Abstract
Ghrelin is a powerful orexigenic peptide predominantly secreted by the stomach. Blood concentration of ghrelin increases before meals and fall postprandial. Its regulation appears to be influenced by the type of macronutrient ingested, the vagus nerve stimulation and by other post-meal stimulated hormonal factors. However, the direct role of nutrients (amino acids or lipids), neuronal (vagal neurotransmitter acetylcholine) and satiety-inducing factor such as CCK are not known. To study this we applied amino acids, lipids, acetylcholine and CCK via vascular perfusion to the isolated stomachs and found that amino acids significantly reduced ghrelin release from the isolated stomach by approximately ~30% vs the control while lipids (10% Intralipid) had no affect. Acetylcholine (1μM) increased ghrelin release from the stomach by ~37% whereas insulin (10nM) decreased it by ~30% vs the control. Interestingly, CCK (100nM) potently increased ghrelin release by ~200% vs the control. Therefore it appears that ghrelin secretion from the stomach is under direct influence of amino acids, neurotransmitter acetylcholine and hormones such as insulin and CCK.
Keywords: ghrelin, glucose, amino acid, lipid, acetylcholine, CCK and insulin
Introduction
The stomach is the major source of circulating ghrelin in rodents and humans [2, 18]. Circulating ghrelin concentrations rise before meals [7] and fall after meals or gastrointestinal nutrient infusion [38, 40]. Also, during negative energy balance, such as insulin-induced hypoglycemia, chronic leptin infusion, and low-protein diets increased gastric ghrelin gene expression, whereas high caloric diets decreased [36]. In rats, carbohydrates more potently decreased gastric ghrelin gene and peptide levels compared to fats. In humans, although both carbohydrates and fats decreased ghrelin levels, protein diet increased [11, 32]. Macronutrients can stimulate the release of several gastrointestinal (GI) peptides (e.g. insulin, glucagon, CCK, peptide YY (PYY), gastric inhibitory peptide (GIP) and glucagon like peptide-1 (GLP-1)); moreover insulin and glucagon can directly reduce or stimulate ghrelin release, respectively, from isolated perfused stomach [9, 19]. However, it is unclear whether a specific nutrient directly affects ghrelin release from the stomach.
Recent reports suggest that CCK inhibits peripheral ghrelin’s orexigenic effect by attenuating the neuronal activity in the arcuate nucleus (ARC) of the hypothalamus [9, 20]. Peripheral ghrelin as well as CCK mediate their orexigenic and anorexigenic effects, respectively, via the vagus afferent terminals, where GHS-R and CCK-AR (CCK receptor type A) are co-localized, and also when centrally administrated [3, 6, 9]. Protein, fats and glucose stimulate plasma CCK levels in rodents and humans [5, 23]. In addition, CCK delays gastric emptying whereas ghrelin stimulates [10, 13]. Functionally, ghrelin and CCK seem to antagonize one another; however a direct role of CCK on ghrelin in the stomach has not been tested.
Peripheral ghrelin administration stimulates food intake and this effect is abolished by blocking the vagal afferent nerves by capsaicin treatment [8]. Vagotomy disrupted fasting-induced elevation of plasma ghrelin but not the post-meal suppression of ghrelin. Also, antagonism of the muscarinic receptors substantially inhibited ghrelin release in fasted rats suggesting that ghrelin release is under the influence of the vagus nerve [41]. Cholinergic activation also stimulates release of other GI peptides such as insulin, somatostatin and glucagon that have been shown to influence ghrelin release [17, 19, 28]. Therefore, in order to determine the direct effect of cholinergic stimulation, nutrients and hormones on ghrelin release from the stomach, we examined the affect of acetylcholine, amino acids, lipids, and CCK on ghrelin release in the vascularly perfused isolated rat stomach.
