Abstract
Enterohemorrhagic Escherichia coli O157:H7 (EHEC O157:H7) outbreaks have revealed the need for improved analytical techniques for environmental samples. Ultrafiltration (UF) is increasingly recognized as an effective procedure for concentrating and recovering microbes from large volumes of water and treated wastewater. This study describes the application of hollow-fiber UF as the primary step for concentrating EHEC O157:H7 seeded into 40-liter samples of surface water, followed by an established culture/immunomagnetic-separation (IMS) method and a suite of real-time PCR assays. Three TaqMan assays were used to detect the stx1, stx2, and rfbE gene targets. The results from this study indicate that approximately 50 EHEC O157:H7 cells can be consistently recovered from a 40-liter surface water sample and detected by culture and real-time PCR. Centrifugation was investigated and shown to be a viable alternative to membrane filtration in the secondary culture/IMS step when water quality limits the volume of water that can be processed by a filter. Using multiple PCR assay sets to detect rfbE, stx1, and stx2 genes allowed for specific detection of EHEC O157:H7 from strains that do not possess all three genes. The reported sample collection and analysis procedure should be a sensitive and effective tool for detecting EHEC O157:H7 in response to outbreaks of disease associated with contaminated water.
Several highly publicized outbreaks of gastrointestinal diseases caused by enterohemorrhagic Escherichia coli O157:H7 (EHEC O157:H7) have highlighted the threat this pathogen poses to public health (1, 2, 3, 14). Although the predominant mode of transmission to humans appears to be contaminated meat or meat products, there have been a number of outbreaks associated with contaminated water (18). Microbiological, epidemiological, and environmental studies have found an association between EHEC O157:H7 outbreaks and recreational water, drinking water, crop irrigation, and wastewater (1, 2, 14). These investigations have also revealed that enhanced rapid analytical techniques are needed to improve the speed and effectiveness of these types of investigations.
Hollow-fiber ultrafiltration (UF) is a sampling technique that is emerging as an option for recovering diverse microbes from large-volume water samples (8, 9, 12, 13, 15). There have been reports of the successful application of UF for surface water as well as for other E. coli strains (8, 13), but additional data are needed to evaluate the robustness of UF for surface water and its ability to effectively concentrate EHEC O157:H7 in the presence of background microbes. The presence of competitive microbes has been shown to significantly alter the growth rate and maximal density of EHEC O157:H7 in broth culture (5).
EHEC O157:H7 is generally detected in water samples by using membrane filtration, selective broth enrichment, immunomagnetic-separation (IMS), and isolation on selective agar culture plates, followed by confirmatory tests such as PCR or serological tests (6, 7). However, sensitive detection of EHEC O157:H7 in surface waters can be difficult due to high levels of competing background microorganisms (7). Membrane filtration can also limit the volume processed for turbid surface waters due to filter clogging. Centrifugation is an alternative to membrane filtration and has an advantage of not being subject to potential sample volume processing constraints for turbid water samples, so the technique could potentially increase the sensitivity of detection. A number of PCR assays have been developed for detection of EHEC O157:H7 that target a variety of virulence genes (17). Testing multiple gene targets is necessary for accurate detection because certain non-EHEC O157:H7 serotypes and other bacterial species are known to possess the target genes; therefore, the isolate cannot be determined to be EHEC O157:H7 unless multiple assays show a positive signal (19).
The goals of this study were to evaluate (i) the effectiveness of a previously reported UF method (8) for application to recovering EHEC O157:H7, (ii) the effectiveness of the culture/IMS technique performed in conjunction with primary UF concentration, (iii) the effectiveness of centrifugation as an alternative for membrane filtration in the culture/IMS method, and (iv) the ability of three previously reported real-time PCR assays to accurately detect EHEC O157:H7 in surface waters (16, 17).
METHODS AND MATERIALS
Water samples.
Forty-liter surface water samples were obtained in cubitainers from two different locations in Georgia between February and June 2008. River and lake water samples were collected from the Chattahoochee River and Murphy Candler Lake, respectively. All water samples were stored at 4°C and used within 1 week of collection.
Water quality testing.
