Abstract
Among the most difficult bacterial infections encountered in treating patients are wound infections, which may occur in burn victims, patients with traumatic wounds, necrotic lesions in people with diabetes, and patients with surgical wounds. Within a wound, infecting bacteria frequently develop biofilms. Many current wound dressings are impregnated with antimicrobial agents, such as silver or antibiotics. Diffusion of the agent(s) from the dressing may damage or destroy nearby healthy tissue as well as compromise the effectiveness of the dressing. In contrast, the antimicrobial agent selenium can be covalently attached to the surfaces of a dressing, prolonging its effectiveness. We examined the effectiveness of an organoselenium coating on cellulose discs in inhibiting Pseudomonas aeruginosa and Staphylococcus aureus biofilm formation. Colony biofilm assays revealed that cellulose discs coated with organoselenium completely inhibited P. aeruginosa and S. aureus biofilm formation. Scanning electron microscopy of the cellulose discs confirmed these results. Additionally, the coating on the cellulose discs was stable and effective after a week of incubation in phosphate-buffered saline. These results demonstrate that 0.2% selenium in a coating on cellulose discs effectively inhibits bacterial attachment and biofilm formation and that, unlike other antimicrobial agents, longer periods of exposure to an aqueous environment do not compromise the effectiveness of the coating.
Among the most difficult bacterial infections encountered in treating patients are wound infections, which may occur in burn victims (10), patients with traumatic wounds (33), people with diabetes (27), and patients with surgical wounds (29, 31). Two of the more common causative agents of wound infections are Staphylococcus aureus and Pseudomonas aeruginosa (10, 27, 29, 31, 33). Such infections often lead to fatality; the mortality rate among patients infected with P. aeruginosa ranges from 26% to 55% (9, 49), while mortality from S. aureus infection ranges from 19% to 38% (28, 46, 50). As opportunistic pathogens, S. aureus and P. aeruginosa cause few infections in healthy individuals but readily cause infection once host defenses are compromised, such as with the removal of skin from burns (10). S. aureus infection originates from the normal flora of either the patient or health care workers (48), while P. aeruginosa is acquired from the environment surrounding the patient (41). Once established on the skin, S. aureus and P. aeruginosa are then able to colonize the wound. Infection results if the organisms proliferate in the wound environment.
Both P. aeruginosa and S. aureus often exist within burn wounds as biofilms (43, 47). A biofilm is presently defined as a sessile microbial community characterized by cells that are irreversibly attached either to a substratum or to each other (16). Biofilms, which can attain over 100 μm in thickness, are made up of multiple layers of bacteria in an exopolysaccharide matrix (12, 16, 42). Sauer et al. showed that P. aeruginosa biofilms form in distinct developmental stages: reversible attachment, irreversible attachment, two stages of maturation, and a dispersion phase (42). Clinically, biofilms present serious medical management problems through their association with different chronic infections (37). During vascular catheter-related infections and sepsis, biofilms serve as a reservoir of bacteria from which planktonic cells detach and spread throughout the tissue and/or enter the circulatory system, resulting in bacteremia or septicemia (15). Factors specific to the bacterium may influence the formation of bacterial biofilms at different infection sites or surfaces. For example, during the initial attachment stage the flagellum, lipopolysaccharide, and possibly outer membrane proteins play a major role in bringing P. aeruginosa into proximity with the surface as well as mediating the interaction with the substratum (12). Using the murine model of thermal injury, we recently showed that P. aeruginosa forms a biofilm within the thermally injured tissues (43). Clinically, the surgeons debride the infected or dead tissues; however, a few microorganisms may remain on the tissue surface and reinitiate biofilm formation.
Antibiotics, silver, or chitosan, attached to or embedded in gauze, have been shown to be efficacious in preventing wound infection (21, 24, 26, 36). However, the resistance of P. aeruginosa and S. aureus to available antibiotics severely limits the choices for antibiotic treatment (13, 40). Additionally, silver compounds, such as silver nitrate and silver sulfadiazine, leaching from dressings are toxic to human fibroblasts even at low concentrations (20, 25). Thus, effective alternative antimicrobial agents that contact the thermally injured/infected tissues and prevent the development of bacterial biofilms are required. Previous studies have shown that selenium (Se) can be covalently bound to a solid matrix and retain its ability to catalyze the formation of superoxide radicals (O2·−) (30). These superoxide radicals inhibit bacterial attachment to the solid surface (30). In this study, we examined the ability of a newly synthesized organoselenium-methacrylate polymer (Se-MAP) to block biofilm formation by both S. aureus and P. aeruginosa. These bacteria were chosen since they cause a major share of wound infections and because drug-resistant forms of these bacteria have become a serious problem in the treatment and management of these wound infections (6, 13, 17, 18, 38). Results of the study show that 0.2% (wt/wt) Se in Se-MAP covalently attached to cellulose discs inhibited P. aeruginosa and S. aureus biofilm formation. This could lead to the development of a selenium-based antimicrobial coating for cotton materials that will prevent the bacterial attachment and colonization that can ultimately lead to bacterial biofilm formation during chronic infections.
MATERIALS AND METHODS
Bacterial strains, media, and reagents.
