grown for 48 h in SDY broth before they were transferred to SDY broth or SDY broth containing 0.4 M NaCl and incubated for a further 24 h. Carbohydrates were extracted and quantified by gas-liquid chromatography (25). (A) Mannitol; (B) glycerol; (C) erythritol; (D) arabitol. The error bars indicate standard deviations from three repeats of the experiment. WT, wild type.
Significant changes in erythritol and arabitol accumulation in the ΔBbhog1 mutant were found when the mycelium was exposed to the osmotic stress. Erythritol is a major solute that accumulates in B. bassiana under osmotic stress conditions (23). When the organisms were stressed with 0.4 M NaCl, the concentration of erythritol increased rapidly in wild-type cells, reaching levels that were 11.7-fold higher than the levels under normal conditions (Fig. 2C). In cells of the ΔBbhog1 mutant, erythritol accumulated at very low levels under normal conditions, reaching only 7.8% (F3,11 = 38.27; least significant difference for the post hoc test, P < 0.001) of the level in the wild-type strain. Although osmotic stress led to an erythritol level in the ΔBbhog1 mutant mycelium that was 8.9-fold-higher than the level under normal conditions, the level was still much lower than that in the wild-type strain (Fig. 2C). Arabitol, which was present at only low levels (2.82 ± 0.20 μmol g−1 [dry weight] mycelium) during growth of B. bassiana under isosmotic conditions, accumulated dramatically in response to hyperosmotic stress, and the level was 17.7-fold greater than the level under normal conditions (Fig. 2D). In contrast, the levels of arabitol in cells of the ΔBbhog1 mutant during growth under normal conditions were significantly lower than those in cells of the wild-type strain (F3,11 = 1935.45; Dunnett T3 for the post hoc test, P < 0.001) and did not increase like the levels in the wild-type strain in response to hyperosmotic stress (Fig. 2D). These results suggested that accumulation of erythritol and accumulation of arabitol were regulated by Bbhog1 in B. bassiana.
Disruption of Bbhog1 leads to a significant decrease in virulence.
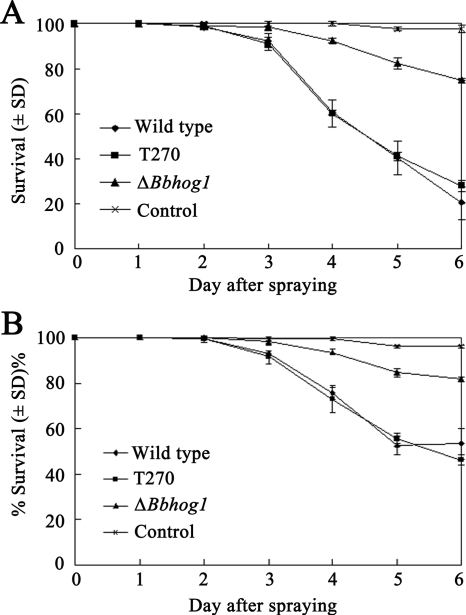
The bioassay results showed that disruption of Bbhog1 in B. bassiana significantly reduced the virulence against M. persicae. Significantly more aphids survived after inoculation of the ΔBbhog1 mutant at concentrations of 1 × 107 and 5 × 107 conidia ml−1 than after inoculation of the wild-type strain. No distinct difference in survival rates was observed for aphids inoculated with the ectopic integration transformant T270 and aphids inoculated with the wild type (Fig. 3). For instance, at day 6 after inoculation, the survival rates for aphids inoculated using concentrations of 5 × 107 and 1 × 107 conidia ml−1 in assays with the ΔBbhog1 mutant were in the range from 75.2 to 81.7% (df = 2 and 8), compared to 20.3 to 53.3% (df = 2 and 8) for assays with the wild-type strain (Fig. 3). Meanwhile, 6 days after inoculation, the corrected mortality rates for M. persicae treated with the ΔBbhog1 mutant using concentrations of 1 × 107 and 5 × 107 conidia ml−1 were reduced by 66.5% and 72.5%, respectively, compared to the mortality rates for M. persicae inoculated with the same concentrations of the wild-type strain. The results suggested that Bbhog1 is an important virulence factor of B. bassiana.
