Abstract
Katiki Domokou is a traditional Greek cheese, which has received the Protected Designation of Origin recognition since 1994. Its microfloras have not been studied although its structure and composition may enable (or even favor) the survival and growth of several pathogens, including Listeria monocytogenes. The persistence of L. monocytogenes during storage at different temperatures has been the subject of many studies since temperature abuse of food products is often encountered. In the present study, five strains of L. monocytogenes were aseptically inoculated individually and as a cocktail in Katiki Domokou cheese, which was then stored at 5, 10, 15, and 20°C. Pulsed-field gel electrophoresis was used to monitor strain evolution or persistence during storage at different temperatures in the case of the cocktail inoculum. The results suggested that strain survival of L. monocytogenes was temperature dependent since different strains predominated at different temperatures. Such information is of great importance in risk assessment studies, which typically consider only the presence or absence of the pathogen.
Listeria monocytogenes is a ubiquitous food-borne pathogen associated with outbreaks of listeriosis from consumption of various food commodities, especially dairy products, seafood, and meat (2, 26). The pathogen is of great health concern for the food industry because it is characterized by high mortality rates, amounting to 20 to 30% (14). Due to the severity of illness, especially for pregnant women, neonates, the elderly, and immunodeficient people, the level of the pathogen in food should remain low to ensure safe food products.
The new regulation of the European Union (EU) for microbiological criteria for L. monocytogenes in foods has set maximum levels of 100 CFU g−1 at the time of consumption for soft cheeses (8). In fact, the new EC 2073/2005 regulation in annex I lists the microbiological criteria for foodstuffs, which are classified into food safety criteria and process hygiene criteria. According to the new EU regulation, food safety criteria are those which “define the acceptability of a product or a batch of foodstuff applicable to products placed on the market” (8).
Legislative amendments regarding the presence of L. monocytogenes in ready-to-eat (RTE) foods are of great importance. Indeed, for the first time RTE foods are legislatively distinguished according to the target population for which they are intended, i.e., whether they are intended for consumption (i) by infants, (ii) by people with special medical conditions (immunocompromised), or (iii) by other target human subpopulations. In the most recent amendment the RTE foods other than those intended for infants or for those with special medical needs are further subdivided into foods that are able to support the growth of L. monocytogenes and those that are not. Products with pH ≤ 5.0 and water activity of ≤0.94 and products with a shelf life of less than 5 days are automatically considered to belong to the category of RTE foods that are unable to support the growth of L. monocytogenes (8). The regulation also states that “other categories of products can also belong to this category, subject to scientific justification.” Last but not least, the food safety criteria for L. monocytogenes are adjusted according to the bacteria's temporal stage in the food chain. Thus, for RTE foods that are able to support the growth of L. monocytogenes, the new regulation demands the absence of the pathogen (in 25 g) “before the food has left the immediate control of the food business operator, who has produced it” but allows up to 100 CFU g−1 in “products placed on the market during their shelf life.” The 100-CFU g−1 limit also applies throughout the shelf life of marketed RTE foods unable to support L. monocytogenes growth (8).
The pH and the water activity of Katiki Domokou (Katiki), a spreadable RTE traditional Greek cheese, are within the limits mentioned in the regulation. This product, a white cheese with a creamy structure, was traditionally produced from goat milk or from a mixture of goat and sheep milk. It has been recognized as a Protected Designation of Origin product since 1994 (www.greekcheese.gr), and its consumption has readily increased in the last few years. The milk is initially pasteurized and cooled at 27 to 28°C. Coagulation is then conducted with or without the addition of rennet, and the mixture is left to stand at 20 to 22°C. The curd is pulped and placed in cloth sacks for draining, with high final moisture (ca. 75%) and low salt content (ca. 1%) and pH (4.3 to 4.5) while it is stored at 4 to 5°C.