The S. aureus subsp. aureus strain ATCC 29213 was obtained from Remel (Lenexa, KS). The prototrophic P. aeruginosa strain PAO1, originally isolated from an infected wound (22), was obtained from S. E. H. West (University of Wisconsin, Madison). P. aeruginosa PA4468ΔsodM and its parent strain, MPAO1, were obtained from The University of Washington Transposon Mutant Collection (Department of Genome Sciences, Seattle, WA).
Coating cellulose discs with polymerized Se-MAP.
The Se-MAP monomer was purchased from Eburon Organics International (catalog no. 700.010; Lubbock, TX). Se-MAP (22% [wt/wt] selenium) stock solution was dissolved and diluted in 99.99% acetoacetoxyethyl methacrylate (AAEMA) to yield different concentrations of selenium (wt/wt): 0.01%, 0.025%, 0.05%, 0.10%, and 0.20%.
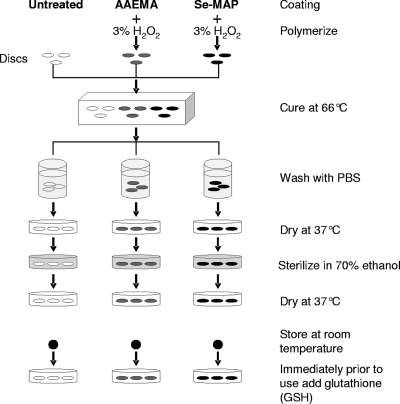
Blank 6-mm cellulose discs (BD Diagnostic Systems, Sparks, MD) were washed once with and soaked in sterile distilled water overnight. The washed cellulose discs were then dried and coated with AAEMA or Se-MAP, as shown in Fig. 1. Briefly, 15 μl of AAEMA or various concentrations of Se-MAP in AAEMA were added onto each disc. Three μl of 3% H2O2 (15 μl/cm2) was added to each disc to initiate free radical polymerization. This has little or no effect on the cellulose material. As a result of the in situ polymerization, the polymer is held in place by a combination of van der Waals forces and physical interlinking with the cellulose material. The discs were then transferred to a 66°C curing oven. After curing, the discs were washed twice (30 min each) in sterile phosphate-buffered saline (PBS, pH 7.4) at 37°C. The discs were then dried, sterilized with 70% ethanol, dried again, and stored at room temperature until used in the assays. Immediately prior to use in every assay, 15 μl of 300 μM glutathione (GSH) was added to each disc.
FIG. 1.
Schematic diagram for coating cellulose discs with AAEMA or Se-MAP. Se-MAP (22% [wt/wt] Se) was diluted in AAEMA to the desired concentrations and applied to 6-mm cellulose discs. Polymerization was initiated with 3% H2O2. Discs, including untreated discs, were cured at 66°C until dry and then washed twice with PBS (pH 7.4). Discs were dried at 37°C and sterilized in 70% ethanol. Discs were dried again at 37°C and stored at room temperature until used in the assays. Immediately prior to use in every assay, 300 μM GSH was added to each disc.
CL assay.
In the presence of GSH and oxygen, Se-MAP is reduced to yield R-Se− anions and superoxide radicals (7). A lucigenin-enhanced chemiluminescence (CL) assay was utilized to detect the presence of superoxide radicals in solution (32). Untreated, AAEMA-coated, or Se-MAP-coated discs were added to 500-μl aliquots of CL assay cocktail (0.05 M sodium phosphate buffer [pH 7.4] with 1 μg/ml lucigenin and 1 μg/ml GSH). The generation of CL was recorded over a 5-min period in a luminometer (Turner Biosystems, Sunnyvale, CA).
Colony biofilm assay.
The abilities of P. aeruginosa and S. aureus to form biofilms on cellulose discs were quantified as follows. P. aeruginosa and S. aureus culture were grown overnight in LB broth at 37°C with shaking. Overnight cultures were washed once with PBS (pH 7.4). Aliquots of fresh LB broth were inoculated with the washed cultures to an optical density at 600 nm (OD600) of 0.02 and then regrown at 37°C with shaking for 4 h to an OD600 0.8 to 0.9. The regrown cultures were washed once with PBS and serially diluted in 10-fold steps to obtain a 1 × 10−5 dilution. Five-microliter aliquots from the 10−5 dilution were added to uncoated cellulose discs, AAEMA-coated discs, and discs coated with Se-MAP at various concentrations of selenium as described above. The inoculated cellulose discs were placed on LB agar plates, and the plates were inverted and incubated at 37°C for 24 h.
Following incubation, each cellulose disc was gently washed twice with sterile PBS to remove any planktonic bacteria. Excess PBS was drained from the disc by touching to sterile filter paper and the discs were transferred to sterile 1.5-ml microtubes containing 1 ml of PBS for enumeration of bacteria within biofilms formed on the discs. To separate the adherent cells from the biofilm matrix and cellulose support, the microtubes were vortexed for 1 min at 2,500 rpm, allowed to rest, and vortexed again for a total of three vortexing steps. Disaggregated bacterial CFU per disc were then enumerated by 10-fold serial dilution in PBS and plating onto LB plates. To confirm complete recovery of the biofilm-associated bacteria from each disc, the discs were placed into new tubes containing PBS and the vortexing process was repeated (three steps). No CFU were recovered from plating of the PBS (data not shown), indicating the efficacy of our recovery protocol. Experiments were conducted in triplicate by inoculating three separate discs per treatment with the inoculum prepared from each culture.