FIG. 3.
Trends for mean survival of M. persicae after spraying. M. persicae peach aphids were sprayed with 1-ml portions of conidial suspensions containing 5 × 107 conidia ml−1 (A) and 1 × 107 conidia ml−1 (B), and the survival was recorded every day after inoculation. Control insects were sprayed with a conidial suspension prepared with the ectopic integration transformant T270 and sterilized water. For each treatment there were three replicates with 30 to 40 aphids each, and the experiments were repeated three times.
Disruption of Bbhog1 decreases spore viability and adherence and impairs appressorium formation.
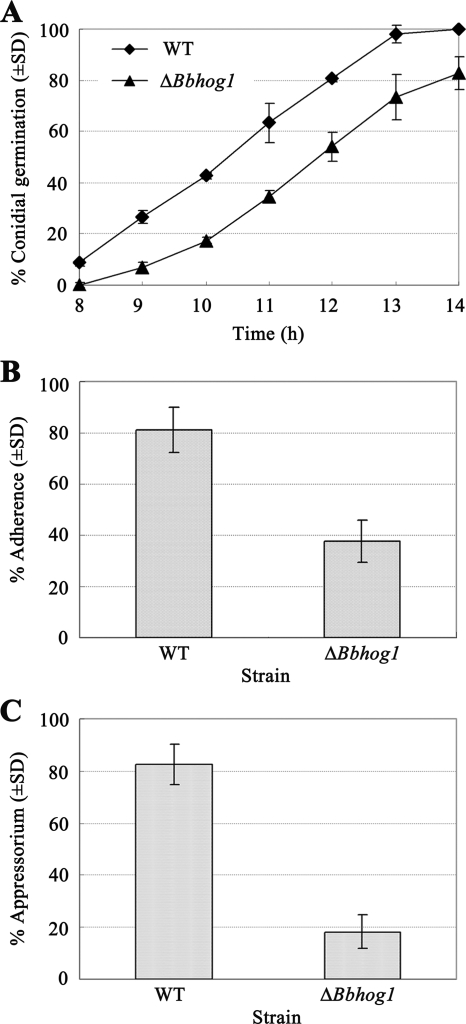
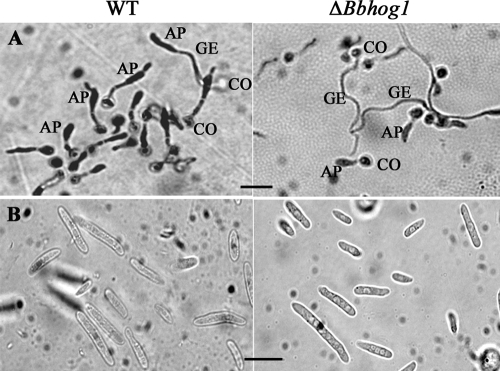
To investigate the effect of Bbhog1 disruption on virulence-related factors, we examined the conidial viability, adherence, appressorium formation, and hyphal body differentiation of ΔBbhog1 mutants. The conidial germination rates of the ΔBbhog1 mutant on agar plates during the 8 to 14 h after inoculation were significantly lower (P < 0.01) than those of the wild-type strain (Fig. 4A). The median germination time of the mutant was ca. 1.50 h longer than that of the wild-type strain. The results suggested that disruption of Bbhog1 in B. bassiana caused a reduction in spore viability.
FIG. 4.
Determination of conidial germination, adherence, and appressorium formation. (A) Conidial germination. Conidia were inoculated onto CZP agar plates, and germinated conidia were counted hourly beginning 8 h after inoculation. (B) Conidial adherence on cicada hind wings. The assay was performed by using a previously described method (48). (C) Frequency of appressorium formation on cicada hind wings after 22 h of incubation. All experiments were repeated three times with three replicates for each repeat. WT, wild type.