The quantitative estimation of kinetic parameters related to growth, survival, and death of L. monocytogenes has been described previously (2, 14, 20). The kinetic parameters of L. monocytogenes during storage at different temperatures have been the subject of many studies since temperature abuse of food products is often encountered (25, 28). However, strain characteristics or viability have not been taken into account (or have not been considered) as yet (20). This may explain the variability of findings in regard to different storage conditions (7, 17). Pulsed-field gel electrophoresis (PFGE) is a powerful subtyping tool, a gold standard for epidemiology, which provides repeatable results. It has the ability to generate profiles of a wide range of microorganisms and to discriminate strains with high fidelity (11, 19). PFGE has been used in several studies to type strains of epidemiological interest as well as to trace contaminants in the food chain (12, 13, 18).
The purpose of the present study was to assess the survival of five strains of L. monocytogenes inoculated either individually or as a cocktail in Katiki cheese. The cheese was stored at 5, 10, 15, and 20°C over a period of 1 month. PFGE was used to monitor the strain(s) that might survive and/or grow at different temperatures in a complex ecosystem like Katiki. The strains used in the study to form the inoculum consisted of two type strains of serotype 4b and three isolates belonging to our laboratory collection that were isolated from soft cheese and the conveyor belt of RTE foods. The strains were chosen on the basis of their source of isolation since this could be crucial to the interpretation of the data. The population was monitored throughout storage with respect to its quantitative as well as its qualitative evolution.
MATERIALS AND METHODS
Strains of L. monocytogenes and cocktail preparation.
L. monocytogenes strains TS125, TS124, TS128, TS131, and TS133 from the strain collection of the Laboratory of Microbiology and Biotechnology of Foods were used in this study. Strains TS124 and TS125 were type strains of serotype 4b. TS124 was the type strain NCTC 10527, and TS125 was the Scott A strain. Strains TS128 and TS133 were recovered from soft cheese, and strain TS131 was isolated from the conveyor belt of RTE foods. Strains were grown overnight in brain heart infusion broth (Lab M, Bury, Lancashire, United Kingdom) at 37°C. Bacterial cultures were centrifuged at 10,000 × g for 5 min and resuspended in Ringer solution (Lab M, Bury, Lancashire, United Kingdom). Cells were washed twice, centrifuged under the same conditions, and resuspended to a final volume of 1 ml of Ringer solution. They were used for the individual strain inocula or the “cocktail” of all five strains where they were mixed in order to obtain a standardized inoculum consisting of approximately the same cell number per strain. This final solution containing all five strains at the same levels was used to inoculate cheese at 106 CFU g−1. Confirmation of the inoculum size before use was obtained by the serial dilution method on PALCAM (polymyxin, acriflavine, lithium chloride, ceftazidime, aesculin, and mannitol) agar (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom).
Katiki cheese storage conditions.
The experiment was performed in triplicate. Each time, for each repetition, eight commercially available Katiki cheese 250-g packs were purchased from the retail market and immediately transferred to the laboratory for analysis. To determine L. monocytogenes and lactic acid bacteria (LAB) populations, samples (10 g) were taken from each pack, homogenized, and plated on PALCAM and MRS (deMann-Rogosa-Sharpe) agar (Biolife, Milan, Italy), respectively. Selective enrichment was applied as well in the cases when the cheese samples were negative by the plate count method for the presence of L. monocytogenes. Subsequently, four packs were opened and inoculated with (1%, vol/wt) of L. monocytogenes (single-strain or cocktail inoculum) to a final population of 5 × 106 CFU g−1. The inoculum was homogeneously applied to the samples by mixing with a sterile spatula before the packs were resealed. One pack was distributed at each temperature (5, 10, 15, and 20°C) (Sanyo MIR-153 incubator; Sanyo Electric Co., Osaka, Japan). The remaining four packs were not inoculated but were treated similarly, i.e., opened, resealed, and placed one each at the above-mentioned temperatures, as controls.
Sampling of the cheese and isolation procedure.
Samples were analyzed throughout storage at regular time intervals. The lactic acid bacteria and the listerial population of the cheese both individually and as a cocktail at each temperature were investigated at the beginning, the middle (3, 4, 6, and 10 days for 20°, 15°, 10°, and 5°C, respectively), and the final point of storage (5, 8, 12, and 18 days for 20°, 15°, 10°, and 5°C, respectively). Each time, 10 g of cheese was aseptically removed from the commercial container, homogenized using Ringer solution in a stomacher (Lab Blender 400; Seward Medical, London United Kingdom), and plated on the appropriate agar plates (MRS for LAB and PALCAM for Listeria).