SEM.
P. aeruginosa and S. aureus biofilms were established on cellulose discs as described above. The cellulose discs were prepared for scanning electron microscopy (SEM) by standard techniques (1, 4). After 24 h of incubation, each cellulose disc and any adherent bacteria were fixed with 2% (wt/vol) glutaraldehyde in filter-sterilized 0.05 M PBS (pH 7.4) at room temperature for 16 h and then rinsed three times for 15 min each in 0.05 M PBS. The fixed cellulose discs were then dehydrated in successive ethanol-water mixtures with increasing ethanol concentrations (20%, 40%, 60%, 80%, and 95% [vol/vol]) for 15 min each and then twice in absolute ethanol for 15 min. The ethanol-dehydrated samples were then placed in an absolute ethanol bath, which was placed in an EMS 850 critical point drier (Electron Microscopy Sciences, Hatfield, PA). The ethanol was replaced by successive additions of liquid carbon dioxide. Once the liquid CO2 had replaced the ethanol, the chamber was heated under pressure to reach the critical evaporation point of carbon dioxide. The chamber was then slowly vented of gaseous CO2 and the dry samples removed. The dried samples were affixed to aluminum mounts with double-sided carbon adhesive tape and sputter-coated with platinum and palladium to a thickness of 18 nm. Observations were performed at 6 to 7 kV with a scanning electron microscope (Hitachi S-570; Japan). Five fields of view at magnifications from ×1,500 were taken at randomly chosen areas from the optic surface of each sample. Each experiment was conducted in triplicate. A biofilm-positive field was defined as being occupied by biofilm over at least half of the visible area. If no biofilm or only scant numbers of cells were seen, examination was then carried out at ×5,000.
Assessing the stability of Se-MAP coating on cellulose discs.
The stability of selenium on the Se-MAP-coated cellulose discs was tested in the following manner. Discs were coated with Se-MAP as described, except that instead of drying after washing twice in PBS (pH 7.4), the discs were placed in glass tubes containing 10 ml of PBS. After incubation at room temperature for 1 week, the discs were then dried, sterilized with 70% ethanol, and dried again. GSH (300 μM) was added to each disc and then the discs were used in the colony biofilm assay.
RESULTS AND DISCUSSION
Selenium in Se-MAP bound to cellulose discs produces superoxide radicals.
To confirm both the presence and function of selenium on the Se-MAP-coated cellulose discs, the generation of the superoxide radical (O2·−) by selenium was measured using a lucigenin-enhanced CL assay (32). Diselenides, such as diselenocystines, are reduced by thiols, forming the selenide anion, RSe− (7). Thiols are compounds that contain a functional group composed of a sulfur atom and a hydrogen atom (-SH), also known as a sulfhydryl group. The catalytic selenide anion, RSe−, reacts with oxygen to form O2·− and a putative thiyl radical, R-Se·, which reacts with two moles of thiol (supplied in the assay as GSH) to regenerate the original selenide anion as shown in the equations below (7, 44):
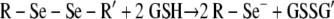 |
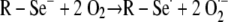 |
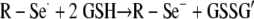 |
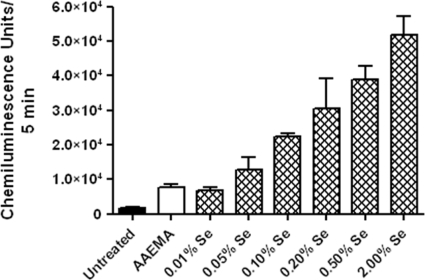
The greater the selenide anion redox or catalytic activity, the greater the amount of O2·− produced in the radical-generating system with a subsequently greater CL activity (32). An increase in CL confirmed the presence of functional selenium on the treated cellulose discs (Fig. 2). Furthermore, the increase correlated with the increase of Se-MAP concentration on the cellulose discs (Fig. 2).
FIG. 2.
Selenium in Se-MAP on cellulose discs generates superoxide in a lucigenin-enhanced CL assay. Untreated, AAEMA-coated, or Se-MAP-coated (with various concentrations of selenium) cellulose discs were placed in a CL assay cocktail containing 1 μg/ml GSH and 1 μg/ml lucigenin in 0.05 M sodium phosphate buffer (pH 7.4), and CL was measured for 5 min. The final concentration of selenium present on the Se-MAP-coated discs is indicated on the graph. Values represent the means of triplicate experiments ± standard errors.
Selenium in Se-MAP bound to cellulose discs inhibits biofilm formation by P. aeruginosa and S. aureus.