Conidial adherence was assayed using cicada hind wings and a method described previously (55). At 8 h after inoculation, only 37.7% ± 9.3% of the mutant conidia remained on the wings and could not be washed off by 0.05% (vol/vol) Tween 20, compared to 81.1% ± 10.5% of the wild-type strain conidia (df = 4; t = 6.17; P < 0.01) (Fig. 4B), indicating that conidial adherence to the insect cuticle was severely impaired by the gene disruption.
Appressoria were induced on cicada hind wings using a method described previously (54). The results showed that although the appressorium structure of the ΔBbhog1 mutant was morphologically similar to that of the wild type (Fig. 5A), the frequency of appressorium formation for the ΔBbhog1 mutant was significantly lower than that for the wild-type strain. Twenty-two hours following inoculation, only 18.2% ± 6.4% of germinated conidia of the mutant differentiated into appressoria, a 77.9% decrease compared to the results for the wild-type strain (82.5% ± 7.5%) (df = 4; t = 27.09; P < 0.001) (Fig. 4C).
FIG. 5.
(A) Appressorium morphology. Appressorium formation was induced on cicada hind wings using a method described previously (54). AP, appressorium; GE, germ tube; CO, conidium. Bar = 20 μm. (B) Hyphal body differentiation at 3 days after injection of conidia into P. brassicae larva. Bar = 20 μm. WT, wild type.
In addition, we also investigated the hyphal body differentiation of ΔBbhog1 mutants inside the insect body. At day 3 after injection, the ΔBbhog1 mutant produced the same amount of hyphal bodies in the hemolymph of the third-instar larvae of P. brassicae as the wild-type strain (Fig. 5B), suggesting that inactivation of Bbhog1 may not influence the postpenetration development of B. bassiana.
Bbhog1 disruption suppresses expression of the hydrophobin-encoding genes hyd1 and hyd2.
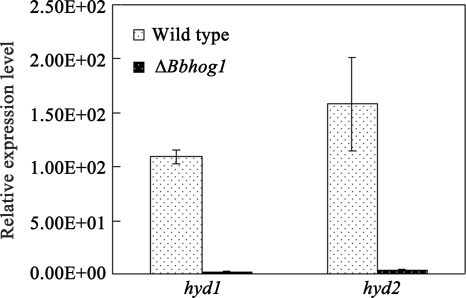
The ranking of the four internal reference genes after determination of gene stability was as follows (from least stable to most stable): actin, 18S rRNA, β-tubulin, Bgpd. Thus, we selected the Bgpd, β-tubulin, and 18S rRNA genes to normalize the levels of gene transcription using geNORM (52). The transcript levels of two hydrophobin-encoding genes, hyd1 and hyd2, were determined by real-time RT-PCR when the fungal cells were grown in the presence of insect cuticle for 3 days. The results showed that the transcript levels of hyd1 and hyd2 in the ΔBbhog1 mutant were 46.4-fold and 41.5-fold lower, respectively, than those in the wild-type strain (Fig. 6). Similar reductions in the levels of expression of hyd1 and hyd2 were observed when ΔBbhog1 mutants were grown in chitin broth (data not shown).
FIG. 6.
Expression of the hydrophobin-encoding genes hyd1 and hyd2 in the ΔBbhog1 mutant and the wild-type strain. Real-time RT-PCR was used to determine the relative levels of expression of hyd1 and hyd2 using Bgpd, β-tubulin, and 18S rRNA as loading controls to normalize samples by a method described previously (52) when the fungal cells were grown in the presence of cicada cuticle for 3 days. The error bars indicate standard deviations.
We also analyzed the transcript levels of the genes encoding two cuticle-degrading enzymes, the Pr1 protease CDEP1 and the chitinase Bbchit1, in ΔBbhog1 mutants and the wild type by real-time RT-PCR using RNA from mycelia induced by using the cicada cuticle. The transcript patterns of these two genes were similar in the wild-type and ΔBbhog1 strains (data not shown), suggesting that inactivation of Bbhog1 in B. bassiana does not affect the expression of genes encoding cuticle-degrading enzymes.