When necessary, selective enrichment was done; briefly, a primary enrichment was performed by suspending 25 g of product samples in 225 ml of 1/2 Fraser Listeria selective enrichment broth (Merck Darmstadt, Germany). Following incubation at 30°C for 24 h (primary enrichment), a 0.1-ml portion of the samples was transferred to 10 ml of Fraser Listeria selective enrichment broth (Merck) and incubated at 35°C for 48 h (secondary enrichment). After each enrichment step the culture was streaked onto PALCAM Listeria agar plates (30°C for 48 h).
In order to monitor strains of the cocktail inoculum, 50 colonies were picked from each replication from the highest dilution at the initial stage of storage of the inoculated cheeses, and 20 colonies were picked for each replication at each time point studied. Out of a total number of 270 colonies picked, 80 isolates were recovered from replication 1, another 80 were from replication 2, and 55 were from replication 3, resulting in a total number of 215 isolates which were grown, purified, and stored at −80°C in tryptic soy broth containing 25% glycerol. Only catalase-positive, gram-positive rods were used for further analyses in the present study, resulting in 159 putative Listeria isolates. The pH of the cheese was also monitored during storage.
Strain differentiation of the cocktail inoculum using PFGE.
All 159 isolates that were gram-positive, catalase-positive rods isolated from PALCAM agar were typed to strain level by PFGE, as described by Kagkli et al. (13). Twenty units of ApaI (New England Biolabs) was used for restriction reaction mixtures. Following digestion, the slices were loaded into wells of a 1% PFGE grade agarose gel (Bio-Rad Laboratories, Hemel Hempstead, Hertfordshire, United Kingdom), and the gel was run in 0.5 mM Tris-borate buffer (45 mM Tris-HCl, 45 mM boric acid, 1 mM EDTA) using a contour-clamped homogeneous electric field electrophoresis apparatus (CHEF II; Bio-Rad) and cooling module at 6 V cm−1 for 18 h, with a pulse time ramped from 1 to 40 s. Gels were then stained with ethidium bromide (0.5 μg ml−1) in water for 2 h and destained for 1 h before being photographed using a GelDoc system (Bio-Rad). Conversion, normalization, and further analysis were performed using the Pearson coefficient and unweighted-pair group method using averages cluster analysis with GelCompar software, version 4.0 (Applied Maths BVBA; Saint-Martens-Latem, Belgium; kindly provided from E. Tsakalidou, Dairy Laboratory, Agricultural University of Athens).
Species confirmation.
The V1-V3 region of the 16S rRNA gene of representative isolates per distinct PFGE pattern was amplified as described previously (24). Primers P1 (5′-GCGGCGTGCCTAATACATGC-3′) and P4 (5′-ATCTACGCATTTCACCGCTAC-3′) were used, and amplification conditions were performed as described by Cocolin et al. (6). PCR products were purified using a QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Purified amplicons were directly sequenced with an ABI 3730 XL automatic DNA sequencer by Macrogen (http://www.macrogen.com). BLAST searches of sequences were performed at the National Center for Biotechnology Information GenBank data library (1).
Data analyses.
All analyses were performed in triplicate. Data were expressed as means ± standard deviations, and they were analyzed using xlstat for Windows software. One-way analysis of variance was performed. Least significant difference multiple-range tests (P ≤ 0.05) were applied to the individual variables to compare means of pH values versus time point and versus temperature and to assess whether a difference was significant.
