Abstract
Lactobacillus plantarum WCFS1 requires both heme and menaquinone to induce respiration-like behavior under aerobic conditions. The addition of these compounds enhanced both biomass production, without progressive acidification, and the oxygen consumption rate. When both heme and menaquinone were present, L. plantarum WCFS1 was also able to reduce nitrate. The ability to reduce nitrate was severely inhibited by the glucose levels that are typically found in L. plantarum growth media (1 to 2% [vol/vol] glucose). In contrast, comparable mannitol levels did not inhibit the reduction of nitrate. L. plantarum reduced nitrate with concomitant formation of nitrite and ammonia. Genes that encode a bd-type cytochrome (cydABCD) and a nitrate reductase (narGHJI) were identified in the genome of L. plantarum. The narGHJI operon is part of a cluster of genes that includes the molybdopterin cofactor biosynthesis genes and narK. Besides a menaquinone source, isogenic mutants revealed that cydA and ndh1 are required for the aerobic-respiration-like response and narG for nitrate reduction. The ndh1 mutant was still able to reduce nitrate. The existence of a nonredundant branched electron transport chain in L. plantarum WCFS1 that is capable of using oxygen or nitrate as a terminal electron acceptor is proposed.
Lactic acid bacteria (LAB) are extensively used for the production of fermented foods from dairy, meat, fruit, and vegetable sources. These fermented foods are valued for their enhanced shelf life, flavor, and structural properties. LAB have been exploited for this purpose for millennia and generally behave as facultative anaerobic, obligate fermentative bacteria.
However, the production of cytochromes, typical constituents of respiratory chains, has been observed in several LAB species when they are grown in the presence of heme. These include Lactococcus lactis (Streptococcus lactis), Leuconostoc mesenteroides, and Enterococcus faecalis (36, 42).
Recently, in L. lactis, generation of a proton motive force by a heme-dependent aerobic electron transport chain was demonstrated (9). In other words, heme induces respiration in L. lactis. L. lactis cells grown under these respiration-permissive conditions have enhanced biomass yields and are more robust (more resistant to oxygen, acid, and cold-storage stress) (15, 18, 31). Respiration-like behavior has also been reported for Streptococcus agalactiae and Oenococcus oeni (43; A. Gruss, unpublished results). However, there have been no published reports of heme-induced respiration-like behavior in any member of the genus Lactobacillus. This genus contains many species that are used extensively in food fermentation, such as Lactobacillus plantarum.
L. plantarum has been isolated from the human gastrointestinal tract and plant surfaces. It is an economically important starter culture bacterium, to initiate food fermentation, and certain strains are even sold as probiotics (2, 3, 13, 40). Improvements in the efficiency of biomass formation and robustness, which are associated with respiration in L. lactis, are desirable traits for starter cultures, as well as probiotics.
In this work, we investigated whether functional electron transport chains are present in L. plantarum. We analyzed the genome for components of electron transport chains and investigated the ability of L. plantarum to exploit extracellular electron acceptors.
MATERIALS AND METHODS
Cultures and growth conditions.
The four strains used in this study were L. plantarum WCFS1 (an isolate from NCIMB8826) and the three NarGΔ, Ndh1Δ, and CydAΔ isogenic strains. These L. plantarum strains were cultivated on MRS broth (Difco) or chemically defined media (38). When indicated, citrate and acetate were omitted, glucose titrated, or replaced by mannitol. The isogenic mutants were grown in the presence of 5 μg/ml chloramphenicol. For the induction of nitrate reductase activity, heme (heme or hemin) was added to a final concentration of 2.5 μg/ml (0.5-mg/ml stock in 0.05 M NaOH; Sigma-Aldrich), vitamin K2 (or menaquinone 4) to a final concentration of 1 μg/ml (2-mg/ml stock in ethanol; Sigma-Aldrich), and NaNO3 (Sigma-Aldrich) to various concentrations. For nitrite reduction assays, NaNO2 (Sigma-Aldrich) was added to a final concentration of 500 mg/liter. Cultures were grown anaerobically under an N2 atmosphere at 37°C.
Escherichia coli (strain E10) was used as the host for constructing plasmids and was cultivated aerobically at 37°C on TYB medium (Difco) with 10 μg/ml chloramphenicol and/or 10 μg/ml erythromycin, as indicated.
pH-controlled batch fermentation.
pH-controlled (pH 5.5; 37°C) batch fermentations were performed with modified MRS broth: 10 mM glucose and no sodium acetate or ammonium citrate. Heme, vitamin K2, or ethanol was added as indicated. One-liter fermentors were filled with 500 ml medium, and the headspaces were flushed with 20 ml/min nitrogen gas.
Mutant construction.