DISCUSSION
Bbhog1 disruption mutants showed sensitivity to hyperosmotic stress, high temperature, and oxidative stress and exhibited a remarkable resistance to the fungicide fludioxonil. These results demonstrate that there is a conserved function of HOG1 MAPKs in regulation of abiotic stress responses. Similar to the situation in S. cerevisiae (48, 57), the sensitivity to osmotic stress of ΔBbhog1 mutants could be alleviated by a higher temperature (32°C) (Fig. 1E), suggesting that a similar mode of response to hyperosmotic conditions and an elevated temperature may be present in B. bassiana. In addition, two strong bands were detected using HOG1 antibody in mycelial extracts of the B. bassiana wild-type strain, but no signal was observed in Bbhog1 disruption mutants (see Fig. 1SD in the supplemental material), demonstrating that both of the bands are related to HOG1 MAPK. This phenomenon has also been observed for Botrytis cinerea (29). The relationship of these two bands is unclear.
Under osmotic stress conditions, fungal cells often accumulate high levels of a compatible solute to maintain cellular turgor (33). The HOG1 pathway is required for regulation of the accumulation of some carbohydrates to increase osmolarity in several fungal species. In S. cerevisiae, hyperosmotic conditions induce an increase in the level of intracellular glycerol (36). In this process, HOG1 MAPK is involved in glycerol synthesis by regulating expression of the glycerol synthesis genes GPD1 and HOR2 (20). In C. albicans, glycerol accumulation under hyperosmotic stress conditions is also partially controlled by this pathway (44). However, in the phytopathogenic fungus M. grisea, it is arabitol and not glycerol that is the major compatible solute for adaptation to acute and chronic hyperosmotic stress. The accumulation of glycerol and turgor generation in appressoria are not altered by disruption of the HOG1 MAPK gene osm1 (12). Disruption of Bbhog1 in B. bassiana resulted in a significant decrease in erythritol and arabitol accumulation under both normal and hyperosmotic stress conditions (Fig. 2). Thus, HOG1-mediated carbohydrate accumulation varies among fungal species.
Although inactivation of Bbhog1 did not result in a measurable defect in the growth of B. bassiana under normal conditions, a significant reduction in conidiation in agar plates was observed for Bbhog1 disruption mutants. This phenomenon was also observed for the rice blast fungus M. grisea, in which disruption of the osm1 gene (a homologue of HOG1) leads to a dramatic reduction in conidial production in standard medium (12). Furthermore, a different MAPK in the corn leaf pathogen Cochliobolus heterostrophus (CHK1, an ortholog of FUS3/KSS1) was required for conidiation (31). However, the mechanism of conidiation regulated by MAPKs is poorly understood. In several filamentous fungi, the heterotrimeric G protein signaling pathway is involved in conidiation, as well as morphogenesis and pathogenesis (3, 16, 30). The regulatory G protein signaling gene Bbrgs1 is also required for conidiation in B. bassiana (15). Although G proteins are generally upstream of MAPK pathways, it is not yet known whether conidiation of B. bassiana mediated by Bbrgs1 occurs through activation of MAPKs. Further experiments are needed to elucidate the cross talk between MAPKs and G protein signal pathways for regulation of conidiation.
Accumulating data have shown that HOG1 kinases are involved mainly in stress responses. However, there is little evidence correlating HOG1 kinases with the virulence or pathogenicity of pathogens. In the phytopathogenic fungus M. grisea, disruption of the osm1 gene homologous to the HOG1 gene does not alter the virulence, and Δosm1 mutants are fully pathogenic (12). In contrast, MgHog1 disruption mutants of the plant pathogen M. graminicola do not infect wheat leaves due to impaired initiation of infectious germ tubes (35). Either hyperactivation or silence of a HOG1 protein, ThHog1, in T. harzianum results in strongly reduced antagonistic activity against the plant pathogens Phoma betae and Colletotrichum acutatum (11). Here we found that Bbhog1 was an important virulence determinant of B. bassiana and influenced at least three aspects of infection, spore viability, adherence to the insect cuticle, and appressorium formation.