RESULTS
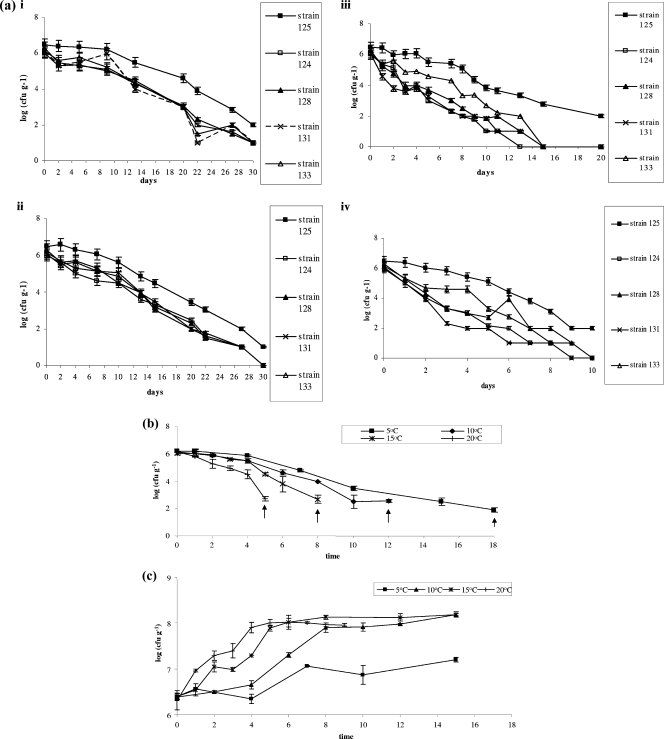
All samples were Listeria free at the beginning of the experiment since no colonies grew on PALCAM agar either before or after the enrichment procedure. The noninoculated cheese remained Listeria negative throughout storage. At the initial time point, the population of the L. monocytogenes cocktail in the inoculated samples was approximately 6 logs (CFU g−1), as calculated by plating the inoculum used. Listerial populations varied with temperature and showed the highest viability at lower temperatures (Fig. 1a and b) since they were detected up to the 18th and 30th days of storage in the cocktail and the single-strain inocula, respectively. Lowest levels were detected at 20°C, where the detection was possible only up to the 10th day of storage in the case of the single strains and up to the 5th day of storage in the cocktail (Fig. 1a and b). Enrichment was applied thereafter to detect the presence of Listeria. The growth of LAB followed the growth of total viable bacteria and increased steadily from 6.2 to 6.5 log CFU g−1 to 8.0 to 8.2 log CFU g−1 (Fig. 1c), whereas a slight decrease in the pH was observed; the initial pH values were in the order of 4.5 to 4.6 and reached levels of 4.2 to 4.3 by the end of storage (data not shown). No statistically significant different pH values were recorded among samples stored at different temperatures.
FIG. 1.
(a) Growth curves of L. monocytogenes individual strains on PALCAM agar at 5°C (i), 10°C (ii), 15°C (iii), and 20°C (iv). Growth curves of L. monocytogenes cocktail strains (b) and LAB (c) on MRS agar over time at the four different temperatures studied, (5, 10, 15, and 20°C). Arrows indicate the end of storage at each temperature.
In monitoring single-strain inocula, differences were observed between low and high temperatures. In all cases, though, strain 125 was the one that survived the longest at all temperatures tested (Fig. 1b). The growth of the rest of the strains was similar at 5 and 10°C even though at 5°C the population at the 30th day was approximately 1 log CFU g−1 compared to growth at 10°C, where it was not detected. At 15 and 20°C, less sharp differences were detected among the single-strain listerial inoculations, at least at the beginning of the storage, until strain TS125 eventually dominated over the rest. Nevertheless, differences were not as pronounced as at the lower temperatures (Fig. 1b).
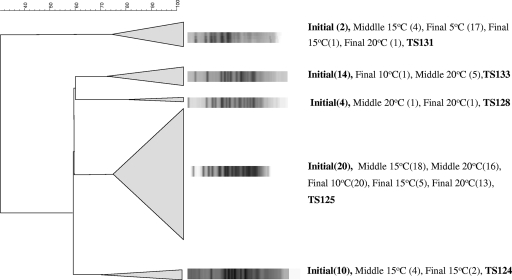
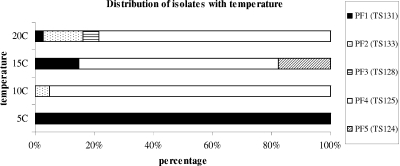
Out of the 215 colonies recovered from the cocktail analysis, only the 159 putative Listeria isolates (74%) were subjected to PFGE to detect specific strain survival during storage (Fig. 2). Fifteen isolates were recovered from replication 1 and 2, and 20 were recovered from replication 3 at the beginning of storage (data not shown). The experiment was designed to have the same levels of all the strains in the cocktail inoculum; PFGE, though, demonstrated that not all strains were present at the same initial levels (Fig. 3). Specifically, strain TS125 prevailed over the others and was present at a percentage of 40% (20 out of 50 initial isolates), while strain TS131 was recovered at only 4%, TS133 at 28%, TS124 at 20%, and TS128 at 8% of the initial inoculum. A representative number of isolates from each cluster of the dendrogram was identified using 16S rRNA gene sequencing and confirmed the species as L. monocytogenes.