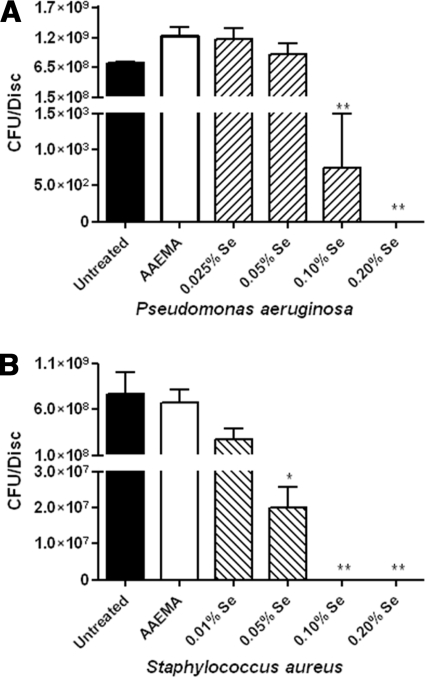
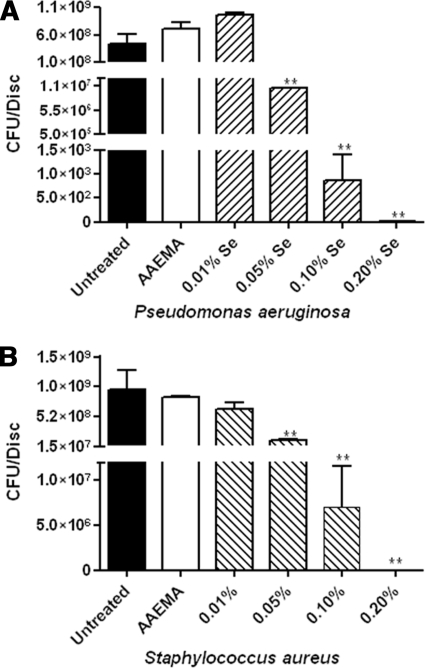
For controls, we examined the ability of P. aeruginosa and S. aureus to form biofilms on uncoated cellulose discs and discs coated with AAEMA alone in the colony biofilm assay. Biofilm formation was measured by determining the CFU adhering to the cellulose discs 24 h after inoculation. For P. aeruginosa, 7 × 108 and 1 × 109 CFU were recovered from untreated cellulose discs and AAEMA-coated discs, respectively (Fig. 3A), while approximately 7 × 108 were recovered from the control discs for S. aureus (Fig. 3B).
FIG. 3.
Selenium in Se-MAP inhibits P. aeruginosa and S. aureus biofilm formation. Untreated, AAEMA-coated, or Se-MAP-coated cellulose discs were prepared as described for Fig. 1 and inoculated with P. aeruginosa (A) or S. aureus (B). Biofilms were allowed to form for 24 h. The discs were gently washed twice in PBS to remove planktonic bacteria. Adherent bacteria (biofilm) were removed from the discs by vortexing in PBS, and CFU were determined by plating 10-fold serial dilutions on LB agar. The final concentrations of selenium present on the Se-MAP-coated discs are indicated on the graphs. Values represent the means of triplicate experiments ± standard errors. A one-way analysis of variance with Dunnett's multiple comparisons post test using the AAEMA-coated discs as the control was done to determine statistical significance. *, P < 0.05; **, P < 0.01.
We then tested the efficacy of different concentrations of selenium bound to cellulose discs as Se-MAP in inhibiting P. aeruginosa and S. aureus biofilms. Cellulose discs coated with 0.2%, 0.1%, 0.05%, and 0.025% Se (as Se-MAP in AAEMA) were inoculated with P. aeruginosa as described in Materials and Methods. At 0.2% Se, total inhibition of biofilm formation was achieved (no CFU recovered), while 0.1% Se significantly (P < 0.001) reduced the CFU of P. aeruginosa recovered from the cellulose discs (Fig. 3A). At 0.05% and 0.025% Se, there were no significant differences observed in the CFU of P. aeruginosa recovered from control discs or Se-MAP-treated discs (Fig. 3A). Inhibition of S. aureus was examined in the same way, except the lowest concentration tested was 0.01% Se. At a Se concentration of 0.1%, total inhibition of biofilm formation was observed, while at 0.05% Se, the number of CFU was significantly reduced (P < 0.05) (Fig. 3B). However, at 0.01% Se no significant difference was observed (Fig. 3B). The results indicate that relatively low concentrations of Se, 0.2% and 0.1%, are efficient in inhibiting biofilm formation by both P. aeruginosa and S. aureus.
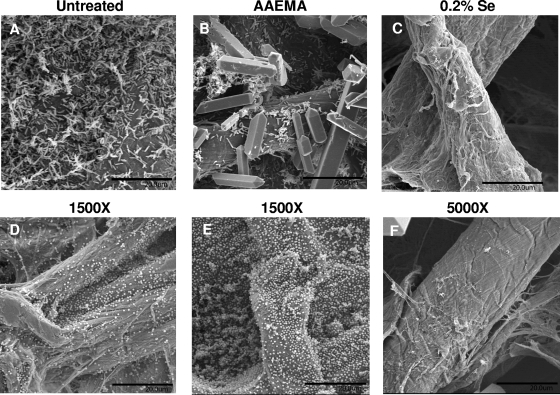
To confirm the results of the previous experiments, P. aeruginosa and S. aureus biofilm formation on uncoated, AAEMA-coated, and Se-MAP-coated cellulose discs was analyzed by SEM. P. aeruginosa and S. aureus were inoculated onto the discs in the same manner as for the colony biofilm assay. On untreated discs and discs coated with AAEMA only, P. aeruginosa formed typical biofilms characterized by the presence of microcolonies (Fig. 4A and B). Similar results were obtained with selenium concentrations of 0.01% (data not shown), while almost no bacteria were seen on the cellulose discs coated with 0.2% selenium (Fig. 4C). S. aureus formed evenly distributed sheets of bacteria on untreated discs and AAEMA-coated discs (Fig. 4D and E). These sheets of bacteria were also present at 0.01% selenium (data not shown), but bacteria were only rarely seen when the discs were coated with 0.2% selenium (Fig. 4F).