All water samples were characterized within 1 day of sampling using the following water quality parameters: temperature, pH, turbidity, specific conductance (SC), alkalinity, total hardness, total organic carbon (TOC), total suspended solids (TSS), and E. coli. Sample pH was measured with a Fisher Scientific Accumet Research AR25 pH/mV/°C/ion selective electrode meter. Turbidity was measured using a Hach model 2100N laboratory turbidimeter (Hach Company). SC and temperature were measured with an Oakton CON 100 conductivity/°C meter. Alkalinity was determined using the Hach alkalinity test method 8203 and AL-DT digital titrator. Total hardness was measured using the Hach total hardness test method 8213 and AL-DT digital titrator. TOC was measured using a Hach low-range TOC reagent set and a Hach DR/2400 portable spectrophotometer. TSS was determined using Standard Methods for the Examination of Water and Wastewater (4). E. coli was enumerated by membrane filtration and a modified membrane-thermotolerant E. coli agar method according to EPA method 1603 (20).
Microbial stock preparation.
E. coli O157:H7 (ATCC 43895) stocks were produced according to ATCC guidance and enumerated by a five-tube most probable number (MPN) enrichment in modified tryptic soy broth with novobiocin (Merck, Darmstadt, Germany). MPN estimates were determined using Standard Methods for the Examination of Water and Wastewater (4). This particular isolate was chosen because it produces both Shiga-like toxin I and Shiga-like toxin II, therefore enabling differentiation between the seeded strain and naturally occurring E. coli present in water samples. In preparation for seeding, the frozen EHEC O157:H7 stocks (9.2 × 108 MPN/ml) were diluted using 10-fold dilutions and vigorous vortex mixing in a diluent containing 0.01 M phosphate-buffered saline (PBS; Dulbecco's modification, pH 7.40), 0.01% (wt/vol) Tween 80 (Fisher), and 0.001% (wt/vol) antifoam Y-30 emulsion (Sigma) in order to disperse bacterial cells. Microbial seeding was performed at target levels of 200, 50, and 10 bacterial cells per 40 liters of surface water. MPN testing of experiment seed stocks indicated that the experiments received geometric mean seeding levels of 180, 48, and 9.2 MPN/ml.
Hollow-fiber UF procedure.
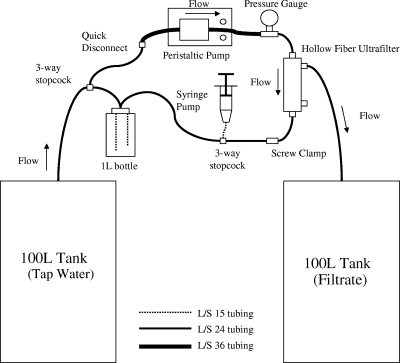
The filtration unit setup is shown in Fig. 1, as previously described (8). Fresenius F200NR ultrafilters were blocked using 5% calf serum (Invitrogen catalog no. 16170-078) and allowed to rotate overnight on a rotisserie (8). Prior to beginning the UF procedure, sodium polyphosphate (Sigma-Aldrich) was added to the water sample to achieve a final concentration of 0.01% (wt/vol) (8). After UF was completed and the retentate was collected, a 500-ml elution solution consisting of 0.01% Tween 80, 0.01% sodium polyphosphate, and 0.001% antifoam Y-30 emulsion was recirculated through the ultrafilter and allowed to reduce in volume (8). The final combined retentate and elution UF samples from this procedure were 475 ± 56 ml.
FIG. 1.
40-Liter UF setup. Schematic of hollow-fiber UF procedure. L/S, laboratory standard.
Secondary sample processing.