FIG. 2.
Dendrogram demonstrating cocktail strains of L. monocytogenes at four different temperatures. Fragments were generated using PFGE after digestion with ApaI. Initial, middle, and final stages of storage and appropriate temperatures are shown. Numbers in parentheses indicate the number of isolates.
FIG. 3.
Distribution of isolates recovered from the cocktail in relation to temperature and to the PFGE profiles (PF).
PFGE also revealed interesting information regarding strain occurrence at the different temperatures. At 5°C only strain TS131 could survive after 18 days of incubation, and all isolates recovered were attributed to this strain, showing that the others were not able to survive at this temperature. No listerial isolates were recovered at 5°C at the end of storage in the third replication since none of the colonies grown on PALCAM agar corresponded to the listerial characteristics of the genus regarding catalase, Gram staining, and microscopic observation (data not shown).
Twenty-one putative listerial isolates that were recovered from the final stage of storage at 10°C (Fig. 2) belonged to two different strains, TS133 and TS125, at percentages of 5 and 95%, respectively. Only eight listerial isolates (27% of the total colonies picked) were present at the end of storage at 15°C, and these belonged to three strains: TS125, TS124, and TS131. The percentages varied between 12.5 and 62.5%, and TS125 was the most prevalent one. The most interesting point about this temperature was that the majority of the isolates (73%) belonged to Bacillus spp. and Lactobacillus spp., which outgrew the listerial ones (data not shown). The enrichment method failed to detect any listerial isolates and only Bacillus spp. isolates were recovered as determined by the phenotypic characterization of the colonies recovered from PALCAM agar.
Fifteen isolates were detected at the end of storage at 20°C; high variability regarding strain recovery was observed, which was similar to the findings at 15°C. Surprisingly, the TS125, TS128, and TS131 strains were present at the end of storage, while strain TS124 was not present at any stage of storage even though it was present at 15°C. TS133 was detected in the middle of storage but did not survive to the end of storage. The rest of the isolates did not belong to the Listeria genus but were gram-positive, catalase-negative rods.
DISCUSSION
The kinetic parameters related to L. monocytogenes, i.e., growth, survival, and inactivation in different foods or food systems (e.g., broths and model systems) have been derived mainly from experiments where single-strain inocula were applied (15, 16, 22, 25). Exceptions to these approaches include the studies of Mataragas et al. (20) and Panagou (23), where mathematical models and neural networks were developed for Katiki cheese stored at various temperatures and using a five-strain cocktail. The latter studies concluded that such models can be useful in risk assessment studies especially when the probability of illness at the time of consumption can be assessed in conjunction with data derived from consumption patterns of the product, retail and home time-temperature profiles, and storage times of the product. Such risk assessment(s) could have been of greater value if there were information on the contribution of individual strains used for the development of these models or the neural network approach (20, 23). The significance of this issue was exemplified in the study of Lianou et al. (17). That study indicated that the risks predicted from the extrapolation of findings on L. monocytogenes behavior obtained with a single strain could not be generalized to include other strains. It was also concluded that test strains should be applied in food products when risk assessments are to be conducted. In a more recent study conducted in sausages, it was demonstrated that Listeria survival was strain dependent as well as starter culture dependent (27).
The above-mentioned information was taken into account in the present study. The contribution of PFGE was of great importance since it could provide information on the recovery of strains with regard to the storage temperatures. PFGE has been widely used to monitor L. monocytogenes from dairy products manufactured with raw milk (10), leading to the conclusion that raw milk and raw milk products could be a potential risk for the consumer. Katiki cheese is not produced with raw milk, but postcontamination of the product under poor hygienic conditions could constitute a potential danger.