FIG. 4.
SEM analysis of P. aeruginosa (A to C) and S. aureus (D to F) biofilm formation on untreated, AAEMA-coated, or Se-MAP-coated (0.2% selenium) cellulose discs. Biofilms were allowed to form as described for Fig. 3. After 24 h of incubation at 37°C, the discs were fixed, dried, affixed to aluminum mounts, and sputter coated with platinum and palladium. Observations were performed at 6 to 7 kV with a scanning electron microscope. Five fields of view were examined from randomly chosen areas from the optical surface of each sample at magnification of ×1,500 for untreated and AAEMA-coated discs and at ×5,000 for the 0.2% selenium Se-MAP-coated discs. Each experiment was conducted in triplicate. Representative fields of view are shown. Bars, 20 μm. Crystals visible in panels B and F are artifacts of fixation.
The reduction in biofilm formation seen with both microorganisms is probably due to oxidative stress from the production of O2·−, which may damage the bacterial cell walls as well as the DNA (5, 11, 23, 35, 45). Selenium acts as a catalytic generator of O2·− from the oxidation of thiols (7, 44), and thiol-containing compounds such as GSH, homocysteine, and Cys-Gly are present in human plasma (2). Therefore, we used GSH throughout our experiments to mimic the host environment. To determine if reduced thiols present in bacteria (8) could activate Se-MAP, we compared the inhibition of P. aeruginosa biofilm development in the presence and absence of GSH. At 0.1% Se, the level of inhibition was approximately 3.5 logs greater with GSH than without (1.3 × 105 versus 4.5 × 108, respectively); however, at 0.2% Se the levels of inhibition were the same regardless of the presence of GSH (data not shown). Thus, at concentrations of 0.1% and lower, Se requires GSH supplementation to inhibit P. aeruginosa biofilm development efficiently.
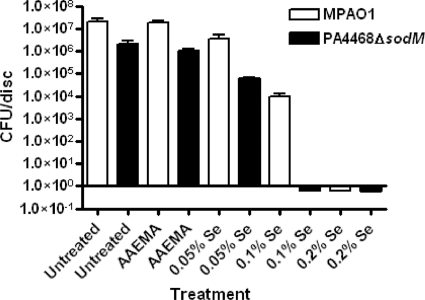
Based on their analysis in Escherichia coli, Bebien et al. (3) suggested that, under aerobic conditions, resistance to selenium toxicity is due mainly to the function of superoxide dismutase. In comparison with their parent strain, E. coli mutants defective in the superoxide dismutase genes sodA and sodB were severely impaired in their tolerance to selenium (3). P. aeruginosa contains two superoxide dismutase genes, sodM (sodA) and sodB, that are highly homologous to the E. coli sodA and sodB (19). The genes were isolated through the complementation analysis of an E. coli sodAB mutant (19). To determine if selenium affects the development of a PAO1 biofilm through superoxide dismutase, we utilized the P. aeruginosa strain PA4468ΔsodM, which carries defective sodM. Since the development of P. aeruginosa biofilm was completely inhibited by 0.2% selenium but partially inhibited by 0.05 and 0.1% selenium (Fig. 3A), we compared the effect of 0.05, 0.1, and 0.2% selenium on the development of biofilms by PA4468ΔsodM and its parent strain, MPAO1. On untreated as well as AAEMA-treated discs (controls), PA4468ΔsodM formed biofilm less efficiently than MPAO1, due to the differences in the growth rate between the two strains (Fig. 5). As previously demonstrated for the E. coli sodAB mutants (3), planktonic cells of PA4468ΔsodM grew more slowly than those of MPAO1 (data not shown). However, 0.05% selenium inhibited biofilm formation by PA4468ΔsodM more efficiently than MPAO1 biofilm (Fig. 5). More importantly, while 0.1% selenium partially interfered with the development of biofilm by MPAO1 (reducing the CFU/disc from 1.9 × 107 to 9.2 × 103), it almost prevented the development of PA4468ΔsodM biofilm (reducing the CFU/disc from 7.1 × 104 to 0.6 × 10−1) (Fig. 5). These results suggest that aerobically, selenium produces much of its inhibitory effect on P. aeruginosa biofilm formation through superoxide dismutase.
FIG. 5.
Comparison of the effectiveness of cellulose discs coated with Se-MAP in inhibiting the development of biofilms formed by the P. aeruginosa strain MPAO1 and its sodM mutant, PA4468ΔsodM. Biofilm development and analysis were conducted as described for Fig. 3. Values represent the means of triplicate experiments ± standard errors.