Materials and Methods
Animals
Male Sprague-Dawley (SD) rats (Harlam, N.C.), weighing 400–450 grams, were housed in conventional hanging cages with a 12 hrs light/12 hrs dark photoperiod (lights on at 07:00) in a temperature-controlled room (21–22 °C). Rats had ad libitum access to standard chow (Purina 5001, Purina Mills, Richmond, IN, USA) and water. All animal procedures were conducted in accordance with established guidelines of the University of Georgia Institutional Animal Care and Use Committee.
Nutrients and drugs
Intralipid emulsion (10%), MEM amino acid solution (50X), Insulin and Acetylcholine were purchased from Sigma-Aldrich (MO, USA). CCK-octapeptide (26–33) was purchased from Peptides International (KY, USA).
Preparation of isolated stomach perfusion
After 36 hours of food deprivation rats were anesthetized with an intramuscular (i.m.) injection of a cocktail of ketamine: xylazine: atropine solution (75:0.04:10 mg/kg of body weight) at a dose of 0.13 ml/100 g body weight. However, in the experiment where the effect of acetylcholine on ghrelin release from the stomach was studied, atropine was not included in the cocktail solution, only ketamine: xylazine with similar ratio and dose was used to anesthetize the rats. Following anesthetization, a ~2 cm midline laparotomy was made and the connective tissues surrounding the gastric artery and gastric vein were carefully removed and the vagus nerve was excised. To the exposed side of the stomach and the gastric vessels, 1 mL heparin solution (10U heparin in 1mL saline) was dispensed to prevent clotting. Subsequently, the gastric artery and the gastric vein were cannulated with stretched polyethylene tubes (10 PE). Tubing inserted into the gastric artery was perfused at a rate of 0.58ml/min with basal media containing 5.5mM glucose in Krebs-Ringer bicarbonate buffer at pH at 7.4 and warmed at 37°C. Basal media also included heparin at 5U/ml and was continuously gassed with a mixture of 95% O2 and 5% CO2. The exposed stomach and the peritoneum were at all times covered with warm saline-drenched gauze to prevent from drying due to the heat lamp that was placed over the stomach to maintain its temperature at 37°C.
Experimental design
An optimization period of 30 min was performed with the basal media (containing 5.5mM glucose in Krebs-Ringer bicarbonate buffer at pH at 7.4); the first 15 min of the optimization period was done with the basal media that included heparin (5U/mL) but the remaining incubation did not include heparin. Following optimization, a further 10 min perfusion was done with the latter media (not including heparin) for measurement of basal level of total ghrelin release into the effluent. Total ghrelin was measured in this study because it has been shown to serve as good surrogate for measurement of the biologically active form ser-3 octanylated ghrelin. Two major forms of ghrelin are released from stomach, octanylated and des-octanylated and their ratio is generally found to remain constant across a wide range of physiological conditions that are known to affect ghrelin levels [1, 26, 27]. After an equilibration period of 30 min and a basal period of 10 min, the treatments was added to the perfusate for 15 min followed by another 10 min perfusion without the treatments. All treatment drugs and nutrients were added to the basal media and the glucose concentration was always maintained at 5.5mM. Treatments included amino acids (Table 1), 10% Intralipid (Table 2), CCK (100nM), Insulin (10nM), or acetylcholine (1μM). Treatment doses were determined based on previous studies [15, 19, 29, 34, 39]. The viability of isolated stomachs was monitored at the end of each experiment by infusion of potassium chloride (60mM) and measurement of the increase of ghrelin [14, 24]. In each stomach, only one experimental condition was examined. Following each treatment, a 10 min wash out period was included. All venous effluent were collected in eppendorf tubes containing EDTA/2Na (1.5mg/mL), aprotinin (500U/mL), and 1N HCl (26.5μL/mL) and stored at −80°C until assay.
Table 1.