Two approaches were investigated for secondary processing of the UF concentrates: membrane filtration and centrifugation. For membrane filtration, UF concentrate was filtered using 0.45-μm-pore-size mixed cellulose ester filters according to Standard Methods for the Examination of Water and Wastewater (4). Because of filter clogging, only 10 ml of UF concentrate could be processed through a membrane filter. For each experiment, four 10-ml aliquots were processed to increase the total sample volume tested by membrane filtration. Negative controls were included by filtering PBS. For centrifugation, 150 ml of UF concentrate was centrifuged at 4,000 × g at 4°C for 30 min, the supernatant was discarded, and the pellet was resuspended using PBS. All filters and resuspended pellets were incubated at 42°C in 150-ml bottles containing 100 ml of modified tryptic soy broth with novobiocin (Merck, Darmstadt, Germany) with agitation for 20 to 24 h (6). One-milliliter aliquots of the enriched samples were analyzed using IMS in combination with growth on selective culture plates (6). Dynabeads anti-E. coli O157 (Dynal Biotech, Oslo, Norway) were used for IMS purification. Dynabeads were used according to the manufacturer's instructions. The immunomagnetically concentrated samples were transferred to selective sorbitol-MacConkey agar plates (Merck, Darmstadt, Germany), supplemented with cefixime-tellurite and incubated at 37°C for 20 to 24 h (6).
Nucleic acid extraction.
One or two suspect colorless (non-sorbitol fermenting) colonies from each plate were confirmed using EHEC O157:H7-specific real-time PCR assays. Nucleic acid extraction was performed using a previously reported noncommercial lysis buffer (8). A colony was picked and suspended in 350 μl of nuclease-free water and 350 μl of lysis buffer. The sample was vortexed for 30 s, transferred to a silica spin column (Omega Biotek, Norcross, GA), and passed through the column by centrifugation at 10,000 × g for 1 min. The column was washed once with 500 μl of 100% ethanol (and centrifuged at 10,000 × g for 1 min) and then again with 75% ethanol (and spun at 10,000 × g for 1 min). The column was centrifuged again to remove any excess ethanol. The column was then transferred to a clean microcentrifuge tube, and nucleic acid was eluted by adding 80 μl of Tris-EDTA buffer and centrifuging the column for 1 min at 10,000 × g.
Real-time PCR.
Amplification of DNA targets was performed using an iCycler iQ5 Real-time PCR Detection System (Bio-Rad, Hercules, CA) in a 96-well plate format. Three different TaqMan assays were performed, detecting Shiga toxin 1, Shiga toxin 2, and the O antigen for O157 serotypes. The Shiga toxin 1 (stx1) assay and Shiga toxin 2 (stx2) assay have been previously reported by Sharma et al. (17). The O antigen assay (rfbE) was reported by Sharma et al. (16) as both a reverse transcription-PCR and PCR assay. For this study, only the PCR assay was used. For each assay, the TaqMan probe was used at a final concentration of 100 nM, and the primers were used at a final concentration of 250 nM each.
Real-time PCR amplifications were performed under the following conditions: denaturation at 95°C for 15 min, followed by 45 cycles of denaturation at 94°C for 10 s, annealing at 55°C for 30 s, and extension at 72°C for 20 s. Each 20-μl reaction mixture contained 10 μl of 2× Master Mix (QuantiTect Probe PCR kit; Qiagen, Valencia, CA) and 2 μl of DNA template for all the target analytes. Each water sample was tested twice using these molecular assays.
RESULTS
Water quality.
A total of thirteen, 40-liter samples were collected from the two surface water locations. A wide range of water quality tests were performed in order to characterize the chemical and biological quality of the water samples used in the study. As shown in Table 1, the average pH of the surface water used in this study was similar for the lake and river water, i.e., 7.34 and 7.38, respectively. Turbidity of the study water varied widely, with river water averaging 3.60 nephelometric turbidity units (NTU) and the lake water averaging 18.4 NTU. Alkalinity and total hardness results indicated that both water types had very low buffering capacities and mineral content. TOC results showed that the river (4.46 mg/liter) and lake (8.01 mg/liter) waters also had low levels of organic matter. The E. coli concentrations varied greatly for both water types, as shown by the large standard deviations in Table 1. The lake water E. coli concentrations ranged from 3 to 107 CFU per 100 ml of water, and the river water counts ranged from 26 to 136 CFU/100 ml.
TABLE 1.