Se-MAP remains bound to cellulose discs in aqueous solution.
To determine whether Se-MAP bound to cellulose discs remains stable when in prolonged contact with aqueous environments, Se-MAP-coated discs were soaked in 10 ml of PBS (pH 7.4) at room temperature for 7 days. The discs were dried and their effectiveness in inhibiting biofilm formation by P. aeruginosa and S. aureus was determined as described above. As shown in Fig. 6, cellulose discs coated with Se-MAP retained their biofilm-inhibitory activity against P. aeruginosa and S. aureus. Total inhibition of biofilm formation was seen at 0.2% Se-MAP for both P. aeruginosa and S. aureus, whereas partial inhibition was seen at 0.1% Se-MAP for both microorganisms. The overall effect of incubation in PBS for 1 week on the antibiofilm activity of the discs was minimal. This indicates that use of Se-MAP-coated cellulose gauze would be suitable for long-term dressing of wounds.
FIG. 6.
Se-MAP coating on cellulose discs remains stable for 1 week in aqueous solution. Cellulose discs were prepared and coated with AAEMA or Se-MAP in AAEMA (Fig. 1) and soaked for 1 week as described in Materials and Methods. The discs were then dried and tested for the ability to inhibit biofilm formation by P. aeruginosa (A) and S. aureus (B) as described for Fig. 3. The final concentrations of selenium present on the Se-MAP-coated discs are indicated on the graphs. Values represent the means of triplicate experiments ± standard errors. A one-way analysis of variance with Dunnett's multiple comparisons post test using the AAEMA-coated discs as the control was done to determine statistical significance. **, P < 0.01.
The paradox of selenium (Se) is that it is both essential and toxic. According to the World Health Organization, the recommended daily dietary selenium intake is 40 μg Se/day (34). While high blood levels of selenium (greater than 1,000 μg/liter) can result in selenosis (14), selenium is not toxic at high levels of dietary intake (3,200 μg/day) (39). To check for the possibility that selenium leaching from the Se-MAP-treated cellulose discs could have reached a toxic level, the effect of the PBS solution in which the discs were soaked was examined by a commercial testing laboratory (Toxikon, Bedford, MA). None of the solutions was found to be cytotoxic on monolayers of L929 mouse fibroblast cells in the agar diffusion test (data not shown).
Conclusions.
This study revealed several important points regarding the clinical application of this organoselenium methacrylate polymer on cellulose as a potential wound dressing: (i) a low-percentage (0.2%) selenium coating was effective in preventing P. aeruginosa or S. aureus from establishing biofilms on cellulose; (ii) the amount of selenium leaching out of the cellulose over the course of 1 week was not cytotoxic against mammalian cells; and (iii) the selenium coating is stable, since incubation in PBS for a week showed little change in the selenium content or the antibacterial activity. Thus, the application of Se-MAP-coated gauze to the debrided tissues of burn wounds may prevent the development of biofilms and facilitate wound healing. Additionally, Se-MAP has the potential to inhibit bacterial biofilm formation, not only on wound dressings but also on tampons, catheters, and other surfaces where bacterial biofilms are a medical problem.
Acknowledgments
We thank Joanna Swickard for her assistance in editing the manuscript. We also thank The Texas Tech University Imaging Center, Department of Biological Sciences, Texas Tech University, Lubbock, for use of their facilities.
This study was supported in part by Selenium Ltd., Austin, TX.
Footnotes
Published ahead of print on 3 April 2009.
REFERENCES
- 1.Araujo, J. C., F. C. Teran, R. A. Oliveira, E. A. Nour, M. A. Montenegro, J. R. Campos, and R. F. Vazoller. 2003. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J. Electron Microsc. (Tokyo) 52:429-433. [DOI] [PubMed] [Google Scholar]
- 2.Bayle, C., E. Causse, and F. Couderc. 2004. Determination of aminothiols in body fluids, cells, and tissues by capillary electrophoresis. Electrophoresis 25:1457-1472. [DOI] [PubMed] [Google Scholar]
- 3.Bebien, M., G. Lagniel, J. Garin, D. Touati, A. Vermeglio, and J. Labarre. 2002. Involvement of superoxide dismutases in the response of Escherichia coli to selenium oxides. J. Bacteriol. 184:1556-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braet, F., R. De Zanger, and E. Wisse. 1997. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J. Microsc. 186:84-87. [DOI] [PubMed] [Google Scholar]
- 5.Cabiscol, E., J. Tamarit, and J. Ros. 2000. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 3:3-8. [PubMed] [Google Scholar]