Amount of the amino acids that was included in the amino acids media perfused into the isolated stomach of rats
| Components of Amino Acids | |
|---|---|
| amino acids | g/L |
| L-Arginine.HCl | 0.632 |
| L-Cystine | 0.1565 |
| L-Histidine·HCL·H2O | 0.21 |
| L-Isoleucine | 0.2625 |
| L-Leucine | 0.26 |
| L-Lysine·HCL | 0.3625 |
| L-Methionine | 0.0755 |
| L-Phenylalanine | 0.165 |
| L-Threonine | 0.238 |
| L-Tryptophan | 0.051 |
| L-Tyrosine | 0.18 |
| L-Valine | 0.234 |
| L-Glutamine | 1.46 |
Table 2.
Amount of the lipids and its constituent fatty acids expressed in percentage in the 10% Intralipid media perfused into the isolated stomach of rats.
| components of 10% Intralipid | |
|---|---|
| Soybean oil | 20% |
| egg yolk phospholipids | 1.20% |
| glycerin | 2.25% |
| cholesterol | N/A |
| water | 76.55% |
| major component of fatty acids | |
| linoleic | 44–62% |
| oleic | 1–30% |
| plamitic | 7–14% |
| linolenic | 4–11% |
| stearic | 1.4–5.5% |
Radioimmunoassay (RIA)
Total ghrelin levels were determined by a commercially available rat total ghrelin radioimmunoassay kit (RIA) from Linco Research (Millipore, MA, USA), using rat ghrelin as standards. All samples were run in triplicates and only samples with the intra assay coefficient of variation below 10% were considered for statistical analysis.
Statistical analysis
Due to the number of animals (n=4) included in each experimental group, a paired t-test was used on data that passed the normality test (basal media vs treatment media on each animal) and those data that did not pass the normality test Wilcoxon Signed Ranks Test was chosen to statistically analyze the data. Effects due to treatments were considered significant when p values were less than 0.05. Statistical analysis was performed using the Sigma Plot (ver.11, Systat Software Inc, CA, USA).
Results
Effect of nutrients on ghrelin release in the isolated stomach perfusion
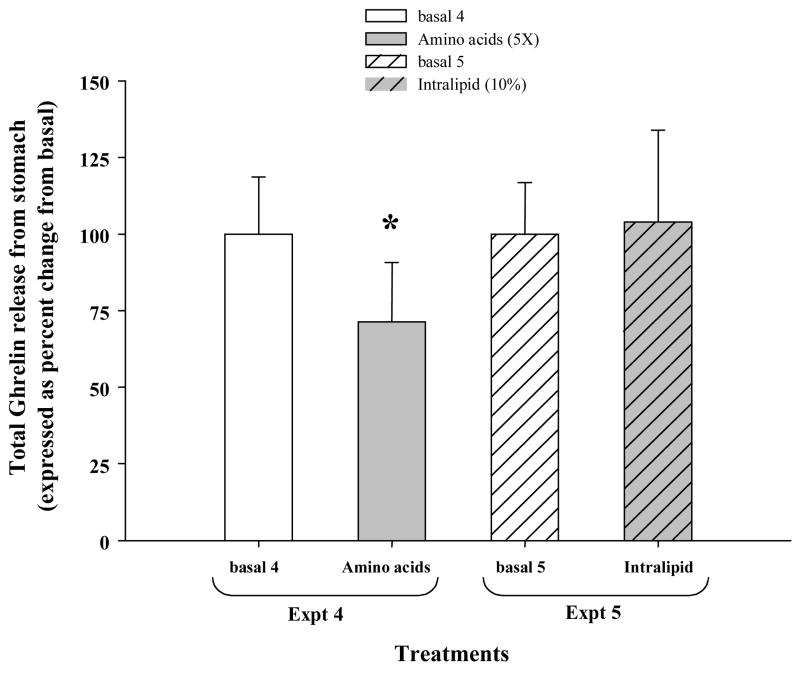
Amino acids added in the basal media significantly reduced total ghrelin release by approximately ~30% compared to that during the 10 min of basal media perfusion (Fig 2, Expt 4, p=0.011). However, 10 % Intralipid perfusion did not change the total ghrelin release from the basal level (Fig 2, Expt 5). KCl (60mM) challenge for 15 min markedly increased the ghrelin release in the isolated stomach (>200 % of basal level ghrelin release), suggesting that the tissue was viable during the perfusion experiments (data not shown).