Water quality data
| Sample source (n)a | pH | Turbidity (NTU) | Field SC (μS/cm at 25°C) | Total hardness (mg/liter of CaCO3) | Alkalinity (mg/liter of CaCO3) | TOC (mg/liter of C) | TSS (mg/liter) | Free chlorine (mg/liter of Cl2) | E. coli (CFU/100 ml) |
|---|---|---|---|---|---|---|---|---|---|
| Lake water (8) | 7.34 ± 0.32 | 18.45 ± 13.58 | 81.90 ± 10.91 | 28.34 ± 4.10 | 25.76 ± 4.09 | 8.01 ± 2.14 | 0.0053 ± 0.0038 | 0.021 ± 0.013 | 39.32 ± 38.36 |
| River water (5) | 7.38 ± 0.18 | 3.60 ± 1.06 | 61.52 ± 6.33 | 17.2 2 ± 1.85 | 16.00 ± 1.41 | 4.46 ± 1.10 | 0.0016 ± 0.0015 | 0.01 ± 0.01 | 53.43 ± 40.49 |
n, number of samples.
Secondary method comparison.
As shown in Table 2, at the high-seed and mid-seed (∼200 and ∼50 MPN, respectively) levels, EHEC O157:H7 was consistently detected in 40-liter samples of lake and river water, regardless of the concentration method. One to three isolates were picked for each experiment to determine the limit of detection for the respective secondary concentration technique. At the mid-seed level (∼50 MPN) in lake water, only seven out of nine suspect isolates picked from the membrane-filtered experiments were determined to be EHEC O157:H7 by real-time PCR. Similarly, four of seven isolates picked from river water membrane filtration experiments at the mid-seed level were determined to be EHEC O157:H7. In the lake water at the low-seed level (∼10 MPN), EHEC O157:H7 was detected in two out of two experiments (and four of four isolates tested) in the centrifuged UF concentrate and in zero out of two when membrane filtration was used. For the river water samples, EHEC O157:H7 was detected in only one of three low-seed experiments (and one of seven isolates tested) when centrifugation was used for secondary concentration; it was not detected in any of the membrane filtration experiments. Two nonseeded experiments were preformed for each water type to ensure that there was no EHEC O157:H7 present in the sample initially.
TABLE 2.
Limits of EHEC O157:H7 detection for different secondary concentration methods
| Water type | No. of samples | Seed level (MPN) | Detection by membrane filtration
|
Detection by centrifugation
|
||
|---|---|---|---|---|---|---|
| No. of positive expts/total no. of expts (%) | No. of positive isolates/total no. of isolates tested (%) | No. of positive expts/total no. of expts (%) | No. of positive isolates/total no. of isolates tested (%) | |||
| Lake | 4 | 200 | 4/4 (100) | 6/6 (100) | 4/4 (100) | 6/6 (100) |
| 2 | 50 | 2/2 (100) | 7/9 (78) | 2/2 (100) | 6/6 (100) | |
| 2 | 10 | 0/2 (0) | 0/6 (0) | 2/2 (100) | 4/4 (100) | |
| 2 | None | 0/2 (0) | 0/6 (0) | 0/2 (0) | 0/6 (0) | |
| River | 2 | 200 | 2/2 (100) | 7/8 (88) | 2/2 (100) | 5/5 (100) |
| 2 | 50 | 2/2 (100) | 4/7 (57) | 2/2 (100) | 3/3 (100) | |
| 3 | 10 | 0/3 (0) | 0/13 (0) | 1/3 (33) | 1/7 (14) | |
| 2 | None | 0/2 (0) | 0/8 (0) | 0/2 (0) | 0/4 (0) | |
Real-time PCR.
Suspect isolates were determined to be the EHEC O157:H7 seeded strain only if all three assays showed a positive signal, as shown in Table 3 for all isolates tested (i.e., membrane filtration and centrifugation experiments combined). For the lake water high-seed (∼200 MPN), mid-seed (∼50 MPN), and control (no seeding) experiments, the isolates were either positive for all three real-time PCR assays or negative for these assays. For the low-seed level (∼10 MPN) lake water experiments, four isolates were positive using the stx2 assay but negative for the stx1 and rfbE assays. For the river water experiments, one isolate at the high-seed level was positive for both stx1 and stx2 but could not be determined to be a positive EHEC O157:H7 because the sample was negative for the rfbE gene. For the mid- and low-seeding experiments and control (no seeding) experiments using river water, there were a number of isolates that were positive for one or two of the genes (but not all three), so these were determined not to be the seeded EHEC O157:H7.