Molecular cloning techniques were carried out in accordance with standard laboratory procedures (34). For the construction of a mutant lacking a functional narG (NarGΔ), the plasmid pNZ5319_NarG_KO was constructed, derived from plasmid pNZ5319. Plasmid pNZ5319 was specifically developed for the creation of double-crossover replacement mutants (23). The plasmid pNZ5319_NarG_KO contains two ∼1-kb regions that are identical to DNA sequences that flank narG on the chromosome. These flanking regions, one upstream and one downstream of narG, were amplified with PCR techniques using genomic L. plantarum WCFS1 DNA as a template (Table 1 lists the primers used). The amplified DNA fragments were cloned, using Escherichia coli as the host strain, and blunt ended in vector pNZ5319 digested with SwaI (upstream fragment) and Ecl36II (downstream fragment) to produce the knockout vector pNZ5319_NarG_KO. The knockout plasmid was subsequently transformed in L. plantarum WCFS1. A chloramphenicol replacement of narG by a double-crossover event was isolated: NarGΔ (Cmr Erys). This procedure for construction of the narG mutant follows the general procedures described previously (23). The isogenic mutants lacking a functional ndh1 or cydA were constructed in a similar process using primers described in Table 1.
TABLE 1.
Primers used in this study
| PCR amplification 1 kb: | Forward primer
|
Reverse primer
|
||
|---|---|---|---|---|
| Name | Sequence | Name | Sequence | |
| Upstream of ndh1 | P93 | GGCAAATCAGCATAGTGTTCCTG | P92 | GGCTCGCATCCCTGCGTAAC |
| Downstream of ndh1 | P94 | GGAACGGTCTTCAGTAAAGG | P95 | CGCATCAATGAATCAGTCCATG |
| Upstream of narG | P101 | CCAGTCAGTAATAGCTGCTAA | P100 | CGATAAGACCTCCTTTATCAC |
| Downstream of narG | P102 | CGGAAGTTAAAGAAGGTGAAC | P103 | CGAATTCTGAGCAGCTTCCA |
| Upstream of cydA | P111 | GCTGAATCGGTCGTTGATTTAG | P110 | CGCCAAGGATAAAATACTTAATC |
| Downstream of cydA | P112 | GCGTTCAGTCATGAGTAATCT | P113 | CGCACCAGTGATACTGTCGT |
Analytical determinations.
Nitrate and nitrite were determined by photometric endpoint determination, using the nitrite/nitrate colorimetric method and Nitrate NO3− kits (Roche Diagnostics GmbH, Mannheim, Germany), as described previously (4, 6, 22).
Nitrate reduction was further confirmed via high-pressure liquid chromatography. Ions were separated on a Dionex column (Ionpac AS9-SC) with an eluent consisting of 1.8 mM Na2CO3 and 1.7 mM NaHCO3 at a flow rate of 1 ml/min at room temperature. The anions were detected with suppressed conductivity. Ammonia was quantified using the standard glutamate dehydrogenase-based method (Roche Diagnostics GmbH, Mannheim, Germany).
HPLC analyses.
Organic acids were also measured by high-performance liquid chromatography (HPLC) techniques (37). Sugars were analyzed by HPLC using a chromatographic system consisting of a precolumn packed with a cation-exchange resin, AG50W-X4, 400 mesh (Bio-Rad, Hercules, CA), and AG3-X4A, 200/400 mesh (35:65; Bio-Rad), and a cation exchanger in a prepacked column (RT 300-7.8 Polyspher CHPb; 300 by 7.8 mm; Merck, Darmstadt, Germany). The samples were eluted with an isocratic pump system (Shimadzu Corporation, Kyoto, Japan) using water as the mobile phase. Detection was carried out using a refractive index detector, ERC-7512 (Erma). Organic acids were analyzed by HPLC using a chromatographic system consisting of a cation-exhange column (Rezex ROA-Organic Acid H+ [8%]; 300 by 7.8 mm; Phenomenex), and the samples were eluted with an isocratic pump system (Shimadzu Corporation, Kyoto, Japan) using 0.005 M H2SO4 as the mobile phase. Detection was carried out using a refractive index detector, ERC-7512 (Erma), and by spectrophotometrically measuring UV light absorbance at 210 nm and 290 nm (Spectra Physics UV1000).
Oxygen uptake measurements.
A biological oxygen monitor (YSI model 5300; YSI Scientific, OH) with a Clark-type polarographic oxygen probe and a 15-ml sample chamber were used to measure dissolved oxygen at room temperature (20°C). Cells were washed three times in 50 mM potassium phosphate (pH 5.0) prior to each measurement. The dissolved oxygen of air-saturated buffer was calibrated using air-saturated water. The electrode was allowed to equilibrate in air-saturated buffer for 5 min before the start of each experiment. After this equilibration, cells were added to a final optical density at 600 nm (OD600) of 1.0. After approximately 5 min, glucose was added to a final concentration of 13 mM, and the decrease in oxygen levels was followed for 10 min. At the end of this analysis, 10 ml of the cell suspension from the chamber was used for dry-weight determination.