Figure 2.
Paired sampling between basal and treatment was performed within each experimental group, separately; Expt 4, Treatments: basal 4 and amino acid (5X) and Expt 5, Treatments: basal 5 and Intralipid (10%). At the end of the experiments a 15 min KCl (60mM) challenge was performed to test the viability of the isolated stomach. A significant increase in ghrelin release was determined in all the experiments indicating that the stomach was viable during treatments (data not shown for clarity). Data is represented as percent change compared to the mean of the basal group, (n=4 rats/group). Asterisk indicates p <0.05 compared to its paired basal group.
Effect of Acetylcholine, CCK and insulin on ghrelin release in the isolated stomach perfusion
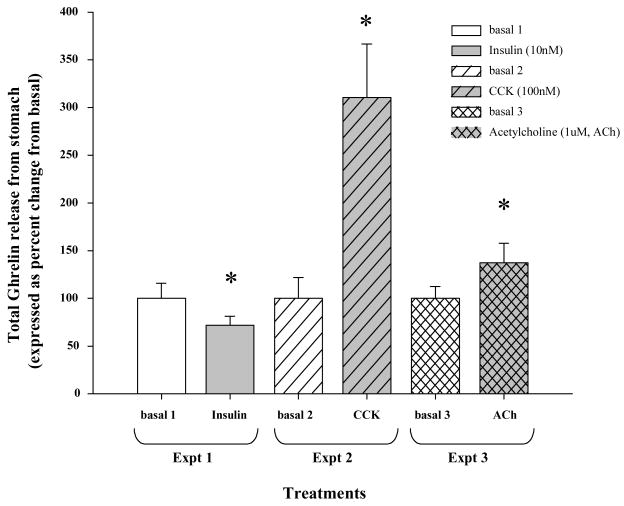
Acetylcholine chloride (1μM) added in the basal media increased total ghrelin release in the isolated stomach with respect to basal level by ~37% (Fig 1, Expt 3, p=0.017). Insulin (10nM) on the other hand decreased total ghrelin release by ~30% (Fig 1, Expt 1, p=0.043). Interestingly, CCK (100nM) treatment most potently increased total ghrelin release from the stomach by ~200% (Fig 1, Expt 2, p=0.018). KCl (60mM) challenge for 15 min markedly increased the ghrelin release in the isolated stomach (>300% of basal level ghrelin release), suggesting that isolated stomach was viable during the perfusion experiments (data not shown).
Figure 1.
Paired sampling between basal and treatment was performed within each experimental group, separately; Expt 1, Treatments: basal 1 and Insulin (10nM), Expt 2, Treatments: basal 2 and CCK (100nM), and finally Expt 3, Treatments: basal 3 and Acetylcholine (1μM). At the end of the experiments a 15 min KCl (60mM) challenge was performed to test the viability of the isolated stomach. A significant increase in ghrelin release was determined in all the experiments indicating that the stomach was viable during treatments (data not shown for clarity). Data is represented as percent change compared to the mean of the basal group, (n=4 rats/group). Asterisk indicates p <0.05 compared to its paired basal group.
Discussion
We found that perfusion of amino acids into the isolated stomach significantly reduced ghrelin release. This finding correlates with previous reports where amino acids infused into the duodenum or jejunum potently suppressed ghrelin levels [29]. However, in humans, oral protein load did not affect ghrelin levels whereas glucose and lipid meals decreased them [16]. In another report, enriched protein diet increased ghrelin secretion compared to carbohydrate or fat diets in rats [39]. The reason behind these conflicting reports is unclear. However, the difference between our isolated stomachs versus whole animal studies could be due to the direct effect of amino acids on ghrelin cells independent of other factors from the GI tract that are influenced by protein or amino acid content in circulation in the latter.