TABLE 3.
Real-time PCR assay results for suspect EHEC O157:H7 isolates by both concentration methods
| Water type | No. of samples | Seed level (MPN) | PCR result (no. of positive isolates/no. of isolates tested [%])
|
|||
|---|---|---|---|---|---|---|
| TaqMan assay for:
|
EHEC O157:H7 detection | |||||
| stx1 | stx2 | rfbE | ||||
| Lake | 4 | 200 | 12/12 (100) | 12/12 (100) | 12/12 (100) | 12/12 (100) |
| 2 | 50 | 13/15 (87) | 13/15 (87) | 13/15 (87) | 13/15 (87) | |
| 2 | 10 | 4/10 (40) | 8/10 (80) | 4/10 (40) | 4/10 (40) | |
| 2 | None | 0/12 (0) | 0/12 (0) | 0/12 (0) | 0/12 (0) | |
| River | 2 | 200 | 13/13 (100) | 13/13 (100) | 12/13 (92) | 12/13 (92) |
| 2 | 50 | 7/10 (70) | 9/10 (90) | 7/10 (70) | 7/10 (70) | |
| 3 | 10 | 2/20 (10) | 4/20 (20) | 3/20 (15) | 2/20 (10) | |
| 2 | None | 0/12 (0) | 4/12 (33) | 1/12 (8) | 0/12 (0) | |
DISCUSSION
UF has been shown in previous studies to effectively recover a diverse set of waterborne microbes in large-volume water samples (8, 9, 12, 13, 15). This study was designed to investigate the use of UF and alternative culture/IMS methods for secondary sample processing and analysis for EHEC O157:H7. In order to simulate normal environmental conditions, this study focused on the recovery of low organism seed levels from 40-liter surface water samples. Many studies have reported successful recovery of EHEC O157:H7 by the IMS/culture method in surface water, but these studies typically limited the analyses to sample volumes of ∼100 ml of water and reported limits of detection ranging from 1 to 6 CFU/100 ml (10, 11, 18). One study in The Netherlands processed 1-liter samples and reported a limit of detection of 4 MPN/liter (7). With the addition of UF to the established culture/IMS approach for EHEC O157:H7, the volume of water that can be analyzed was greatly increased. Using the UF technique prior to culture/IMS, the results from this study indicate that approximately 50 EHEC O157:H7 cells can be consistently recovered from a 40-liter surface water sample and detected by real-time PCR.
The results of this study also indicate that centrifugation is a more effective alternative to membrane filtration when water quality hinders the volume of water that can be passed through a 0.45-μm-pore-size filter. Additionally, the data indicate that centrifugation can increase the sensitivity of the method even with increased levels of bacterial background flora. This was anticipated because 150 ml of UF concentrate could be concentrated by centrifugation, whereas membrane filtration could process only 10 ml of UF concentrate before a filter became clogged. Also, according to the manufacturer and other publications, Dynal IMS beads are able to separate 100 E. coli O157 cells in the presence of 106 background flora cells (7). Centrifugation greatly minimized the number of subsamples that were necessary when membrane filtration was used. The entire UF concentrate volume (∼500 ml) could conceivably be centrifuged and enriched in one bottle of broth; however, this was not explored in this study.
Initially, a multiplex SYBR green PCR assay (21) was investigated for confirmatory testing of EHEC O157:H7. The SYBR Green assay produced good results for individual bacterial testing, but when applied to environmental isolates it resulted in numerous false positives (data not shown). A suite of three TaqMan assays was then selected to target the stx1, stx2, and rfbE gene targets (16, 17). The data from the present study indicate that the stx1, stx2, and rfbE TaqMan assays can be effectively used for detecting the presence of E. coli O157:H7 in surface water.
The stx2 assay yielded a higher percentage of positives than the other real-time PCR assays (Table 3), possibly due to horizontal transmission of stx genes between E. coli strains and also in non-E. coli enterobacteria such as Citrobacter and Enterobacter (14). In addition, the detection of the stx1 and/or stx2 gene(s) may indicate the presence of other Shiga toxin-producing E. coli in the environment, which in itself would be of importance in regard to public health protection and outbreak response.