RESULTS
The L. plantarum WCFS1 aerobic electron transport chain.
A cydABCD operon that encodes a bd-type cytochrome was found in the genome of L. plantarum WCFS1. However, supplementation with heme alone did not result in a respiration-like phenotype (increase in biomass) in aerobic non-pH-controlled batch cultures. This supplementation with heme did induce catalase (peroxidase) activity (data not shown), as reported for other LAB (1).
Apparently, heme-cultivated L. plantarum still lacks components essential for restoration of the aerobic electron transport chain. Indeed, the genome of L. plantarum lacks a complete (mena)quinone biosynthesis gene set. Menaquinones are common membrane-integral components of bacterial electron transport chains (39).
When aerobic cultures of L. plantarum were supplemented with both heme and a menaquinone source, in the form of vitamin K2, and incubated for 48 h, a greater biomass and higher final pH were observed (Table 2). The higher final pH, induced by the combined addition of heme and menaquinone, coincided with a pronounced shift from lactic to acetic acid production (data not shown). This metabolic shift clearly indicated that a more oxidative metabolism had occurred.
TABLE 2.
Aerobic phenotypes of L. plantarum WCFS1 wild-type and CydAΔ and Ndh1Δ isogenic mutant cells
| Cell | Additiona
|
OD600b | pHb | O2 consumption (mg O2/g [dry wt]/min)b | |
|---|---|---|---|---|---|
| Heme | K2 | ||||
| Wild type | + | + | 9.88 ± 0.25 | 4.51 ± <0.01 | 36.4 ± 3.1 |
| Wild type | + | − | 6.75 ± 0.22 | 3.96 ± 0.01 | NDc |
| Wild type | − | + | 7.55 ± 0.11 | 3.96 ± 0.01 | ND |
| Wild type | − | − | 7.04 ± 0.08 | 3.96 ± 0.01 | 12.2 ± 0.7 |
| CydAΔ | + | + | 5.56 ± 0.17 | 3.94 ± 0.02 | 8.9 ± 1.2 |
| CydAΔ | − | − | 5.69 ± 0.13 | 3.96 ± 0.01 | ND |
| Ndh1Δ | + | + | 7.22 ± 0.2 | 3.96 ± 0.01 | 15.2 ± 0.4 |
| Ndh1Δ | − | − | 6.79 ± 0.1 | 3.95 ± <0.01 | ND |
+, added; −, not added.
The values are averages ± standard deviations.
ND, not done.
Loss of heme and menaquinone stimulation in CydAΔ and Ndh1Δ strains.
The aerobic branch of the L. lactis electron transport chain terminates in a cydABCD-encoded bd-type cytochrome (9, 15). A mutant of L. plantarum WCFS1 in which the cydA gene was replaced by an antibiotic resistance marker lost the heme- and menaquinone-induced phenotype (Table 2). Furthermore, in the genome of L. plantarum, two putative NADH dehydrogenase genes (ndh1 and ndh2) have been annotated. As aerobic electron transport chains canonically use NADH as an electron donor, these genes are likely candidates to encode this activity. The predicted ndh1 gene product has no membrane-spanning helices, although it lies in an operon-like structure upstream of a gene encoding an integral membrane protein, with which it may be associated (Fig. 1). The acetate kinase gene (ack2) is positioned upstream of ndh1. Activation of the aerobic electron transport chain induced a higher metabolite flux toward acetate. We constructed a mutant lacking a complete ndh1 gene, which also failed to be stimulated by heme and menaquinone (Table 2).
FIG. 1.
Genomic region of ndh1 and ack2.ndh1 (NADH dehydrogenase) forms part of an operon-like structure with a transcription factor (c) and an integral membrane protein (d) that neighbors the acetate kinase gene (ack2). ack2 itself is flanked by another integral membrane protein (a) and a putative acetyl transferase (b).
The final biomass of the two isogenic mutants (CydAΔ and Ndh1Δ) at most equaled that of unsupplemented wild-type cells, while their final pH was unaffected by supplementation. Furthermore, heme and menaquinone addition approximately tripled the oxygen consumption rate of wild-type cells (Table 2). Again, heme and menaquinone addition did not induce a comparable change in phenotype of the CydAΔ and Ndh1Δ isogenic mutants.
The L. plantarum WCFS1 genome codes for nitrate reductase genes.