The rate of conidial germination is an important indicator of virulence in entomopathogenic fungi and is to some degree correlated with virulence (2, 26, 43, 46). It was demonstrated previously that physical manipulation of growth conditions can significantly modify the endogenous compounds synthesized and channeled into the propagules of fungi (33, 38). Optimization of erythritol and glycerol contents in conidia of entomopathogenic fungi, such as B. bassiana, Metarhizium anisopliae, and Paecilomyces farinosus, has increased the germination rate and improved the pathogenicity at a low relative humidity (22, 23, 33). A reduction in the spore viability of ΔBbhog1 mutants may be due to lower levels of some compatible solutes in mature conidia compared to the levels in the wild-type strain (Fig. 2).
Cuticle attachment has been considered a reliable determinant of pathogenicity (2, 24, 55). Fungal cell attachment to the cuticle may involve specific receptor-ligand and/or nonspecific hydrophobic and electrostatic mechanisms (4, 5, 13). Hydrophobins are generally thought to be ubiquitous proteins of fungal walls. These proteins form an outer layer of hyphae and conidia and play a role in a broad range of processes in the growth and development of filamentous fungi. They are involved in the formation of aerial structures and in the attachment of hyphae to hydrophobic surfaces (9, 56). For instance, the hydrophobin MPG1 of M. grisea directs formation of a rodlet layer on conidia, which contributes to the hydrophobicity of the surface. A Δmpg1 mutant showed reduced infectivity and an inability to form appressoria (50). Recently, two hydrophobins, Hyd1 and Hyd2, were also isolated from B. bassiana, and Hyd2 has been identified as the major component of the rodlet layer in the aerial conidium surface (9). Here we found that transcript levels of hyd1 and hyd2 were markedly reduced in a ΔBbhog1 mutant when the fungal cells were grown in the presence of insect cuticle (Fig. 6), suggesting that Bbhog1 may influence the expression of some genes associated with hydrophobicity or adherence. In addition, hyd2 is expressed more strongly than hyd1 both in wild-type strain Bb0062 and in a ΔBbhog1 mutant, which is not consistent with the data described previously (9). The difference may be due to genetic diversity of different isolates.
Like the conidia of many plant-pathogenic fungi, conidia of entomopathogenic fungi, such as M. anisopliae and B. bassiana, often differentiate to form appressoria on the host surface. The formation of appressoria is pivotal for penetrating the host cuticle and establishing a pathogenic relationship with the host (10). Consequently, impairment of appressorium formation reduces the virulence of the fungal pathogen to host. It has been demonstrated that attachment is required to trigger the appropriate stimuli necessary to initiate appressorium development (50). Therefore, we may reasonably speculate that impairment of appressorium formation in ΔBbhog1 mutants is partially due to the reduction in attachment which is to a certain extent mediated by hydrophobins.
Supplementary Material
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (grants 2009CB118904 and 2006AA10A212), the Natural Science Foundation of China (grant 30471174), and the Program for the First Excellent Talents of University in Chongqing.
Footnotes
Published ahead of print on 10 April 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abbott, W. S. 1925. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 18:265-267. [Google Scholar]
- 2.Altre, J. A., J. D. Vandenberg, and F. A. Cantone. 1999. Pathogenicity of Paecilomyces fumosoroseus isolates to diamondback moth, Plutella xylostella: correlation with spore size, germination speed, and attachment to cuticle. J. Invertebr. Pathol. 73:332-338. [DOI] [PubMed] [Google Scholar]
- 3.Bölker, M. 1998. Sex and crime: heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 25:143-156. [DOI] [PubMed] [Google Scholar]
- 4.Boucias, D. G., and J. C. Pendland. 1991. Attachment of mycopathogens to cuticle, p. 101-127. In G. T. Cole and H. C. Hoch (ed.), The fungal spore and disease initiation in plants and animals. Plenum Press, New York, NY.