The findings of this study indicate that storage conditions may affect the diversity of the microbial population as well as the survival and growth of different L. monocytogenes strains in a real food system. Such an observation becomes even more evident by the survival of the single listerial strains themselves; when strains were grown individually, only one strain (TS125) maintained high levels, whereas in a mixed cocktail culture differences were observed among strains, depending on the storage temperature. That observation could be explained by the presence of diverse microbiota at different temperatures since different storage temperatures may influence the growth of the indigenous microbiota as well as the survival of one strain over the others. In the present study, at higher temperatures, which would putatively permit growth of a broader range of microorganisms, Bacillus spp. grew on PALCAM medium at 15 and 20°C. This suggested differences in the microfloras between low and high temperatures since such a result was not obtained at low storage temperatures. The presence of Bacillus spp. became evident once the enrichment step was applied, i.e., after the middle of the storage period. In contrast, listerial isolates were recovered for a longer period at the lower temperatures, both in the single-strain and cocktail inocula. This suggests putatively lower competition for nutrients in the cheese since the spectrum of microorganisms able to grown in Katiki at these temperatures was less broad.
With respect to strain diversity of the listerial isolates, surprising results were obtained for the cocktail analysis. The most important finding was that not all strains survived at all temperatures, and differences were observed even within the low and high temperatures. Moreover, strains which were detected at low levels at the initial inoculum managed to survive, e.g., TS131, which was unable to survive when inoculated as a single strain. This finding could be due to the fact that this particular strain probably did not readily adapt to the conditions of Katiki cheese, e.g., low pH, and as a result could not be recovered initially using a plate count method. Strain TS131 has been previously isolated from the conveyor belt of RTE foods, and this could explain why it was not immediately adapted to the cheese ecosystem. The pH values in cheese were close to the tolerance limit for growth of L. monocytogenes (www.greekcheese.gr), and inhibition of the pathogen by low pH has been reported previously for other soft cheeses (21, 24).
Whether this finding is related to the characteristics of each strain, its pathogenicity, serotype, etc., would be interesting to determine in the future. We would expect similar floras to be present, at least between the high temperatures, since the main microflora of the cheese consists of LAB and since the elevated temperatures (15 and 20°C) would allow the lactobacillus population to grow as well. Even though we did not study the LAB population with respect to strain diversity, the fact that Listeria survived for a shorter period at 20°C than at 15°C could indicate a higher competition in the first instance since lactobacillus isolates grew on PALCAM agar (data not shown). Moreover, it has been demonstrated that the growth of fungi in feta cheese or yogurt has an effect on the survival rate of Listeria innocua by altering the pH of the food product (4). Whether bactericidal compounds are produced by the LAB population present, thus eliminating certain susceptible strains, would be an interesting point of future work since it has been shown that several LAB can inhibit growth of L. monocytogenes through the production of bactericidal or bacteriostatic compounds (3, 21).
The present study provides an analysis of a spreadable RTE European cheese that has not been studied so far in regard to the survival of L. monocytogenes under conditions usually encountered in home refrigerators. It has given insights regarding differential strain survival, demonstrating that the lack of recovery of listerial isolates at a certain temperature does not necessarily imply that similar results would be obtained if another strain were to be used. Moreover, it gives an overview of how the strains behave when cocultured in single-strain or cocktail inoculum. The fact that in the present study different strains were recovered from different temperatures is an observation of great importance. There are certainly some strains which have better survival capacity, but the rest of the strains studied did not show the same behavior and, when inoculated individually, were more or less affected by the presence of other strains. In addition, it is worth pointing out that the most persistent strain in all cases, both individually or mixed with other listerial strains, was TS125, a serotype 4b strain, which is in accordance with previous observations regarding strain serotypes and food-borne illnesses (5, 9). This fact demonstrates the importance of the selection criteria of one strain versus another when risk assessment analysis is conducted. Whether the environment in which Listeria is found, e.g., cheese or meat, could influence the survival rate might be another important factor to take into consideration since it has been shown in several models that microorganisms have a different behavior in broth culture than in a complex food matrix (22, 28). Relevant findings would contribute further to the applicability of EU regulation 2073/2005 (8).