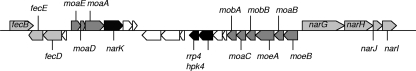
Genes that encode a putative nitrate-reductase complex (narGHJI) were identified in the genome of L. plantarum WCFS1 (21). The homologous genes in Bacillus subtilis and E. coli encode a membrane-bound nitrate reductase that permits anaerobic nitrate respiration. This active nitrate reductase A contains several cofactors: a heme moiety, a molybdenum-pterin cofactor, and iron-sulfur clusters. Although L. plantarum WCFS1 does not produce heme, molybdopterin cofactor biosynthesis genes (moaABEDA, mobAB, and moeAB) were found in close proximity to the narGHJI operon (Fig. 2). In addition, genes coding for an iron transport complex (fecBED), a nitrite extrusion protein (narK), and a kinase/response regulator system (rrp4 and hpk4) were found in its vicinity (8, 35). The gene product of narK may also promote nitrate import into the cytoplasm, in addition to its role in nitrite excretion (10).
FIG. 2.
The genomic region upstream of the nar operon (narGHJI) in L. plantarum WCFS1. Genes coding for molybdopterin cofactor biosynthesis (moaABEDA, mobAB, and moeAB) (dark grey), for iron transport (fecBED and narGHJI) (light grey), and for nitrite extrusion (narK) and a kinase/response regulator system (rrp4 and hpk4) (black) are found in this region. Putative genes are indicated by white arrows.
Growth conditions that allow nitrate reduction.
Anaerobic overnight cultivation of L. plantarum WCFS1 in standard MRS broth supplemented with nitrate did not lead to production of detectable amounts of nitrite. Indeed, as mentioned above, the narGHJI operon is predicted to encode a nitrate reductase A type that in other microorganisms is associated with electron transport (7, 17). Reconstitution of the L. plantarum electron transport chain by the addition of heme and menaquinone (vitamin K2) did lead to an observable, but low, level of nitrite production. When the glucose levels (normally present at 20 g/liter [110 mM] in MRS broth) were reduced, however, nitrite production was stimulated (Fig. 3).
FIG. 3.
Nitrate reduction and nitrite formation by L. plantarum WCFS1 in the presence of glucose. The bars indicate the average values ± the standard deviation. Wild-type cells were grown overnight in anaerobic batch cultures in modified MRS broth. Acetate and citrate were omitted from the standard MRS broth recipe. This medium was supplemented with heme, menaquinone, 16 mM nitrate, and various concentrations of glucose (glu): 0, 5, 10, 20, and 40 mM.
The requirement for both heme and menaquinone (vitamin K2) in order to induce nitrite production was verified in chemically defined medium with 10 mM glucose. When heme or menaquinone alone was added to the culture medium, only minimal amounts of nitrite were formed (26 ± 1.3 μM). Addition of both compounds increased nitrite formation by 25-fold to 728 ± 77.3 μM. Next, to demonstrate the requirement for the narGHJI operon in order to reduce nitrate, a narG knockout mutant (NarGΔ) was constructed. In this case, no nitrite production by the NarGΔ strain could be measured when it was grown in the presence of heme and menaquinone. As mentioned above, traces of nitrite were still produced by the wild-type cells when either heme or menaquinone was omitted. Clearly, the nitrate reductase complex has a much reduced activity, but it still retains some residual activity even without these cofactors.
Finally, we tested whether the ndh1 gene product, required for the activity of the aerobic electron transport chain, is also involved in the ability of cells to use nitrate as a redox sink. Using anaerobic batch conditions that stimulated nitrite formation in the wild type, we observed that the Ndh1Δ mutant still produced high levels of nitrite (at about 67% of wild-type levels).
Effect of mannitol on nitrate reductase activity.
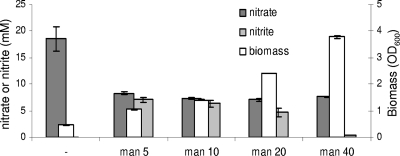
As shown above, the presence of high glucose levels resulted in a clearly decreased level of (anaerobic) nitrate reduction (Fig. 3). In contrast, high concentrations of the reduced sugar mannitol did not seem to repress nitrate reduction (Fig. 4). Anaerobic growth with the reduced sugar mannitol as the main carbon source requires a proportional concentration of an electron acceptor (27). Standard MRS broth contains citrate, which can be used for this purpose. Extensive anaerobic growth of L. plantarum in MRS broth (in which glucose was replaced with mannitol) required the presence of citrate or a combination of heme, vitamin K2, nitrate, and a functional narG gene (Table 3). In addition, these results indicate that the residual nitrate reductase activity observed in the presence of only heme or menaquinone does not sustain anaerobic growth on mannitol. Thus, only the fully reconstituted anaerobic electron transport chain of L. plantarum is able to use nitrate as an electron sink.
FIG. 4.
Nitrate reduction and nitrite formation by L. plantarum WCFS1 in the presence of mannitol. The bars indicate the average values ± standard deviations. Wild-type cells were grown overnight in anaerobic batch cultures in MRS broth from which acetate, citrate, and glucose were omitted (modified MRS broth). This medium was further supplemented with heme, menaquinone, nitrate, and various concentrations of mannitol (man) (in mM). Nitrate reduction is not repressed at high mannitol concentrations.