Although the rfbE assay is suitable for specific detection of EHEC O157:H7, it can produce false-positive results for those E. coli strains that possess the O157-type O antigen but do not belong to the EHEC O157:H7 serogroup. Similarly, PCR assays targeting Shiga toxin-encoding genes stx1 and stx2 are not specific for EHEC O157:H7 as various combinations of these two genes are present in all Shiga toxin-producing E. coli strains. However, the use of multiple primer-probe sets for rfbE-, stx1-, and stx2-specific assays allows specific detection and profiling of virulence markers of EHEC O157:H7 in complex environmental samples.
This study has shown that a UF-based procedure performed in conjunction with secondary concentration and culture/IMS can detect 50 cells of EHEC O157:H7 in 40-liter environmental water samples. This sample volume size and sensitivity of detection have not been demonstrated previously using other sample processing methods. In conjunction with the UF-culture/IMS procedure, a suite of real-time PCR assays can be effectively used for specific detection of EHEC O157:H7, a pathogen that continues to be of public health importance. This sampling and analytical procedure should be a useful tool for monitoring water resources for EHEC O157:H7 and responding to outbreaks of disease associated with contaminated water.
Acknowledgments
We thank Patricia Fields (Centers for Disease Control and Prevention, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, Division of Food-borne, Bacterial and Mycotic Diseases) for providing guidance regarding the PCR assays used in this study.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention or the U.S. Department of Health and Human Services.
Footnotes
Published ahead of print on 10 April 2009.
REFERENCES
- 1.Ackman, D., S. Marks, P. Mack, M. Caldwell, T. Root, and G. Birkhead. 1997. Swimming-associated haemorrhagic colitis due to Escherichia coli O157:H7 infection: evidence of prolonged contamination of a fresh water lake. Epidemiol. Infect. 119:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bopp, D. J., B. D. Sauders, A. L. Waring, J. Ackelsberg, N. Dumas, E. Braun-Howland, D. Dziewulski, B. J. Wallace, M. Kelly, T. Halse, K. A. Musser, P. F. Smith, D. L. Morse, and R. J. Limberger. 2003. Detection, isolation, and molecular subtyping of Escherichia coli O157: H7 and Campylobacter jejuni associated with a large waterborne outbreak. J. Clin. Microbiol. 41:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalmers, R. M., H. Aird, and F. J. Bolton. 2000. Waterborne Escherichia coli O157. Symp. Ser. Soc. Appl. Microbiol. 2000:124S-132S. [DOI] [PubMed] [Google Scholar]
- 4.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC.
- 5.Duffy, G., R. C. Whiting, and J. J. Sheridan. 1999. The effect of a competitive microflora, pH and temperature on the growth kinetics of Escherichia coli O157: H7. Food Microbiol. 16:299-307. [Google Scholar]
- 6.Environment Agency of the United Kingdom. 2002. The microbiology of drinking water (2002). Methods for the isolation and enumeration of coliform bacteria and Escherichia coli (including E. coli O157:H7). Standing Committee of Analysts Blue Book no. 179. Environment Agency, London, United Kingdom.