Acknowledgments
The present study was part of Truefood, Traditional United Europe Food, an integrated project financed by the European Commission under the 6th Framework Programme for RTD (contract no. FOOD-CT-2006-016264).
The information in this document reflects only the authors' views, and the European Community is not liable for any use that may be made of the information contained therein.
D.-M. Kagkli thanks E. Panagou and A. Nisiotou for critical reading and their contribution to the statistical analysis of the manuscript.
Footnotes
Published ahead of print on 17 April 2009.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelidis, A. S., and K. Koutsoumanis. 2006. Prevalence and concentration of Listeria monocytogenes in sliced ready-to-eat meat products in the Hellenic retail market. J. Food Prot. 69:938-942. [DOI] [PubMed] [Google Scholar]
- 3.Arqués, J. L., J. Fernández, P. Gaya, M. Nuñez, E. Rodríguez, and M. Medina. 2004. Antimicrobial activity of reuterin in combination with nisin against food-borne pathogens. Int. J. Food Microbiol. 95:225-229. [DOI] [PubMed] [Google Scholar]
- 4.Belessi, C. I., S. Papanikolaou, E. H. Drosinos, and P. N. Skandamis. 2008. Survival and acid resistance of Listeria innocua in feta cheese and yogurt, in the presence or absence of fungi. J. Food Prot. 71:742-749. [DOI] [PubMed] [Google Scholar]
- 5.Borucki, M. K., and D. R. Call. 2003. Listeria monocytogenes serotype identification by PCR. J. Clin. Microbiol. 41:5537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocolin, L., M. Manzano, C. Cantoni, and G. Comi. 2001. Denaturing gradient gel electrophoresis analysis of the 16S rRNA gene V1 region to monitor dynamic changes in the bacterial population during fermentation of Italian sausages. Appl. Environ. Microbiol. 67:5113-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Jesus, A. J., and R. C. Whiting. 2003. Thermal inactivation, growth, and survival studies of Listeria monocytogenes strains belonging to three distinct genotypic lineages. J. Food Prot. 66:1611-1617. [DOI] [PubMed] [Google Scholar]
- 8.European Commission. 2005. Commission regulation (EC) no. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Official J. Eur. Union L338:1-26. [Google Scholar]
- 9.Evans, M. R., B. Swaminathan, L. M. Graves, E. Altermann, T. R. Klaenhammer, R. C. Fink, S. Kernodle, and S. Kathariou. 2004. Genetic markers unique to Listeria monocytogenes serotype 4b differentiate epidemic clone II (hot dog outbreak strains) from other lineages. Appl. Environ. Microbiol. 70:2383-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamdi, T. M., M. Naïm, P. Martin, and C. Jacquet. 2007. Identification and molecular characterization of Listeria monocytogenes isolated in raw milk in the region of Algiers (Algeria). Int. J. Food Microbiol. 116:190-193. [DOI] [PubMed] [Google Scholar]
- 11.Heir, E., B. A. Lindstedt, O. J. Røtterud, T. Vardund, G. Kapperud, and T. Nesbakken. 2004. Molecular epidemiology and disinfectant susceptibility of Listeria monocytogenes from meat processing plants and human infections. Int. J. Food Microbiol. 96:85-96. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, L., J. Chen, J. Xu, X. Zhang, S. Wang, H. Zhao, K. Vongxay, and W. Fang. 2008. Virulence characterization and genotypic analyses of Listeria monocytogenes isolates from food and processing environments in eastern China. Int. J. Food Microbiol. 121:53-59. [DOI] [PubMed] [Google Scholar]
- 13.Kagkli, D. M., M. Vancanneyt, P. Vandamme, C. Hill, and T. M. Cogan. 2007. Contamination of milk by enterococci and coliforms from bovine faeces. J. Appl. Microbiol. 103:1393-1405. [DOI] [PubMed] [Google Scholar]