Recently, a report demonstrated that ghrelin secretion was reduced in fasted rats by intragastric infusion of fats, protein and carbohydrates [15]. The report also demonstrated that intravenous infusion of triglyceride emulsion or dextrose decreased ghrelin secretion. Elsewhere, total parenteral nutrition (TPN), given intravenously, including lipids, amino acids, glucose and minerals and vitamins, significantly reduced plasma ghrelin levels compared to fasted as well as fed rats, suggesting that nutritional status may be more important than actual presence of food or nutrients in the GI tract [30]. Eight days on TPN induced a hyperlipidemic state in the experimental animals but no change in insulin or glucose was found, suggesting that hyperlipidemia may contribute to lower serum ghrelin levels. However, our results show that fat emulsion (Intralipid 10%) administered directly into the gastric circulation in the isolated stomach of rats did not affect the ghrelin release. The reason for this lack of effect may be that lipids may not directly affect ghrelin producing (Gr) cells in the stomach but probably do affect post-absorption from the intestines, via factors secreted by other entero-endocrine cells influenced by lipids. This possibility is absent in our study because intestines were surgically separated during the isolation of the stomach from the GI tract. Dietary fat can activate secretion of CCK [22, 33] and administration of soybean trypsin inhibitor (SBTI), a secretagogue for intestinal CCK secretion has been shown to significantly reduce ghrelin secretion in fasted rats [15]. Also a fat diet can activate PYY and GLP-1, and both have been shown to decrease ghrelin levels in vivo and from isolated stomach [4, 24].
Acetylcholine potently and significantly increased ghrelin release from isolated stomachs of rats. This is in line with other reports that cholinergic blockade by atropine significantly decreased plasma ghrelin in overnight fasted rats [25] or by sub-diaphragmatic vagotomy [41]. In contrast, others have reported that ghrelin secretion is regulated by cholinergic neurons of the vagus and that cholinergic activity suppresses ghrelin secretion in sheep [34].
Interestingly, we found that CCK (100nM) significantly and potently increased ghrelin release from the isolated stomach. A similar effect was reported where a single bolus injection of CCK (20μg/kg body weight) in rats significantly increased ghrelin release by approximately 4-fold, comparable to 12h fasted ghrelin levels [26]. After adjusting for the doses, approximately 1.5 μg of CCK (since ~10 ml of 100nM CCK was actually perfused) was used in our study, which would be approximately 1/10th the dose used in the study by Murakami and collaborators [26]. Because of the lower dose used in our study we may have observed a slightly lower stimulation on ghrelin release, i.e. 2-fold compared to ~ 4–fold increase seen in their report. However, we agree that it is not possible to accurately determine the actual amount of CCK that the stomach may have been exposed to in their report. Nevertheless, the results are congruent and suggest that CCK may directly stimulate ghrelin secretion from the stomach.
A recent report elegantly demonstrated that ghrelin levels were significantly lower in CCK-AR (−/−) BR(−/−) mice than in wild-type mice, and no change in response to fasting was observed [31]. A similar phenomenon was reported in that the lack of both CCK and gastrin, but not gastrin alone, attenuated fasting-induced ghrelin gene expression and secretion. Also CCK administration increased ghrelin expression in the fundus, where the Gr cells express the CCK-A receptors [12]. Our result is in agreement with these reports in that CCK directly stimulates ghrelin release from the stomach. However, the physiological interpretation of this is challenging. CCK is well documented as a satiety factor, released post-absorption of nutrients, such as fats and proteins. In contrast ghrelin, an orexigenic factor, increases during pre-meal and decreases post-meal, suggesting functional antagonism between the two factors. Therefore, how is it possible that a satiety factor, CCK, increases production and secretion of an orexigenic factor, ghrelin? Interestingly enough the satiety signal CCK, appears to have a rather unconventional role on ghrelin secretion. However, a scenario could be imagined where CCK, stimulated by diets with high fat and protein, could induce satiety as well as stimulate ghrelin secretion, which has being shown to inhibit fat utilization [35, 37], thus promoting fat accumulation with diets rich in fat and protein.