- 5.Boucias, D. G., J. C. Pendland, and J. P. Latge. 1988. Nonspecific factors involved in attachment of entomopathogenic deuteromycetes to host insect cuticle. Appl. Environ. Microbiol. 54:1795-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braga, G. U. L., S. D. Flint, C. L. Messias, A. J. Anderson, and D. W. Roberts. 2001. Effect of UV-B on conidia and germlings of the entomopathogenic hyphomycete Metarhizium anisopliae. Mycol. Res. 105:874-882. [DOI] [PubMed] [Google Scholar]
- 7.Brewster, J. L., T. de Valoir, N. D. Dwyer, E. Winter, and M. C. Gustin. 1993. An osmosensing signal transduction pathway in yeast. Science 259:1760-1763. [DOI] [PubMed] [Google Scholar]
- 8.Butt, T. M., C. Jackson, and N. Magan. 2001. Fungi as biocontrol agents: progress, problems and potential, p. 1-8. In T. M. Butt, C. Jackson, and N. Magan (ed.), Fungi as biocontrol agents: progress, problems and potential. CAB International, Oxford, United Kingdom.
- 9.Cho, E. M., B. H. Kirkland, D. J. Holder, and N. O. Keyhani. 2007. Phage display cDNA cloning and expression analysis of hydrophobins from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology 153:3438-3447. [DOI] [PubMed] [Google Scholar]
- 10.Clarkson, J. M., and A. K. Charnley. 1996. New insights into the mechanisms of fungal pathogenesis in insect. Trends Microbiol. 4:197-203. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Jarana, J., S. Sousa, F. González, M. Rey, and A. Llobell. 2006. ThHog1 controls the hyperosmotic stress response in Trichoderma harzianum. Microbiology 152:1687-1700. [DOI] [PubMed] [Google Scholar]
- 12.Dixon, K. P., J. R. Xu, N. Smirnoff, and N. J. Talbot. 1999. Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 11:2045-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doss, R. P., S. W. Potter, G. A. Chastagner, and J. K. Christian. 1993. Adhesion of nongerminated Botrytis cinerea conidia to several substrata. Appl. Environ. Microbiol. 59:1786-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang, W., B. Leng, Y. Xiao, K. Jin, J. Ma, Y. Fan, J. Feng, X. Yang, Y. Zhang, and Y. Pei. 2005. Cloning of Beauveria bassiana chitinase gene Bbchit1 and its application to improve fungal strain virulence. Appl. Environ. Microbiol. 71:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang, W., L. R. Scully, L. Zhang, Y. Pei, and M. J. Bidochka. 2008. Implication of a regulator of G protein signaling (BbRGS1) in conidiation and conidial thermotolerance of the insect pathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 279:146-156. [DOI] [PubMed] [Google Scholar]
- 16.Fang, W., Y. Pei, and M. J. Bidochka. 2007. A regulator of a G protein signalling (RGS) gene, cag8, from the insect-pathogenic fungus Metarhizium anisopliae is involved in conidiation, virulence and hydrophobin synthesis. Microbiology 153:1017-1025. [DOI] [PubMed] [Google Scholar]
- 17.Fang, W., Y. Zhang, X. Yang, X. Zheng, H. Duan, Y. Li, and Y. Pei. 2004. Agrobacterium tumefaciens-mediated transformation of Beauveria bassiana using an herbicide resistance gene as a selection marker. J. Invertebr. Pathol. 85:18-24. [DOI] [PubMed] [Google Scholar]
- 18.Fang, W., Y. Zhang, X. Yang, Z. Wang, and Y. Pei. 2002. Cloning and characterization of cuticle degrading enzyme CDEP-1 from Beauveria bassiana. Acta Genet. Sin. 29:278-282. [PubMed] [Google Scholar]