TABLE 3.
Residual nitrate concentration and biomass of L. plantarum WCFS1 wild type and NarGΔ grown anaerobically with mannitol as a carbon source
| Cell | Addition(s) | Residual NO3 (mM)a,b | Biomassa (OD600) |
|---|---|---|---|
| Wild type | Heme, K2, NO3 | 10.12 ± 0.82 | 3.88 ± 0.51 |
| Wild type | Heme, NO3 | 16.69 ± 1.42 | 0.88 ± 0.02 |
| Wild type | K2, NO3 | 18.94 ± 1.59 | 0.85 ± 0.03 |
| Wild type | Heme, K2 | 0.46 ± 0.46 | 0.95 ± 0.02 |
| NarGΔ | Heme, K2, NO3 | 19.34 ± 0.53 | 0.91 ± 0.02 |
The values are averages ± standard deviations.
20 mM of nitrate was used as the starting concentration.
At higher mannitol concentrations (10 mM to 40 mM), progressively less nitrite was formed, while nitrate consumption remained relatively stable. As growth on mannitol requires an electron sink, it appears that nitrite can be actively consumed as an electron sink, as well (Fig. 4). We have experimentally verified that L. plantarum cells actively remove nitrite and that the contribution of chemical degradation to this removal is minimal. Furthermore, during pH-controlled anaerobic growth on 40 mM mannitol with nitrate as a redox sink, nitrite was mostly transient, and high levels of ammonia were formed (10.7 ± 1.03 mM). In comparison, only 1.35 ± 0.63 mM of ammonia was produced when citrate was supplied as a redox sink. The reduction of nitrite appears not to be negatively affected by a high glucose concentration and, in contrast to the reduction of nitrate, does not require heme, menaquinone, or a functional narG (data not shown).
DISCUSSION
L. plantarum, as a typical LAB, has traditionally been considered an obligate fermenter. Here, we propose that L. plantarum in fact contains a branched electron transport chain capable of using oxygen or nitrate as an extracellular electron acceptor. The genome of L. plantarum WCFS1 indeed contains the cydABCD genes, encoding a bd-type cytochrome, and the narGHJI genes, encoding a nitrate reductase A (21). The electron transport chain requires activation by the addition of the heme cofactor and a menaquinone pool in the form of vitamin K2.
The proposed aerobic electron transport chain is simple and nonredundant and consists of an NADH dehydrogenase (Ndh1), a menaquinone pool (vitamin K2), and a bd-type cytochrome (Fig. 5). We also propose a simple structure of the nitrate-reducing chain, consisting of at least three parts, a dehydrogenase, a menaquinol pool, and the nitrate reductase complex. As the Ndh1Δ mutant was still able to reduce nitrate, another dehydrogenase must be involved in menaquinone reduction in the anaerobic branch. Anaerobic growth on mannitol as the main carbon source requires the presence of a proportional amount of an extracellular electron acceptor (27). The putative candidate for the dehydrogenase of the anaerobic electron transport chain is therefore likely to oxidize (indirectly) one of the intermediates of primary metabolism. In any case, to our knowledge, this is the first report of nitrate reduction by a LAB that is associated with an electron transport chain.
FIG. 5.
Proposed branched electron transport chain of L. plantarum WCFS1 that terminates in either a nitrate reductase A or a bd-type cytochrome complex. The dashed arrows represent the extracellular origin of menaquinone (vitamin K2) and heme. Red. sub., reduced substrate; Oxid. sub., oxidized substrate; mQ, menaquinone; mQH2, menaquinol.
The bd-type cytochrome consists of two subunits (CydA and CydB). CydC and CydD are required for assembly of the oxidase (12). This bd-type cytochrome is found in various (facultative) aerobic bacteria (19), where it functions as a proton motive force generating an (alternative) terminal electron acceptor. Its ability to generate a proton motive force and to permit respiration has also been shown in L. lactis MG1363 (9). This cytochrome is capable of working (and being activated) particularly under low-oxygen conditions (20, 33). Activation of this aerobic electron transport chain leads to a significant increase in biomass formation in non-pH-controlled batch cultures of L. plantarum WCFS1.
The second branch of the electron transport chain is active under anaerobic conditions and terminates in a nitrate reductase A complex. This type of nitrate reductase A has been extensively studied in E. coli. It consists of three subunits (α, β, and γ) encoded by narG, narH, and narI, respectively. The α and β subunits form a complex that contains a molybdenum-pterin cofactor and four iron-sulfur clusters (5, 26). The narI subunit contains two heme moieties, provides binding to the membrane, and interacts with the quinone pool (32, 44). narJ is required for full activity of the membrane-bound nitrate reductase (14). It has an established function in an anaerobic electron transport chain and generates a proton motive force (24, 39, 41, 45). The nitrate reductase complex and the bd-type cytochrome of E. coli are assumed not to be actual proton pumps themselves, since scalar chemistry alone could account for the 2H+/NO3 and 2H+/O2 translocation efficiencies (17, 29).