We also found that insulin significantly reduced ghrelin release in isolated stomach. This is in congruence with a previous report where insulin decreased ghrelin release from the isolated stomach [19]. However, others have reported that insulin injected twice daily for 3 days resulted in increased stomach and plasma ghrelin levels in vivo [21]. It is unclear at this time what could have accounted for this conflict, we think that perhaps the subsequent insulin injection-induced hypoglycemia, may have accounted for the stimulation of ghrelin release in the latter study.
In conclusion, our results show that nutrients can directly influence ghrelin secretion and that the Gr cells in the stomach may be stimulated directly by cholinergic system via its neurotransmitter, acetylcholine.
Acknowledgments
This work was supported by the National Institute of Health (NIDDK-DK 59836). The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ariyasu H, Takaya K, Hosoda H, Iwakura H, Ebihara K, Mori K, et al. Delayed short-term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology. 2002;143:3341–50. doi: 10.1210/en.2002-220225. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–8. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–60. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 5.Burton-Freeman B, Davis PA, Schneeman BO. Interaction of fat availability and sex on postprandial satiety and cholecystokinin after mixed-food meals. The American Journal of Clinical Nutrition. 2004;80:1207–14. doi: 10.1093/ajcn/80.5.1207. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–12. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 8.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, et al. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146:3518–25. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- 10.Dornonville de la Cour C, Lindstrom E, Norlen P, Hakanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul Pept. 2003;116:101–7. doi: 10.1016/s0167-0115(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 12.Friis-Hansen L, Wierup N, Rehfeld JF, Sundler F. Reduced ghrelin, islet amyloid polypeptide, and peptide YY expression in the stomach of gastrin-cholecystokinin knockout mice. Endocrinology. 2005;146:4464–71. doi: 10.1210/en.2004-0938. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda H, Mizuta Y, Isomoto H, Takeshima F, Ohnita K, Ohba K, et al. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurones in rats. Scand J Gastroenterol. 2004;39:1209–14. [PubMed] [Google Scholar]
- 14.Giraudo SQ, Mullen BJ, Seerley RW, Azain MJ, Martin RJ. Somatostatin and growth hormone-releasing factor release from Zucker rat hypothalamic tissue. Brain research bulletin. 1992;29:853–8. doi: 10.1016/0361-9230(92)90155-q. [DOI] [PubMed] [Google Scholar]
- 15.Gomez G, Englander EW, Greeley GH., Jr Nutrient inhibition of ghrelin secretion in the fasted rat. Regul Pept. 2004;117:33–6. doi: 10.1016/j.regpep.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Greenman Y, Golani N, Gilad S, Yaron M, Limor R, Stern N. Ghrelin secretion is modulated in a nutrient- and gender-specific manner. Clin Endocrinol (Oxf) 2004;60:382–8. doi: 10.1111/j.1365-2265.2004.01993.x. [DOI] [PubMed] [Google Scholar]
- 17.Honey R, Weir G. Acetylcholine stimulates insulin, glucagon, and somatostatin release in the perfused chicken pancreas. Endocrinology. 1980;107:1065–8. doi: 10.1210/endo-107-4-1065. [DOI] [PubMed] [Google Scholar]
- 18.Hosoda H, Kangawa K. Ghrelin and GH secretion. Nippon Ronen Igakkai Zasshi. 2003;40:341–3. doi: 10.3143/geriatrics.40.341. [DOI] [PubMed] [Google Scholar]
- 19.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept. 2004;119:77–81. doi: 10.1016/j.regpep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Kobelt P, Tebbe JJ, Tjandra I, Stengel A, Bae HG, Andresen V, et al. CCK inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol. 2005;288:R751–8. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- 21.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH., Jr Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143:185–90. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- 22.Lewis L, Williams JA. Regulation of cholecystokinin secretion by food, hormones, and neural pathways in the rat. AmJPhysiol. 1990;258:G512–G8. doi: 10.1152/ajpgi.1990.258.4.G512. [DOI] [PubMed] [Google Scholar]