- 14.Koutsoumanis, K., and A. Angelidis. 2007. Probabilistic modeling approach for evaluating the compliance of ready-to-eat foods with new European Union safety criteria for Listeria monocytogenes. Appl. Environ. Microbiol. 73:4996-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koutsoumanis, K. P., and J. N. Sofos. 2004. Comparative acid stress response of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella Typhimurium after habituation at different pH conditions. Lett. Appl. Microbiol. 38:321-326. [DOI] [PubMed] [Google Scholar]
- 16.Koutsoumanis, K. P., P. A. Kendall, and J. N. Sofos. 2003. Effect of food processing-related stresses on acid tolerance of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7514-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lianou, A., J. D. Stopforth, Y. Yoon, M. Wiedmann, and J. N. Sofos. 2006. Growth and stress resistance variation in culture broth among Listeria monocytogenes strains of various serotypes and origins. J. Food Prot. 69:2640-2647. [DOI] [PubMed] [Google Scholar]
- 18.Lopez, V., S. Ortiz, A. Corujo, P. López, J. Navas, R. Moreno, and J. V. Martínez-Suárez. 2007. Traceback identification of an ingredient (pork dewlap) as the possible source of Listeria monocytogenes serotype 4b contamination in raw chicken products. J. Food Prot. 70:1513-1517. [DOI] [PubMed] [Google Scholar]
- 19.Martin, P., C. Jacquet, V. Goulet, V. Vaillant, H. De Valk, and participants in the PulseNet Europe feasibility study. 2006. Pulsed-field gel electrophoresis of Listeria monocytogenes strains: the PulseNet Europe feasibility study. Foodborne Pathog. Dis. 3:303-308. [DOI] [PubMed] [Google Scholar]
- 20.Mataragas, M., V. Stergiou, and G.-J. E. Nychas. 2008. Modeling survival of Listeria monocytogenes in a traditional Greek soft cheese “Katiki” J. Food Prot. 71:1835-1845. [DOI] [PubMed] [Google Scholar]
- 21.Murdock, C. A., J. Cleveland, K. R. Matthews, and M. L. Chikindas. 2007. The synergistic effect of nisin and lactoferrin on the inhibition of Listeria monocytogenes and Escherichia coli O157:H7. Lett. Appl. Microbiol. 44:255-261. [DOI] [PubMed] [Google Scholar]
- 22.Pal, A., T. P. Labuza, and F. Diez-Gonzalez. 2008. Comparison of primary predictive models to study the growth of Listeria monocytogenes at low temperatures in liquid cultures and selection of fastest growing ribotypes in meat and turkey product slurries. Food Microbiol. 25:460-470. [DOI] [PubMed] [Google Scholar]
- 23.Panagou, E. Z. 2008. A radial basis function neural network approach to determine the survival of Listeria monocytogenes in Katiki, a traditional Greek soft cheese. J. Food Prot. 71:750-759. [DOI] [PubMed] [Google Scholar]
- 24.Paramithiotis, S., D.-M. Kagkli, V. A. Blana, G.-J. E. Nychas, and E. H. Drosinos. 2008. Identification and characterization of Enterococcus spp. in Greek spontaneous sausage fermentation. J. Food Prot. 71:1244-1247. [DOI] [PubMed] [Google Scholar]
- 25.Sivarooban, T., N. S. Hettiarachchy, and M. G. Johnson. 2007. Inhibition of Listeria monocytogenes using nisin with grape seed extract on turkey frankfurters stored at 4 and 10 degrees C. J. Food Prot. 70:1017-1020. [DOI] [PubMed] [Google Scholar]
- 26.Skandamis, P. N., Y. Yoon, J. D. Stopforth, P. A. Kendall, and J. N. Sofos. 2008. Heat and acid tolerance of Listeria monocytogenes after exposure to single and multiple sublethal stresses. Food Microbiol. 25:294-303. [DOI] [PubMed] [Google Scholar]
- 27.Tolvanen, R., S. Hellström, D. Elsser, H. Morgenstern, J. Björkroth, and H. Korkeala. 2008. Survival of Listeria monocytogenes strains in a dry sausage model. J. Food Prot. 71:1550-1555. [DOI] [PubMed] [Google Scholar]
- 28.Xanthiakos, K., D. Simos, A. S. Angelidis, G.-J. Nychas, and K. Koutsoumanis. 2006. Dynamic modeling of Listeria monocytogenes growth in pasteurized milk. J. Appl. Microbiol. 100:1289-1298. [DOI] [PubMed] [Google Scholar]