We have shown conclusively that heme and menaquinone can reconstitute an active aerobic and anaerobic electron transport chain in L. plantarum. Whether a proton motive force can be formed via these, and possibly other, electron transport chains and thus enable a respiratory metabolism will form the subject of further research.
As shown here, high mannitol levels do not inhibit nitrate reductase activity, in contrast to high glucose levels. This specific sensitivity to glucose suggests an active glucose-induced repression. The location of the response regulator and the sensor protein (rrp4 and hpk4) that are found on the genome in close proximity to the nar genes makes them promising candidates to be involved in transcriptional regulation of the nitrate reductase genes.
The use of either oxygen or nitrate as an extracellular electron acceptor by L. plantarum only in the presence of heme and menaquinone may seem to be an artificially induced phenotype. However, these compounds are also present in more “natural” environments, such as plant- and meat-based food fermentation and the human gastrointestinal (GI) tract. First, nitrate is a natural compound found in green plants and drinking water and is also used as a curing salt in meat fermentation (16, 25, 28). Second, live L. plantarum cells, present in many fermented food products, are consumed by humans. Third, heme, as a component of cytochromes, is present in all meat and plant products that are consumed. Finally, menaquinones are produced in the GI tract by the indigenous microflora (11). The combination of nitrate (and perhaps also oxygen), live Lactobacillus spp., heme, and menaquinones in the human GI tract, for example, may be quite common.
It would be extremely interesting, and relevant, to study the positive or negative influence of nitrate reduction on the (probiotic) effect of Lactobacillus strains on human health. Furthermore, as in the case of L. lactis, exploitation of the aerobic-respiration-like phenotype for commercial purposes, such as the starter culture industry, appears likely (30; P. Duwat, A. Gruss, Y. Le Loir, and P. Gaudu, 1999, French patent application FR2798670).
Acknowledgments
We acknowledge Pascal Hols for his help in initiating this project by sharing his observation of a heme- and menaquinone-induced altered aerobic phenotype in L. plantarum WCFS1.
The Kluyver Centre for Genomics of Industrial Fermentation is part of The Netherlands Genomics Initiative.
Footnotes
Published ahead of print on 3 April 2009.
REFERENCES
- 1.Abriouel, H., A. Herrmann, J. Starke, N. M. Yousif, A. Wijaya, B. Tauscher, W. Holzapfel, and C. M. Franz. 2004. Cloning and heterologous expression of hematin-dependent catalase produced by Lactobacillus plantarum CNRZ 1228. Appl. Environ. Microbiol. 70:603-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adawi, D., S. Ahrne, and G. Molin. 2001. Effects of different probiotic strains of Lactobacillus and Bifidobacterium on bacterial translocation and liver injury in an acute liver injury model. Int. J. Food Microbiol. 70:213-220. [DOI] [PubMed] [Google Scholar]
- 3.Ahrne, S., S. Nobaek, B. Jeppsson, I. Adlerberth, A. E. Wold, and G. Molin. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Appl. Microbiol. 85:88-94. [DOI] [PubMed] [Google Scholar]
- 4.Arneth, W., and B. Herold. 1988. Nitrat/Nitrit-Bestimmung in Wurstwaren nach enzymatischer Reduction. Fleischwirtschaft 68:761-764. [Google Scholar]
- 5.Augier, V., M. Asso, B. Guigliarelli, C. More, P. Bertrand, C. L. Santini, F. Blasco, M. Chippaux, and G. Giordano. 1993. Removal of the high-potential [4Fe-4S] center of the beta-subunit from Escherichia coli nitrate reductase. Physiological, biochemical, and EPR characterization of site-directed mutated enzymes. Biochemistry 32:5099-5108. [DOI] [PubMed] [Google Scholar]
- 6.Beutler, H.-O., B. Wurst, and S. Fisher. 1986. Eine neue Methode zur enzymatischen Bestimmung van Nitrat in Lebensmitteln. Dtsch. Lebensmittel-Rundschau 82:283-289. [Google Scholar]
- 7.Bonnefoy, V., and J. A. Demoss. 1994. Nitrate reductases in Escherichia coli. Antonie van Leeuwenhoek 66:47-56. [DOI] [PubMed] [Google Scholar]