- 19.Feng, M. G., T. J. Poprawski, and G. G. Khachatourians. 1994. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci. Technol. 4:3-34. [Google Scholar]
- 20.Gustin, M. C., J. Albertyn, M. Alexander, and K. Davenport. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62:1264-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajek, A. E., and R. J. St. Leger. 1994. Interactions between fungal pathogenesis and insect hosts. Annu. Rev. Entomol. 39:293-322. [Google Scholar]
- 22.Hallsworth, J. E., and N. Magan. 1994. Effects of KCl concentration on accumulation of acyclic sugar alcohols and trehalose in conidia of three entomopathogenic fungi. Lett. Appl. Microbiol. 18:8-11. [Google Scholar]
- 23.Hallsworth, J. E., and N. Magan. 1995. Manipulation of intracellular glycerol and erythritol enhances germination of conidia at low water availability. Microbiology 141:1109-1115. [DOI] [PubMed] [Google Scholar]
- 24.Holder, D. J., and N. O. Keyhani. 2005. Adhesion of the entomopathogenic fungus Beauveria (Cordyceps) bassiana to substrata. Appl. Environ. Microbiol. 71:5260-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu, L., B. Guo, H. Lu, X. Zou, Y. Li, and X. Jiang. 2004. Gas chromatographic analysis of sugar and sugar alcohol derivatives through 1-methlimidazole catalyzed acetylation. Chin. Bull. Bot. 21:689-699. [Google Scholar]
- 26.James, R. R. 2001. Effects of exogenous nutrients on conidial germination and virulence against the silverleaf whitefly for two hyphomycetes. J. Invertebr. Pathol. 77:99-107. [DOI] [PubMed] [Google Scholar]
- 27.Jin, K., Y. Zhang, Z. Luo, Y. Xiao, Y. Fan, D. Wu, and Y. Pei. 2008. An improved method for Beauveria bassiana transformation using phosphinothricin acetlytransferase and green fluorescent protein fusion gene as a selectable and visible marker. Biotechnol. Lett. 30:1379-1383. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki, L., O. Sánchez, K. Shiozaki, and J. Aguirre. 2002. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol. Microbiol. 45:1153-1163. [DOI] [PubMed] [Google Scholar]
- 29.Kojima, K., Y. Takano, A. Yoshimi, C. Tanaka, T. Kikuchi, and T. Okuno. 2004. Fungicide activity through activation of a fungal signalling pathway. Mol. Microbiol. 53:1785-1796. [DOI] [PubMed] [Google Scholar]
- 30.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lev, S., A. Sharon, R. Hadar, H. Ma, and B. A. Horwitz. 1999. A mitogen-activated protein kinase of the corn leaf pathogen Cochliobolus heterostrophus is involved in conidiation, appressorium formation, and pathogenicity: diverse roles for mitogenactivated protein kinase homologs in foliar pathogens. Proc. Natl. Acad. Sci. USA 96:13542-13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luz, C., I. G. Silva, C. M. T. Cordeiro, and M. S. Tigano. 1998. Beauveria bassiana (Hyphomycetes) as a possible agent for biological control of Chagas disease vectors. J. Med. Entomol. 35:977-979. [DOI] [PubMed] [Google Scholar]
- 33.Magan, N. 2001. Physiological approaches to improving the ecological fitness of fungal biocontrol agents, p. 239-251. In T. M. Butt, C. Jackson, and N. Magan (ed.), Fungi as biocontrol agents: progress, problems and potential. CAB International, Oxford, United Kingdom.