- 6.Carrier, M., R. Marchand, P. Auger, Y. Hebert, M. Pellerin, L. P. Perrault, R. Cartier, D. Bouchard, N. Poirier, and P. Page. 2002. Methicillin-resistant Staphylococcus aureus infection in a cardiac surgical unit. J. Thorac. Cardiovasc. Surg. 123:40-44. [DOI] [PubMed] [Google Scholar]
- 7.Chaudiere, J., O. Courtin, and J. Leclaire. 1992. Glutathione oxidase activity of selenocystamine: a mechanistic study. Arch. Biochem. Biophys. 296:328-336. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H., J. Hu, P. R. Chen, L. Lan, Z. Li, L. M. Hicks, A. R. Dinner, and C. He. 2008. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc. Natl. Acad. Sci. USA 105:13586-13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheong, H. S., C. I. Kang, Y. M. Wi, E. S. Kim, J. S. Lee, K. S. Ko, D. R. Chung, N. Y. Lee, J. H. Song, and K. R. Peck. 2008. Clinical significance and predictors of community-onset Pseudomonas aeruginosa bacteremia. Am. J. Med. 121:709-714. [DOI] [PubMed] [Google Scholar]
- 10.Church, D., S. Elsayed, O. Reid, B. Winston, and R. Lindsay. 2006. Burn wound infections. Clin. Microbiol. Rev. 19:403-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooksley, C., P. J. Jenks, A. Green, A. Cockayne, R. P. Logan, and K. R. Hardie. 2003. NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J. Med. Microbiol. 52:461-469. [DOI] [PubMed] [Google Scholar]
- 12.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decousser, J.-W., P. Pina, F. Picot, C. Delalande, B. Pangon, P. Courvalin, P. Allouch, and the ColBVH Study Group. 2003. Frequency of isolation and antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections: a French prospective national survey. J. Antimicrob. Chemother. 51:1213-1222. [DOI] [PubMed] [Google Scholar]
- 14.Deore, M. D., A. K. Srivastava, and S. K. Sharma. 2005. Effect of reduced glutathione treatment on selenosis, blood selenium concentration and glutathione peroxidase activity after repeated short-term selenium exposure in buffalo calves. Toxicology 213:169-174. [DOI] [PubMed] [Google Scholar]
- 15.Donelli, G. 2006. Vascular catheter-related infection and sepsis. Surg. Infect. (Larchmont) 7(Suppl. 2):S25-S27. [DOI] [PubMed] [Google Scholar]
- 16.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuya, E. Y., and F. D. Lowy. 2006. Antimicrobial-resistant bacteria in the community setting. Nat. Rev. Microbiol. 4:36-45. [DOI] [PubMed] [Google Scholar]
- 18.Giacometti, A., O. Cirioni, A. M. Schimizzi, M. S. Del Prete, F. Barchiesi, M. M. D'Errico, E. Petrelli, and G. Scalise. 2000. Epidemiology and microbiology of surgical wound infections. J. Clin. Microbiol. 38:918-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassett, D. J., W. A. Woodruff, D. J. Wozniak, M. L. Vasil, M. S. Cohen, and D. E. Ohman. 1993. Cloning and characterization of the Pseudomonas aeruginosa sodA and sodB genes encoding manganese- and iron-cofactored superoxide dismutase: demonstration of increased manganese superoxide dismutase activity in alginate-producing bacteria. J. Bacteriol. 175:7658-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hidalgo, E., R. Bartolome, C. Barroso, A. Moreno, and C. Dominguez. 1998. Silver nitrate: antimicrobial activity related to cytotoxicity in cultured human fibroblasts. Skin Pharmacol. Appl. Skin Physiol. 11:140-151. [DOI] [PubMed] [Google Scholar]
- 21.Holder, I. A., P. Durkee, A. P. Supp, and S. T. Boyce. 2003. Assessment of a silver-coated barrier dressing for potential use with skin grafts on excised burns. Burns 29:445-448. [DOI] [PubMed] [Google Scholar]
- 22.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:2907-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imlay, J. A., and S. Linn. 1988. DNA damage and oxygen radical toxicity. Science 240:1302-1309. [DOI] [PubMed] [Google Scholar]
- 24.Kuroyanagi, Y., A. Shiraishi, Y. Shirasaki, N. Nakakita, Y. Yasutomi, Y. Takano, and N. Shioya. 1994. Development of a new wound dressing with antimicrobial delivery capability. Wound Repair Regen. 2:122-129. [DOI] [PubMed] [Google Scholar]
- 25.Lee, A. R., and H. K. Moon. 2003. Effect of topically applied silver sulfadiazine on fibroblast cell proliferation and biomechanical properties of the wound. Arch. Pharm. Res. 26:855-860. [DOI] [PubMed] [Google Scholar]
- 26.Lin, S. S., S. W. Ueng, S. S. Lee, E. C. Chan, K. T. Chen, C. Y. Yang, C. Y. Chen, and Y. S. Chan. 1999. In vitro elution of antibiotic from antibiotic-impregnated biodegradable calcium alginate wound dressing. J. Trauma 47:136-141. [DOI] [PubMed] [Google Scholar]
- 27.Lipsky, B. A., A. R. Berendt, H. G. Deery, J. M. Embil, W. S. Joseph, A. W. Karchmer, J. L. LeFrock, D. P. Lew, J. T. Mader, C. Norden, and J. S. Tan. 2006. Diagnosis and treatment of diabetic foot infections. Plast. Reconstr. Surg. 117:212S-238S. [DOI] [PubMed] [Google Scholar]