- 8.Braun, V., and C. Herrmann. 2007. Docking of the periplasmic FecB binding protein to the FecCD transmembrane proteins in the ferric citrate transport system of Escherichia coli. J. Bacteriol. 189:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooijmans, R. J., B. Poolman, G. K. Schuurman-Wolters, W. M. de Vos, and J. Hugenholtz. 2007. Generation of a membrane potential by Lactococcus lactis through aerobic electron transport. J. Bacteriol. 189:5203-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clegg, S., F. Yu, L. Griffiths, and J. A. Cole. 2002. The roles of the polytopic membrane proteins NarK, NarU and NirC in Escherichia coli K-12: two nitrate and three nitrite transporters. Mol. Microbiol. 44:143-155. [DOI] [PubMed] [Google Scholar]
- 11.Conly, J. M., and K. Stein. 1992. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog. Food Nutr. Sci. 16:307-343. [PubMed] [Google Scholar]
- 12.Cruz-Ramos, H., G. M. Cook, G. Wu, M. W. Cleeter, and R. K. Poole. 2004. Membrane topology and mutational analysis of Escherichia coli CydDC, an ABC-type cysteine exporter required for cytochrome assembly. Microbiology 150:3415-3427. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham-Rundles, S., S. Ahrne, S. Bengmark, R. Johann-Liang, F. Marshall, L. Metakis, C. Califano, A. M. Dunn, C. Grassey, G. Hinds, and J. Cervia. 2000. Probiotics and immune response. Am. J. Gastroenterol. 95:S22-S55. [DOI] [PubMed] [Google Scholar]
- 14.Dubourdieu, M., and J. A. DeMoss. 1992. The narJ gene product is required for biogenesis of respiratory nitrate reductase in Escherichia coli. J. Bacteriol. 174:867-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duwat, P., S. Sourice, B. Cesselin, G. Lamberet, K. Vido, P. Gaudu, Y. Le Loir, F. Violet, P. Loubiere, and A. Gruss. 2001. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J. Bacteriol. 183:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangolli, S. D., P. A. van den Brandt, V. J. Feron, C. Janzowsky, J. H. Koeman, G. J. Speijers, B. Spiegelhalder, R. Walker, and J. S. Wisnok. 1994. Nitrate, nitrite and N-nitroso compounds. Eur. J. Pharmacol. 292:1-38. [DOI] [PubMed] [Google Scholar]
- 17.Garland, P. B., J. A. Downie, and B. A. Haddock. 1975. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem. J. 152:547-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudu, P., K. Vido, B. Cesselin, S. Kulakauskas, J. Tremblay, L. Rezaiki, G. Lamberret, S. Sourice, P. Duwat, and A. Gruss. 2002. Respiration capacity and consequences in Lactococcus lactis. Antonie van Leeuwenhoek 82:263-269. [PubMed] [Google Scholar]
- 19.Jünemann, S. 1997. Cytochrome bd terminal oxidase. Biochim. Biophys. Acta 1321:107-127. [DOI] [PubMed] [Google Scholar]
- 20.Kita, K., K. Konishi, and Y. Anraku. 1986. Purification and properties of two terminal oxidase complexes of Escherichia coli aerobic respiratory chain. Methods Enzymol. 126:94-113. [DOI] [PubMed] [Google Scholar]
- 21.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretzschmar, R., and T. Kretschmar. 1988. Enzymatische Nitrat-Bestimmung in kommunalen Abwasser. Vom Abwasser 70:119-128. [Google Scholar]
- 23.Lambert, J. M., R. S. Bongers, and M. Kleerebezem. 2007. Cre-Lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl. Environ. Microbiol. 73:1126-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanciano, P., A. Magalon, P. Bertrand, B. Guigliarelli, and S. Grimaldi. 2007. High-stability semiquinone intermediate in nitrate reductase A (NarGHI) from Escherichia coli is located in a quinol oxidation site close to heme bD. Biochemistry 46:5323-5329. [DOI] [PubMed] [Google Scholar]
- 25.Linseisen, J., S. Rohrmann, T. Norat, C. A. Gonzalez, M. Dorronsoro Iraeta, P. Morote Gomez, M. D. Chirlaque, B. G. Pozo, E. Ardanaz, I. Mattisson, U. Pettersson, R. Palmqvist, B. Van Guelpen, S. A. Bingham, A. McTaggart, E. A. Spencer, K. Overvad, A. Tjonneland, C. Stripp, F. Clavel-Chapelon, E. Kesse, H. Boeing, K. Klipstein-Grobusch, A. Trichopoulou, E. Vasilopoulou, G. Bellos, V. Pala, G. Masala, R. Tumino, C. Sacerdote, M. Del Pezzo, H. B. Bueno-de-Mesquita, M. C. Ocke, P. H. Peeters, D. Engeset, G. Skeie, N. Slimani, and E. Riboli. 2006. Dietary intake of different types and characteristics of processed meat which might be associated with cancer risk—results from the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 9:449-464. [DOI] [PubMed] [Google Scholar]