- 34.McCluskey, K. 2003. The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52:245-262. [DOI] [PubMed] [Google Scholar]
- 35.Mehrabi, R., L. H. Zwiers, M. A. de Waard, and G. H. J. Kema. 2006. MgHog1 regulates dimorphism and pathogenicity in the fungal wheat pathogen Mycosphaerella graminicola. Mol. Plant-Microbe. Interact. 19:1262-1269. [DOI] [PubMed] [Google Scholar]
- 36.Nevoigt, E., and U. Stahl. 1997. Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 21:231-241. [DOI] [PubMed] [Google Scholar]
- 37.Nishida, E., and Y. Gotoh. 1993. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem. Sci. 18:128-131. [DOI] [PubMed] [Google Scholar]
- 38.Pascual, S., P. Melgarejo, and N. Magan. 2003. Water availability affects the growth, accumulation of compatible solutes and the viability of the biocontrol agent Epicoccum nigrum. Mycopathologia 156:93-100. [DOI] [PubMed] [Google Scholar]
- 39.Raeder, U., and P. Broda. 1985. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1:17-20. [Google Scholar]
- 40.Rangel, D. E. N., G. U. L. Braga, S. D. Flint, A. J. Anderson, and D. W. Roberts. 2004. Variations in UV-B tolerance and germination speed of Metarhizium anisopliae conidia produced on insects and artificial substrates. J. Invertebr. Pathol. 87:77-83. [DOI] [PubMed] [Google Scholar]
- 41.Roberts, D. W., and R. J. St. Leger. 2004. Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv. Appl. Microbiol. 54:1-70. [DOI] [PubMed] [Google Scholar]
- 42.Robinson, M. J., and M. H. Cobb. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180-186. [DOI] [PubMed] [Google Scholar]
- 43.Safavi, S. A., F. A. Shah, A. K. Pakdel, G. R. Rasoulian, A. R. Bandani, and T. M. Butt. 2007. Effect of nutrition on growth and virulence of the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 270:116-123. [DOI] [PubMed] [Google Scholar]
- 44.San José, C., R. Alonso Monge, R. Pérez-Díaz, J. Pla, and C. Nombela. 1996. The mitogen-activated protein kinase homologue HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 178:5850-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaeffer, H. J., and M. J. Webber. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah, F. A., C. S. Wang, and T. M. Butt. 2005. Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol. Lett. 251:259-266. [DOI] [PubMed] [Google Scholar]
- 47.Sharma, P., N. Meena, M. Aggarwal, and A. K. Mondal. 2005. Debaryomyces hansenii, a highly osmo-tolerant and halo-tolerant yeast, maintains activated Dhog1p in the cytoplasm during its growth under severe osmotic stress. Curr. Genet. 48:162-170. [DOI] [PubMed] [Google Scholar]
- 48.Siderius, M., E. Rots, and W. H. Mager. 1997. High-osmolarity signalling in Saccharomyces cerevisiae is modulated in a carbon-source-dependent fashion. Microbiology 143:3241-3250. [DOI] [PubMed] [Google Scholar]
- 49.St. Leger, R. J., L. Joshi, M. J. Bidochka, and D. W. Roberts. 1996. Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc. Natl. Acad. Sci. USA 93:6349-6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talbot, N. J., M. J. Kershaw, G. E. Wakley, O. M. H. De Vries, J. G. H. Wessels, and J. E. Hamer. 1996. MPG1 encodes a fungal hydrophobin involved in surface interactions during infection-related development of Magnaporthe grisea. Plant Cell 8:985-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vandenberg, J. D. 1996. Standardized bioassay and screening of Beauveria bassiana and Paecilomyces fumosoroseus against the Russian wheat aphid (Homoptera: Aphididae). J. Econ. Entomol. 89:1418-1423. [Google Scholar]
- 52.Vandesompele, J., K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe, and F. Speleman. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:RESEARCH0034.1-RESEARCH0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan, C. H., and T. A. Wilkins. 1994. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal. Biochem. 223:7-12. [DOI] [PubMed] [Google Scholar]
- 54.Wang, C. S., and R. J. St. Leger. 2005. Developmental and transcriptional responses to host and nonhost cuticles by the specific locust pathogen Metarhizium anisopliae var. acridum. Eukaryot. Cell 4:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, C. S., and R. J. St. Leger. 2007. The MAD1 adhesion of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesion enables attachment to plants. Eukaryot. Cell 6:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whiteford, J. R., and P. D. Spanu. 2002. Hydrophobins and the interactions between fungi and plants. Mol. Plant Pathol. 3:391-400. [DOI] [PubMed] [Google Scholar]
- 57.Wojda, I., R. Alonso-Monge, J. Bebelman, W. H. Mager, and M. Siderius. 2003. Response to high osmotic conditions and elevated temperature in Saccharomyces cerevisiae is controlled by intracellular glycerol and involves coordinate activity of MAP kinase pathways. Microbiology 149:1193-1204. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, Y., R. Lamm, C. Pillonel, S. Lam, and J.-R. Xu. 2002. Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 68:532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.