- 7.Heijnen, L., and G. Medema. 2006. Quantitative detection of E. coli, E. coli O157 and other Shiga toxin producing E. coli in water samples using a culture method combined with real-time PCR. J. Water Health 4:487-498. [PubMed] [Google Scholar]
- 8.Hill, V. R., A. M. Kahler, N. Jothikumar, T. B. Johnson, D. Hahn, and T. L. Cromeans. 2007. Multistate evaluation of an ultrafiltration-based procedure for simultaneous recovery of enteric microbes in 100-liter tap water samples. Appl. Environ. Microbiol. 73:4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill, V. R., A. L. Polaczyk, D. Hahn, J. Narayanan, T. L. Cromeans, J. M. Roberts, and J. E. Amburgey. 2005. Development of a rapid method for simultaneous recovery of diverse microbes in drinking water by ultrafiltration with sodium polyphosphate and surfactants. Appl. Environ. Microbiol. 71:6878-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himathongkham, S., M. L. Dodd, J. K. Yee, D. K. Lau, R. G. Bryant, A. S. Badoiu, H. K. Lau, L. S. Guthertz, L. Crawford-Miksza, and M. A. Soliman. 2007. Recirculating immunomagnetic separation and optimal enrichment conditions for enhanced detection and recovery of low levels of Escherichia coli O157:H7 from fresh leafy produce and surface water. J. Food Prot. 70:2717-2724. [DOI] [PubMed] [Google Scholar]
- 11.Lejeune, J. T., T. E. Besser, D. H. Rice, and D. D. Hancock. 2001. Methods for the isolation of water-borne Escherichia coli O157. Lett. Appl. Microbiol. 32:316-320. [DOI] [PubMed] [Google Scholar]
- 12.Lindquist, H. D. A., S. Harris, S. Lucas, M. Hartzel, D. Riner, P. Rochele, and R. DeLeon. 2007. Using ultrafiltration to concentrate and detect Bacillus anthracis, Bacillus atrophaeus subspecies globigii, and Cryptosporidium parvum in 100-liter water samples. J. Microbiol. Methods 70:484-492. [DOI] [PubMed] [Google Scholar]
- 13.Morales-Morales, H. A., G. Vidal, J. Olszewski, C. M. Rock, D. Dasgupta, K. H. Oshima, and G. B. Smith. 2003. Optimization of a reusable hollow-fiber ultrafilter for simultaneous concentration of enteric bacteria, protozoa, and viruses from water. Appl. Environ. Microbiol. 69:4098-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muniesa, M., J. Jofre, C. Garcia-Aljaro, and A. R. Blanch. 2006. Occurrence of Escherichia coli 0157:H7 and other enterohemorrhagic Escherichia coli in the environment. Environ. Sci. Technol. 40:7141-7149. [DOI] [PubMed] [Google Scholar]
- 15.Polaczyk, A. L., J. Narayanan, T. L. Cromeans, D. Hahn, J. M. Roberts, J. E. Amburgey, and V. R. Hill. 2008. Ultrafiltration-based techniques for rapid and simultaneous concentration of multiple microbe classes from 100-L tap water samples. J. Microbiol. Methods 73:92-99. [DOI] [PubMed] [Google Scholar]
- 16.Sharma, V. K. 2006. Real-time reverse transcription-multiplex PCR for simultaneous and specific detection of rfbE and eae genes of Escherichia coli O157: H7. Mol. Cell. Probes 20:298-306. [DOI] [PubMed] [Google Scholar]
- 17.Sharma, V. K., and E. A. Dean-Nystrom. 2003. Detection of enterohemorrhagic Escherichia coli O157: H7 by using a multiplex real-time PCR assay for genes encoding intimin and Shiga toxins. Vet. Microbiol. 93:247-260. [DOI] [PubMed] [Google Scholar]
- 18.Shelton, D. R., J. A. Higgins, J. A. S. Van Kessel, Y. A. Pachepsky, K. Belt, and J. S. Karns. 2004. Estimation of viable Escherichia coli O157 in surface waters using enrichment in conjunction with immunological detection. J. Microbiol. Methods 58:223-231. [DOI] [PubMed] [Google Scholar]
- 19.Shelton, D. R., J. S. Karns, J. A. Higgins, J. A. S. Van Kessel, M. L. Perdue, K. T. Belt, J. Russell-Anelli, and C. DebRoy. 2006. Impact of microbial diversity on rapid detection of enterohemorrhagic Escherichia coli in surface waters. FEMS Microbiol. Lett. 261:95-101. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Environmental Protection Agency. 2005. Method 1603: Escherichia coli (E. coli) in water by membrane filtration using modified membrane-thermotolerant Escherichia coli agar (modified mTEC), EPA-821-R-04-025. Office of Water, U.S. Environmental Protection Agency, Washington, DC.
- 21.Yoshitomi, K. J., K. C. Jinneman, and S. D. Weagant. 2006. Detection of Shiga toxin genes stx1, stx2, and the +93 uidA mutation of E. coli O157:H7/H-using SYBR Green I in a real-time multiplex PCR. Mol. Cell. Probes 20:31-41. [DOI] [PubMed] [Google Scholar]