- 26.Magalon, A., M. Asso, B. Guigliarelli, R. A. Rothery, P. Bertrand, G. Giordano, and F. Blasco. 1998. Molybdenum cofactor properties and [Fe-S] cluster coordination in Escherichia coli nitrate reductase A: investigation by site-directed mutagenesis of the conserved His-50 residue in the NarG subunit. Biochemistry 37:7363-7370. [DOI] [PubMed] [Google Scholar]
- 27.McFeeters, R. F., and K. Chen. 1986. Utilization of electron acceptors for anaerobic mannitol metabolism by Lactobacillus plantarum, compounds which serve as electron acceptors. Food Microbiol. 3:73-81. [Google Scholar]
- 28.McKnight, G. M., C. W. Duncan, C. Leifert, and M. H. Golden. 1999. Dietary nitrate in man: friend or foe? Br. J. Nutr. 81:349-358. [DOI] [PubMed] [Google Scholar]
- 29.Miller, M. J., and R. B. Gennis. 1985. The cytochrome d complex is a coupling site in the aerobic respiratory chain of Escherichia coli J. Biol. Chem. 260:14003-14008. [PubMed] [Google Scholar]
- 30.Pedersen, M. B., S. L. Iversen, K. I. Sorensen, and E. Johansen. 2005. The long and winding road from the research laboratory to industrial applications of lactic acid bacteria. FEMS Microbiol. Rev. 29:611-624. [DOI] [PubMed] [Google Scholar]
- 31.Rezaiki, L., B. Cesselin, Y. Yamamoto, K. Vido, E. van West, P. Gaudu, and A. Gruss. 2004. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol. Microbiol. 53:1331-1342. [DOI] [PubMed] [Google Scholar]
- 32.Rothery, R. A., F. Blasco, A. Magalon, M. Asso, and J. H. Weiner. 1999. The hemes of Escherichia coli nitrate reductase A (NarGHI): potentiometric effects of inhibitor binding to NarI. Biochemistry 38:12747-12757. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto, J., E. Koga, T. Mizuta, C. Sato, S. Noguchi, and N. Sone. 1999. Gene structure and quinol oxidase activity of a cytochrome bd-type oxidase from Bacillus stearothermophilus. Biochim. Biophys. Acta 1411:147-158. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 35.Shanmugam, K. T., V. Stewart, R. P. Gunsalus, D. H. Boxer, J. A. Cole, M. Chippaux, J. A. DeMoss, G. Giordano, E. C. Lin, and K. V. Rajagopalan. 1992. Proposed nomenclature for the genes involved in molybdenum metabolism in Escherichia coli and Salmonella typhimurium. Mol. Microbiol. 6:3452-3454. [DOI] [PubMed] [Google Scholar]
- 36.Sijpesteijn, A. K. 1970. Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in Streptococcus lactis and Leuconostoc mesenteroides. Antonie van Leeuwenhoek 36:335-348. [DOI] [PubMed] [Google Scholar]
- 37.Starrenburg, M. J., and J. Hugenholtz. 1991. Citrate fermentation by Lactococcus and Leuconostoc spp. Appl. Environ. Microbiol. 57:3535-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teusink, B., F. H. van Enckevort, C. Francke, A. Wiersma, A. Wegkamp, E. J. Smid, and R. J. Siezen. 2005. In silico reconstruction of the metabolic pathways of Lactobacillus plantarum: comparing predictions of nutrient requirements with those from growth experiments. Appl. Environ. Microbiol. 71:7253-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unden, G., and J. Bongaerts. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim. Biophys. Acta 1320:217-234. [DOI] [PubMed] [Google Scholar]
- 40.Vesa, T., P. Pochart, and P. Marteau. 2000. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment Pharmacol. Ther. 14:823-828. [DOI] [PubMed] [Google Scholar]
- 41.Wang, H., C. P. Tseng, and R. P. Gunsalus. 1999. The napF and narG nitrate reductase operons in Escherichia coli are differentially expressed in response to submicromolar concentrations of nitrate but not nitrite. J. Bacteriol. 181:5303-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winstedt, L., L. Frankenberg, L. Hederstedt, and C. von Wachenfeldt. 2000. Enterococcus faecalis V583 contains a cytochrome bd-type respiratory oxidase. J. Bacteriol. 182:3863-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto, Y., C. Poyart, P. Trieu-Cuot, G. Lamberet, A. Gruss, and P. Gaudu. 2006. Roles of environmental heme, and menaquinone, in Streptococcus agalactiae. Biometals 19:205-210. [DOI] [PubMed] [Google Scholar]
- 44.Zhao, Z., R. A. Rothery, and J. H. Weiner. 2003. Effects of site-directed mutations on heme reduction in Escherichia coli nitrate reductase A by menaquinol: a stopped-flow study. Biochemistry 42:14225-14233. [DOI] [PubMed] [Google Scholar]
- 45.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]