- 28.Malani, P. N., M. M. Rana, M. Banerjee, and S. F. Bradley. 2008. Staphylococcus aureus bloodstream infections: the association between age and mortality and functional status. J. Am. Geriatr. Soc. 56:1485-1489. [DOI] [PubMed] [Google Scholar]
- 29.Markogiannakis, H., N. Pachylaki, E. Samara, M. Kalderi, M. Minettou, M. Toutouza, K. G. Toutouzas, D. Theodorou, and S. Katsaragakis. 2009. Infections in a surgical intensive care unit of a university hospital in Greece. Int. J. Infect. Dis. 13:145-153. [DOI] [PubMed] [Google Scholar]
- 30.Mathews, S. M., J. E. Spallholz, M. J. Grimson, R. R. Dubielzig, T. Gray, and T. W. Reid. 2006. Prevention of bacterial colonization of contact lenses with covalently attached selenium and effects on the rabbit cornea. Cornea 25:806-814. [DOI] [PubMed] [Google Scholar]
- 31.McCaig, L. F., L. C. McDonald, S. Mandal, and D. B. Jernigan. 2006. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg. Infect. Dis. 12:1715-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munzel, T., I. B. Afanas'ev, A. L. Kleschyov, and D. G. Harrison. 2002. Detection of superoxide in vascular tissue. Arterioscler. Thromb. Vasc. Biol. 22:1761-1768. [DOI] [PubMed] [Google Scholar]
- 33.Murray, C. K. 2008. Infectious disease complications of combat-related injuries. Crit. Care Med. 36:S358-S364. [DOI] [PubMed] [Google Scholar]
- 34.Neve, J. 2002. Selenium as a ‘nutraceutical’: how to conciliate physiological and supra-nutritional effects for an essential trace element. Curr. Opin. Clin. Nutr. Metab. Care 5:659-663. [DOI] [PubMed] [Google Scholar]
- 35.Okuno, T., H. Kawai, T. Hasegawa, H. Ueno, and K. Nakamuro. 2001. Enhancement of hydroxyl radical formation from superoxide anion radical in the presence of organic selenium compounds. J. Health Sci. 47:240-247. [Google Scholar]
- 36.Ong, S. Y., J. Wu, S. M. Moochhala, M. H. Tan, and J. Lu. 2008. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 29:4323-4332. [DOI] [PubMed] [Google Scholar]
- 37.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 38.Reddy, S. L., A. D. Grayson, G. Smith, R. Warwick, and J. A. Chalmers. 2007. Methicillin resistant Staphylococcus aureus infections following cardiac surgery: incidence, impact and identifying adverse outcome traits. Eur. J. Cardiothorac. Surg. 32:113-117. [DOI] [PubMed] [Google Scholar]
- 39.Reid, M. E., M. S. Stratton, A. J. Lillico, M. Fakih, R. Natarajan, L. C. Clark, and J. R. Marshall. 2004. A report of high-dose selenium supplementation: response and toxicities. J. Trace Elem. Med. Biol. 18:69-74. [DOI] [PubMed] [Google Scholar]
- 40.Rennie, R. P., R. N. Jones, and A. H. Mutnick. 2003. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn. Microbiol. Infect. Dis. 45:287-293. [DOI] [PubMed] [Google Scholar]
- 41.Rossolini, G. M., and E. Mantengoli. 2005. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin. Microbiol. Infect. 11(Suppl. 4):17-32. [DOI] [PubMed] [Google Scholar]
- 42.Sauer, K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaber, J. A., W. J. Triffo, S. J. Suh, J. W. Oliver, M. C. Hastert, J. A. Griswold, M. Auer, A. N. Hamood, and K. P. Rumbaugh. 2007. Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infect. Immun. 75:3715-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spallholz, J. E. 1994. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 17:45-64. [DOI] [PubMed] [Google Scholar]
- 45.Stadtman, E. R., and R. L. Levine. 2000. Protein oxidation. Ann. N. Y. Acad. Sci. 899:191-208. [DOI] [PubMed] [Google Scholar]
- 46.Thompson, D. S., R. Workman, and M. Strutt. 2008. Contribution of acquired methicillin-resistant Staphylococcus aureus bacteraemia to overall mortality in a general intensive care unit. J. Hosp. Infect. 70:223-227. [DOI] [PubMed] [Google Scholar]
- 47.Trafny, E. A. 1998. Susceptibility of adherent organisms from Pseudomonas aeruginosa and Staphylococcus aureus strains isolated from burn wounds to antimicrobial agents. Int. J. Antimicrob. Agents 10:223-228. [DOI] [PubMed] [Google Scholar]
- 48.van Belkum, A., D. C. Melles, J. Nouwen, W. B. van Leeuwen, W. van Wamel, M. C. Vos, H. F. Wertheim, and H. A. Verbrugh. 2008. Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infect. Genet. Evol. 9:32-47. [DOI] [PubMed] [Google Scholar]
- 49.van Delden, C. 2007. Pseudomonas aeruginosa bloodstream infections: how should we treat them? Int. J. Antimicrob. Agents 30(Suppl. 1):S71-S75. [DOI] [PubMed] [Google Scholar]
- 50.Wyllie, D. H., D. W. Crook, and T. E. Peto. 2006. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997-2003: cohort study. BMJ 333:281. [DOI] [PMC free article] [PubMed] [Google Scholar]