- 23.Liddle RA, Green GM, Conrad CK, Williams JA. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. The American journal of physiology. 1986;251:G243–8. doi: 10.1152/ajpgi.1986.251.2.G243. [DOI] [PubMed] [Google Scholar]
- 24.Lippl F, Kircher F, Erdmann J, Allescher HD, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regul Pept. 2004;119:93–8. doi: 10.1016/j.regpep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Maier C, Schaller G, Buranyi B, Nowotny P, Geyer G, Wolzt M, et al. The cholinergic system controls ghrelin release and ghrelin-induced growth hormone release in humans. J Clin Endocrinol Metab. 2004;89:4729–33. doi: 10.1210/jc.2004-0656. [DOI] [PubMed] [Google Scholar]
- 26.Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, et al. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174:283–8. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- 27.Nakai Y, Hosoda H, Nin K, Ooya C, Hayashi H, Akamizu T, et al. Plasma levels of active form of ghrelin during oral glucose tolerance test in patients with anorexia nervosa. Eur J Endocrinol. 2003;149:R1–3. doi: 10.1530/eje.0.149r001. [DOI] [PubMed] [Google Scholar]
- 28.Norrelund H, Hansen TK, Orskov H, Hosoda H, Kojima M, Kangawa K, et al. Ghrelin immunoreactivity in human plasma is suppressed by somatostatin. Clin Endocrinol (Oxf) 2002;57:539–46. doi: 10.1046/j.1365-2265.2002.01649.x. [DOI] [PubMed] [Google Scholar]
- 29.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146:845–50. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- 30.Qader SS, Salehi A, Hakanson R, Lundquist I, Ekelund M. Long-term infusion of nutrients (total parenteral nutrition) suppresses circulating ghrelin in food-deprived rats. Regul Pept. 2005;131:82–8. doi: 10.1016/j.regpep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai C, Ohta M, Kanai S, Uematsu H, Funakoshi A, Miyasaka K. Lack of ghrelin secretion in response to fasting in cholecystokinin-A (−1), -B (−2) receptor-deficient mice. J Physiol Sci. 2006;56:441–7. doi: 10.2170/physiolsci.RP003306. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez J, Oliver P, Palou A, Pico C. The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient. Endocrinology. 2004;145:5049–55. doi: 10.1210/en.2004-0493. [DOI] [PubMed] [Google Scholar]
- 33.Spannagel AW, Nakano I, Tawil T, Chey WY, Liddle RA, Green GM. Adaptation to fat markedly increases pancreatic secretory response to intraduodenal fat in rats. The American journal of physiology. 1996;270:G128–35. doi: 10.1152/ajpgi.1996.270.1.G128. [DOI] [PubMed] [Google Scholar]
- 34.Sugino T, Yamaura J, Yamagishi M, Kurose Y, Kojima M, Kangawa K, et al. Involvement of cholinergic neurons in the regulation of the ghrelin secretory response to feeding in sheep. Biochem Biophys Res Commun. 2003;304:308–12. doi: 10.1016/s0006-291x(03)00593-x. [DOI] [PubMed] [Google Scholar]
- 35.Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–93. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toshinai K, Mondal MS, Shimbara T, Yamaguchi H, Date Y, Kangawa K, et al. Ghrelin stimulates growth hormone secretion and food intake in aged rats. Mech Ageing Dev. 2007;128:182–6. doi: 10.1016/j.mad.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 38.Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 39.Vallejo-Cremades MT, Gomez-Garcia L, Chacatas-Cortesao M, Moreno C, Sanchez M, De Miguel E, et al. Enriched protein diet-modified ghrelin expression and secretion in rats. Regul Pept. 2004;121:113–9. doi: 10.1016/j.regpep.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology. 2003;144:2765–7. doi: 10.1210/en.2003-0381. [DOI] [PubMed] [Google Scholar]
- 41.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short-and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–7. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]